Enhancing Perovskite Solar Cells With Rare-Earth Metal Doped Zinc Oxide: A Review of Electron Mobility, Stability, and Photocarrier Recombination
Abstract
Zinc oxide (ZnO) is an important electron transfer layer (ETL) material due to its optical and electrical properties, maintaining its pivotal role in advancing perovskite solar cells (PSCs) given its high experimental accessibility and reported high power conversion efficiencies (PCEs). Recent studies reveal that doping ZnO nanomaterials with dual-functioning rare-earth metal (REM) ions can further bolster PCEs of ZnO-based PSCs. This review synthesizes recent empirical studies on REM-doped ZnO, focusing on enhancing PSC electron mobility, stability, and mitigating photocarrier recombination. Additionally, it examines the shift from mesoscopic to planar PSC architectures, underscores synthesis/fabrication strategies, and investigates REMs’ potential in ZnO for up/down conversion processes. Despite potential cost implications, REMs consistently achieve remarkable PCEs of up to 22.9% in ZnO-based devices.
Summary
- •
Explores the dual-functioning rare-earth metal (REM) ions in zinc oxide (ZnO) perovskites solar cell.
- •
Examines the shift from mesoscopic to planar perovskite solar cell (PSC) architectures.
- •
It addresses the challenges related to toxicity concerns, stability issues, and scalability considerations associated with rare earth ion-doped ZnO nanomaterials
1. Introduction
Perovskite solar cells (PSCs) are a promising alternative to traditional silicon-based solar cells, offering high-power conversion efficiency (PCE), cost-effectiveness, and customizable optical and electronic properties [1, 2]. The term “perovskite” originates from the crystal structure of perovskite calcium titanate (CaTiO3), sharing similarities with perovskite materials. Typically, a perovskite material, often a metal halide compound, is positioned between an electron transport layer (ETL) and a hole-transport layer (HTL) in PSC configurations. When exposed to light, the perovskite material absorbs photons, generating free holes and electrons collected by the transport layers to produce an electrical current [3]. Perovskites have the chemical formula ABX3, as depicted in Figure 1, with A representing a monovalent cation, B denoting a divalent metal cation (e., and X corresponding to a halogen anion [e.g., I, Br, Cl]). The distinct crystal structure of perovskites has driven extensive research during the decade prior because of their diverse properties, including superconductivity, ferroelectricity, and photovoltaic activity.
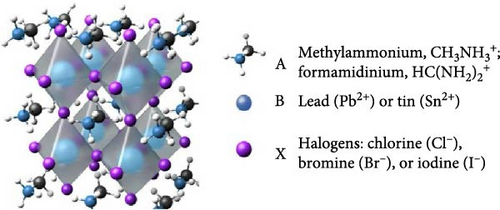
Methylammonium lead iodide (CH3NH3PbI3, abbreviated MAPbI) is prominent amongst these materials [4] because it notably achieves PSCs with remarkable PCEs of up to 25.2% in laboratory settings [2], but with a host of contemporary issues pertaining to PCE and ambient stability. These issues have prompted investigations into modifiers such as rare-earth metal (REM)-doped zinc oxide (ZnO) nanomaterials with the potential to modify their optical, electrical, and charge transport properties [5, 6].
The last few years have seen remarkable progress in PSCs. Initially demonstrated in 2009 with an efficiency of 3.8%, PSCs have achieved efficiencies exceeding 25%, rivaling the best silicon-based solar cells. These advancements stem from a comprehensive approach involving material optimization, device engineering, and deeper insights into the underlying physics and chemistry of PSCs [5].
Despite these strides, several challenges persist before PSCs can achieve commercial viability. Chief among these is the stability of PSC devices. When exposed to heat, light, and moisture, perovskite materials are susceptible to deterioration, impacting their long-term performance and durability. Ongoing research focuses on developing encapsulation techniques, moisture-resistant materials, and robust device architectures to bolster stability and reliability [7, 8]. Scalability is another critical consideration. While most PSCs in literature are lab-scale, large-scale commercial production necessitates efficient and cost-effective fabrication methods. Techniques like scalable deposition processes (e.g., blade coating, slot-die coating), printing methods, and roll-to-roll manufacturing are being explored to enable high-throughput, low-cost production [9, 10]. Moreover, the potential toxicity of certain PSC materials raises environmental and health concerns, particularly due to lead content.
Research is actively exploring lead-free compositions and alternative material systems that maintain efficiency while being environmentally friendly and devoid of toxic elements. This includes investigating lead-free perovskite materials and incorporating REMs and other dopants to enhance performance and reduce toxicity [11, 12]. However, despite these challenges, the promising potential of PSCs as a cost-effective and efficient solar technology has garnered significant attention from academia and industry alike. PSCs offer benefits such as solution processability, tunable bandgaps, and flexibility in substrate choice, paving the way for diverse applications such as wearable electronics, portable electronics, and photovoltaics integrated into buildings. Extensive collaborative efforts between academia and industry are ongoing to surmount remaining hurdles and drive widespread adoption of PSCs as a clean and sustainable energy solution [6, 13].
Hence, this review article concentrates on the synthesis and characterization of rare earth (RE) ion-doped ZnO nanomaterials and their potential role in enhancing PSC performance. By integrating REMs into ZnO nanostructures, material properties such as optical, electrical, and charge transport can be tailored, leading to improved PSC efficiency and stability. The review offers a thorough examination of synthesis techniques for introducing REMs into ZnO nanomaterials, encompassing doping strategies and growth methods. It also scrutinizes characterization techniques used to assess the structural, optical, and electrical properties of these doped nanomaterials, emphasizing parameters relevant to PSC performance. Notably, the article underscores the importance of optimizing synthesis processes to attain precise control over doping concentrations, crystal structures, and morphologies, all crucial for the functionality of RE ion-doped ZnO nanomaterials in PSCs. Additionally, it delves into the effects of RE ion doping on ZnO properties, including bandgap engineering and charge carrier dynamics, elucidating the mechanisms behind enhanced PSC performance. Addressing challenges related to toxicity concerns, stability issues, and scalability considerations associated with RE ion-doped ZnO nanomaterials, this review offers valuable insights for future research and development. Overall, it consolidates current knowledge and advancements in the field, providing guidance to researchers and engineers and contributing to the advancement of next-generation photovoltaic technologies.
1.1. ZnO as an ETL
In PSCs, the ETL plays a pivotal role in facilitating electron movement from the perovskite layer to the electrode [7]. ZnO, a wide-bandgap semiconductor with high electron affinity and exciton binding energy, serves as an excellent ETL candidate in electronic devices [8, 9]. Its superior electron mobility ensures efficient transport and minimizes charge carrier recombination at the ETL-device active layer interface. ZnO boasts unique physical and chemical properties, including a room temperature band gap of ~3.37 eV, making it a promising material for ultraviolet (UV) blue devices such as short-wavelength LEDs and laser diodes [10, 11]. Manipulating ZnO’s particle size can alter its electrical structure, conductivity, melting point, and mechanical characteristics [12, 13]. Various growth processes enable the synthesis of ZnO in diverse nanostructures like nanorods (NRs), nanowires (NWs), and nanotubes, offering versatility for applications [14–18]. ZnO has a wurtzite crystal structure with lattice constants of 3.249 (a) and 5.207 (b). Its strong excitonic binding energy of 60 MeV and precise crystal structure make it suitable for room-temperature UV lasing and blue-UV light emission [19]. Because of its lack of center symmetry, mechanical stress or strain can be converted into electrical voltage and vice versa [19]. Because ZnO single crystals have high electron diffusion coefficients, they have better mobility and improved electron transport, which enhances device performance [20]. ZnO is interesting for several applications, such as semiconductor and laser devices, piezoelectric transducers, transparent electronics, surface acoustic wave devices, spin functional devices, and gas sensing, because of its near UV emission, transparent conductivity, and piezoelectric qualities [21].
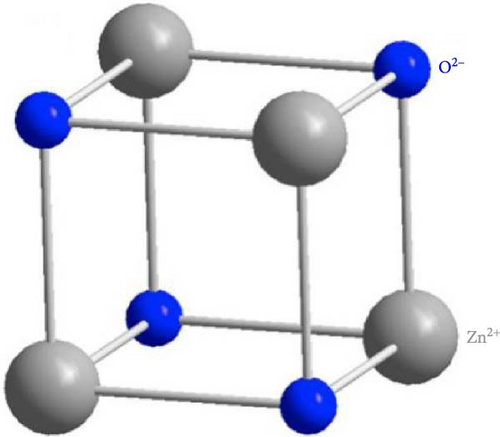
The surge in publication and research interest in ZnO semiconductor materials underscores exponential growth in understanding and exploration. ZnO can be produced on a small or large scale at low cost and is considered nontoxic. The three crystal forms of ZnO are wurtzite, zinc blende, and rock salt (Figure 2). Its hexagonal wurtzite phase, the most common and stable structure, is characterized by four zinc ions (Zn2+) at tetrahedral corners and one oxygen ion (O2−) at the center, and vice versa [23]. Although the zinc blend and rock salt structures are less common, they have their unique arrangements. ZnO’s expanding popularity in optoelectronics stems from its wide band gap of ~3.3 eV (corresponding to a wavelength of 376 nm) at 300 K, which can be tailored by factors like crystallite size, doping, and codoping [24–30].
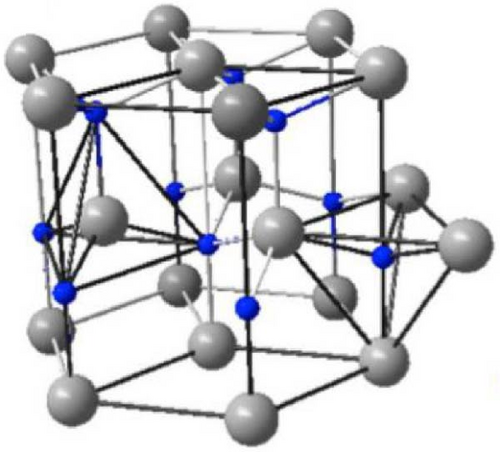

In contrast to their bulk and thin-film counterparts, one-dimensional (1D) ZnO nanostructures, which are distinguished by defect-free high crystallinity, have attracted a lot of attention among ZnO nanomaterials because of their distinct chemical, electrical, physical, and mechanical properties [31]. However, many growth methods either require high-temperature wet-chemical syntheses using hazardous solvents or involve costly manufacturing processes, making it difficult to synthesize defect-free 1D ZnO nanostructures with the desired form and composition. Molecular beam epitaxy (MBE), electrochemical deposition, chemical vapor deposition (CVD), and pulse-laser deposition (PLD) are among the processes used to produce ZnO nanostructures [32]. The transparency of ZnO in the visible (Vis) spectrum enables efficient photon extraction from the active layer, prompting extensive research on ZnO as an ETL in both organic and inorganic solar cells as well as LEDs. Incorporating ZnO as an ETL has resulted in significant enhancements in device performance, including improved power stability, efficiency, and device stability [33–37]. Despite its advantages, pure ZnO has limitations affecting the performance of PSCs. One major challenge is its high carrier concentration, leading to elevated recombination rates and reduced performance [38, 39]. Additionally, controlling the morphology and crystal structure of pure ZnO influences its optical and electronic properties, while its large bandgap limits efficiency in some applications and absorption of photons in the Vis spectrum [40, 41]. To overcome these limitations, various strategies have been proposed, such as doping ZnO with REMs and other elements, surface modification, and the use of hybrid structures. These approaches have shown promising results in optimizing the stability, morphology, and efficiency of ZnO-based materials, leading to the development of new and enhanced applications [41–45]. Researchers have explored the integration of RE ion doping in ZnO to enhance the performance of PSCs. REMs have been found to reduce the carrier concentration of ZnO, increase electron mobility, and extend the absorption range into the Vis spectrum [46–48]. These improvements have resulted in PCEs of up to 22.9% for PSCs incorporating RE ion-doped ZnO ETLs [49]. Moreover, REMs can induce various effects on the properties of ZnO, including improved stability, modified optical and electronic properties, and enhanced performance in different applications.
1.2. The Dual Functionality of REMs in PSCs
REMs, also referred to as inner transition metals or lanthanides, encompass 15 lanthanides ranging from lanthanum (La) to lutetium, alongside scandium and yttrium in group III B [50, 51]. These elements share relatively similar physical and chemical properties, with electronic configurations spanning from 4 F0,2 to 14 5d0,1 6 s2. The presence of abundant 4f orbitals and specific vacant 5d orbitals in REMs enables the creation of a variety of intermediate energy states, endowing them with unique photophysical characteristics. When appropriately doped into materials like metal oxides and perovskites, REMs can alter the electrical properties of the host material. Typically existing in the 3+ or 2+ oxidation state, REMs exhibit strong interactions with negatively charged ions. Incorporating REMs into crystalline materials can influence crystallization dynamics, reduce intra-gap trap states, and modify unit cell characteristics and phase stability by substituting suitable ionic radii for elements within the crystal lattice. In multilayered PSCs, REMs can interact with the photoactive perovskites and charge-transporting metal oxides. Figure 3 illustrates the roles of these components [53–55]. The incorporation of REMs, particularly into perovskites or metal oxides, has a significant impact on the band structures, absorption/luminescence properties, and charge carrier mobility of the resulting materials. This leads to altered well-aligned energy levels and charge transport dynamics in the corresponding PSCs. The strong interaction between REMs and the halides in perovskite precursor also modifies perovskite crystallization, enabling lower formation barriers and customizable thin film morphology. Additionally, REMs can stabilize the crystal phase structure by improving crystal unit binding energy, customizing crystal size and the dimensions, and eliminating defect states. Furthermore, RE elements can enhance light harvesting by extending the spectral response through up conversion, down conversion, or light scattering properties [56]. By down-converting (DC) high-energy light into the Vis light band, they can also reduce light-induced performance deterioration and improve device stability.

RE ion doping in ZnO offers two key advantages: reduction of carrier concentration and enhancement of electron mobility. Pure ZnO typically exhibits high carrier concentration, leading to increased recombination rates and decreased device performance. Introducing REMs into the ZnO lattice effectively reduces carrier concentration, mitigating recombination losses and improving PSC efficiency [46, 48, 56]. Additionally, RE ion doping enhances electron mobility in ZnO by improving crystalline structure and modifying charge transport properties. This results in more efficient electron transport and reduced loss of charge carriers, enhancing device performance and PCEs [56–58]. Hall effect measurements conducted by Derouiche, Salhi, and Baklouti [59] revealed a reduction in resistivity and an increase in electron mobility due to doping, with REMs primarily occupying interstitial positions. Initially, carrier concentration rose with doping until reaching a composition of (5 Er, 5 Yb:ZnO), indicating substitution of Zn2+ ions by RE3+ ions. Further doping caused a decrease in carrier concentration, suggesting introduction of grain boundary defects and confirming n-type conductivity of the doped nanopowders. Soumahoro et al. [60] identified RE atoms in interstitial positions as donor impurities, with carrier concentration increasing up to (5 Er, 5 Yb:ZnO) and then decreasing. This rise in carrier concentration could result from substitution of Zn2+ ions by REMs or incorporation of REMs in interstitial sites [60]. Asikuzun et al. [61] supported this, attributing the decrease in carrier concentration to an increase in grain boundary defects that trapped free carriers. He et al. [62] evaluated electrical properties using Hall measurements, confirming n-type conductivity of ZnO and La-ZnO films. La-ZnO B film showed higher conductivity and carrier density than pure ZnO, but increased La-doping content decreased conductivity and carrier density. The higher conductivity was attributed to higher carrier concentration introduced by La3+ cations [62]. Senol [63] observed decreased carrier concentrations with Er and Yb doping, while Hall mobility and electrical resistivity increased compared to undoped ZnO nano powder at room temperature. Similarly, Chao, Liau, and Chang [64] noted decreased carrier concentration and increased resistivity and mobility with Er doping ratios of 1.1% and higher in ZnO [63].
1.3. Vis Spectrum Extension of Absorption
Another advantage of RE ion doping in ZnO is the extension of the absorption range of the material into the Vis spectrum. Pure ZnO has a relatively large bandgap, which limits its absorption of photons in the Vis region. However, by incorporating REMs into the ZnO lattice, the bandgap can be effectively tuned, allowing for absorption of a broader range of wavelengths, including those in the Vis spectrum. This expanded absorption range enables a more efficient utilization of solar energy, leading to enhanced light harvesting and improved device performance in PSCs [58]. Table 1 presents a comprehensive summary of RE ion-doped ZnO materials for the extension of the absorption range into the Vis spectrum, showcasing their diverse applications in PSCs and related technologies. Various dopants, such as La, Er, Yb, Gd, Eu, Tb, Nd, and Ce, were examined with different concentrations, absorption ranges, and application modes. The key advantage lies in effectively tuning the bandgap of ZnO through RE ion doping, enabling the absorption of a broader range of wavelengths, particularly in the Vis spectrum. This expanded absorption range contributes to enhanced solar energy utilization, leading to improved light harvesting and overall device performance in PSCs. Table 1 shows the versatility of RE ion-doped ZnO and emphasizes the importance of tailored doping strategies for specific applications in energy, optoelectronics, and photocatalysis.
| Dopant materials | Doping concentration | Absorption range (emission peaks) | Applications | Reference |
|---|---|---|---|---|
| La:ZnO | 2%, 5%, 10% | 350–500 nm | Dope in the ZnO electron layer/FTO substrates | [65] |
| Er, Yb: ZnO | 5 mol% | 525–550 and 665 nm | Enhancement of up conversion emission, electrical properties | [59] |
| Gd:ZnO | 2% and 4% | 358–371 nm | Energy generation | [66] |
| Eu:ZnO | 0.2–1.0 mol% | 325, 384, and 600 nm. | Optoelectronics, displays | [67] |
|
|
|
|
[68] |
| La:ZnO | 1, 5, and 10 mol% | 375, 376 and 378 nm | Photocatalyst for organic dye degradation | [69] |
| Y: ZnO | 3% | — | Electronic devices | [70] |
| Ce,Dy:ZnO | 0.01–0.05 M | 200–800 nm | Enhancement of photocatalytic activity | [71] |
| Nd:ZnO | 3%, 6%, and 9% | 387, 412, 438, 452, 477, and 525 nm | Deposition on glass substrate | [72] |
1.4. Improving Stability, Optical, and Electronic Properties
RE ion doping in ZnO presents the advantage of enhancing stability and modifying optical and electronic properties. Incorporating REMs into the ZnO lattice improves material stability against environmental factors like moisture, oxygen, and light exposure, crucial for long-term performance and reliability of PSCs. REMs can also introduce favorable optical and electronic properties to ZnO by altering its energy levels and electronic band structure, leading to improved charge separation, reduced recombination, and enhanced charge transport. Additionally, the luminescence of REMs opens avenues for applications in optoelectronic devices such as light-emitting devices and lasers [51, 58, 73, 74]. Various studies have addressed these issues. For instance, Cao et al. [75] demonstrated that introducing MgO-bridged ethanolamine enhanced ZnO compatibility with perovskite, improving the stability of the ZnO/perovskite interface. Similarly, Azmi et al. [76] utilized alkali metals to modify ZnO, passivating surface defects and establishing balanced energy levels. However, achieving synergy to enhance chemical compatibility between ZnO and perovskite interfaces, matching energy levels, and ensuring outstanding UV stability for further improving PSC efficiency and stability remains a challenge. The thermal stabilities of similar devices were examined by Meng et al. [77] in an inert environment at 85°C. The device with a modified electron transport material (ETM) retained 95% of its original PCE for ~150 h, while the unmodified device experienced a significant 40% drop in PCE under the same conditions. The improved chemical environment of the ZnO surface was credited with the treated device’s increased thermal stability. Additionally, perovskite films that were formed on ZnO and ZnO/CeOx substrates were heated in a nitrogen environment to 85°C. While the ZnO-based perovskite films started to become yellow, the modified film stayed black and underwent no change. This suggests that CeOx not only reduces surface defects on ZnO but also improves the moisture, UV, and thermal stability of PSCs [77]. The structural, optical, and photoluminescence (PL) characteristics of both pure ZnO and ZnO doped with REMs were assessed in this study. Data from thermal gravimetric analysis (TGA) showed that several ZnO systems were stable even at temperatures higher than 450°C. The differential thermal analysis (DTA) curve for ZnO demonstrated its commendable thermal stability even at temperatures higher than 450°C, with doped ZnO samples also exhibiting stable behavior above 450°C, highlighting their excellent thermal stability as reported by Al-Otaibi, Howsawi, and Ghrib [78]. Comparing PSCs with a pure ZnO ETL to those with ZnO/CeOx as the ETL, the optimal efficiency of MAPbI3 PSCs increased from 16.0% to 19.5%. However, challenges related to PSC stability persisted due to perovskite layer degradation caused by high-energy UV radiation. To address this issue, Zhang et al. [79] proposed using Eu3+ and Sm3+ codoped TiO2 as the ETL in planar MAPbI3 PSCs, leveraging the luminescence down-conversion capability, UV blocking and conversion abilities, and efficient energy transfer of REMs [80].
2. Preparation of REM-Doped ZnO
The integration of REMs into the ZnO lattice occurs in diverse synthesis methods to enhance its optical and electronic properties. Various techniques are utilized, each with distinct advantages and challenges, aiming for a uniform distribution of REMs within the ZnO matrix.
2.1. The Coprecipitation Method
The coprecipitation method involves adding a precipitating agent to an aqueous solution of metal ions, followed by calcination to produce the desired oxide material [74]. This approach provides a high yield of material at a reasonable cost and has been successfully used to dope ZnO with REMs such as Nd, Sm, and La [81]. However, it has limitations regarding repeatability and control over particle size and shape. Table 2 summarizes the synthesis of ZnO doped with REM (e.g., Gd, La, Sm, Tb, Dy, Er, and Ce) generally involves the coprecipitation method. RE nitrates (such as gadolinium nitrate, La nitrate, and cerium [Ce] nitrate) were employed as dopants in conjunction with zinc-based precursors such as zinc nitrate or zinc acetate. To aid in the precipitation process, NaOH was used to bring the reaction mixture’s pH down to predetermined levels (such as pH 14). At temperatures ranging from ambient temperature to 100°C, the solutions were swirled for 30 min to 4 h. Filtration, washing, and drying were postsynthesis procedures that frequently took place at temperatures of 80°C or 120°C. The dried precipitates were calcined for two to 3 h at higher temperatures, usually between 250 and 500°C, to improve their structure. The range of dopant concentrations was 1%–5% molar or weight percentages. To improve the precipitation process, organic solvents such as methylene blue or ethanol were occasionally used. To maximize the ZnO material’s structural, thermal, and optical qualities, each synthesis was customized according to the dopant. Table 2 offers a concise summary of coprecipitation’s use in preparing REM-doped ZnO, highlighting the range of dopants and synthesis parameters explored in reviewed studies.
Dopant material |
Chemical reagent/precursor | Doping concentration | Synthesis condition | Reference |
|---|---|---|---|---|
| Gd, La:ZnO | Zinc nitrate hexahydrate [Zn(NO3)2 ·6H2O], Gd(NO3)3·6H2O, La(NO3)3·6H2O | 5 mol% Ga, 3 mol% La | pH = 14 by adding NaOH; precipitate is cleaned, dried at 80°C, and then ground into a powder at 500°C for 2 h | [82] |
| Sm:ZnO | Zn (NO3)2·6H2O, Sm(NO3)3·6H2O, NaOH, methylene blue (C16H18ClN3S), deionized water (DI), and Ethanol | 1%, 3%, and 5% | Stirring for 30 min | [83] |
| Tb, Dy, and Er:ZnO | Zinc acetate dihydrate [(CH3COO)2 Zn·2H2O], terbium (III) nitrate pentahydrate [Tb(NO3)3·5H2O], dysprosium (III) nitrate hydrate [Dy(NO3)3·H2O], and erbium (III) nitrate pentahydrate [Er(NO3)3·5H2O] | 4 wt.% | Precipitate was filtered, cleaned, heated at 500°F for 3 h, and dried at 120°F | [84] |
| Er, Yb:ZnO | Zn(CH3COO)2·2H2O and erbium and ytterbium oxides (Er2O3, Yb2O3) | 1, 4, and 6 wt.% | At 100°C, the mixture was cooked for 4 h. The mixture was centrifuged for 4 h and 30 min at 8500 rpm. Precipitate was rinsed, dried, and heated to 500°C for 1 h before being calcined | [85] |
| Gd:ZnO |
|
1%, 2% | The mixture was kept in an oven at 80°C for 2 h. The created white precipitates are dried for 1 h in a 100°C oven. The powder was annealed at 250°C in air for 2 h | [86] |
| Ce:ZnO | Zn(NO3)2·6H2O, Ce(NO3)3·6H2O, and NaOH | 1.87%, 3.97%, and 5.85% | Heated for 3 h at 60°C and stirred for 30 min. The solution reacted for a day at 23°C and dried precipitate at 120°C for 1 h | [87] |
| Gd:ZnO | Zn(NO3)2, Gd(NO3)3, and sodium hydroxide (NaOH) | 3%,5%, 7% | After 30 min of stirring at room temperature, the mixture was kept at 85°C for 5 h. At 120°C, the precipitate was dried for 60 min | [88] |
| Eu, La:ZnO | Eu(NO3)3•6H2O, La(NO3)3•6H2O, and Zn(NO3)2•6H2O | 1 wt.% | After adding 30 ml of 3 M NaOH to the solution for 12 h, the slurry was dried for around 10 h at 80°C in an oven | [89] |
| Eu:ZnO | Zn(NO3)3·6H2O, Eu(NO3)3·5H2O, NaOH, ethanol, and distilled water | 3%, 6%, and 9% | Stirred for 40 min at room temperature, calcined in a muffle furnace at 500°C, and dried for 19 h in a hot air oven at 80°C | [90] |
2.2. Solid-State Reaction Method
The solid-state reaction involves directly combining ZnO powder with REMs, followed by high-temperature calcination to produce the final product. While this method is straightforward, cost-effective, and scalable, it may introduce contaminants and poses challenges in controlling dopant concentration. Solid-state reaction is commonly employed for synthesizing RE-doped materials, including up-conversion nanoparticles (UCNPs) and down-conversion nanoparticles (DCNPs) [91]. Despite its simplicity and versatility, high-temperature annealing often leads to uneven particles with a wide size distribution, which may not be ideal for regulating up-conversion/down-conversion processes in PSCs. Table 3 summarizes the preparation of RE ion-doped ZnO via the solid-state reaction method, detailing dopants such as Tb, Y, Eu, and Er, along with their chemical reagents, doping concentrations, and synthesis conditions. The primary chemical reagents include ZnO as the host material and RE oxides or salts (e.g., Eu2O3, Er2O3, Tb2O3, Eu(NO3)3·6H2O). Ethanol and bases like NaOH were occasionally used as part of the process. Concentrations ranged from 0.3 to 5 wt.% or more. Several doping levels including 0.3, 0.6, 0.9 and 1.2 wt.% were explored to study the effect of doping on ZnO properties. Frequently, materials were crushed into slurries or pellets and then dried at temperatures of about 100°C. A crucial phase was calcination, which included temperatures ranging from 700 to 1400°C. To maximize crystalline structure, heating and cooling rates were regulated, for example, by 20 and 5°C/min. Pellets were calcined at temperatures as high as 1400°C for up to 60 min. Before being heated further, slurries were made, dried, and refluxed at lower temperatures (e.g., 50°C for 3 h). For certain dopants (e.g., Er), additional reagents such as LiOH·H2O were used. Experiments with varying pressures (e.g., 8 Torr) were also conducted to study the impact on structural and optical properties.
| Dopants material | Chemical reagents | Doping concentration | Synthesis conditions | Reference |
|---|---|---|---|---|
| Tb, Y, and Eu:ZnO | ZnO, terbium oxide (Tb4O7), yttrium oxide (Y2O3), and europium oxide (Eu2O3) | 5% | Heated for 60 min at 1400°C at a rate of 20°C/min. After that, the pellets were grounded for an hour while cooling at of 5°C per min to room temperature | [78] |
| Er:ZnO | ZnO, Er2O3, LiOH·H2O, and ethanol | 0.3, 0.6, 0.9 and 1.2 wt.% | Pellets were put in a quartz boat and, once the slurry had dried at 100°C, burned at 900°C at 8 Torr of pressure | [92] |
| Er:ZnO | ZnO, Er2O3, NaOH, and ethanol | 1%–5% | Slurry was then dried at 100°C and heated for 5 h at 700°C | [93] |
| Eu:ZnO | Eu (NO3)3·6H2O and Zn (Ac)2·2H2O |
|
Heated, then mixed and refluxed at 50°C for 3 h, followed by drying in an oven at 80°C | [94] |
| Tb:ZnO | ZnO and Tb4O7 | 3%, 5%, and 7% | Heated to 1400°C at a rate of 20°C per minute, calcined for 60 min, and then cooled at a rate of 5°C per minute | [95] |
| Er:ZnO | ZnO, Er2O3, and LiOH·H2O | 0.3, 0.6, 0.9, and 1.2 wt.% | Slurry then dried at 100°C | [96] |
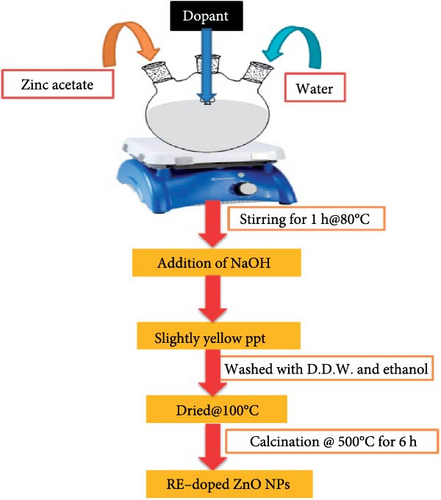
2.3. The Sol–Gel Method
The sol–gel method involves hydrolyzing and condensing metal alkoxides to create a colloidal solution, which is then annealed to form a solid oxide material, allowing precise control over morphology and composition. During gel formation, REMs are integrated into the ZnO lattice [97]. This technique has successfully doped ZnO with various REMs, including Eu, Tb, and Dy [98, 99]. However, it is time-consuming and requires high-temperature annealing, which can introduce impurities and defects. Figure 4 illustrates the synthesis of REM-doped ZnO nanoparticles (NPs) [100]. Table 4 summarizes the preparation of RE ion-doped ZnO via the sol–gel method, detailing dopants such as Ho, Sm, Tb, Tb3, La, Ce, Eu, Er, and Yb, along with their chemical reagents, doping concentrations, and synthesis conditions. For instance, Ho-doped ZnO, zinc acetate, and Ho nitrate were used as precursors, with doping concentrations of 1%, 3%, and 5%. Following a 5-min preheating period at 300°C and a 2-h heating period at 500°C, spin-coating was applied at 3000 rpm. Zinc and samarium acetate were used in Sm-doped ZnO at doping concentrations of 1%, 1.5%, and 2%. The powders were air-annealed for 3 h at 500°C. Zinc and terbium acetate were used as precursors for Tb-doped ZnO, with a doping range of 0.01%–0.3%, and they were agitated for an hour at 60°C. Additional instances include La, Ce, and Eu-doped ZnO, in which metal acetates (1 mol%) were agitated for an hour at 60°C. Zinc acetate and Er chloride were used in Er-doped ZnO, which was annealed at 600°C. ZnO particles doped with Sm were calcined at 500°C after being dried at 120°C. Glycine and nitrates were annealed at 1100°C for La and Y codoped ZnO. Lastly, zinc acetate and ytterbium nitrate were used in quantities of 0.05%–0.25% to create Yb-doped ZnO, which was then mixed and dried for 2 h at 80°C. To obtain the appropriate ZnO doped structures, factors including precursor type, doping level, and heat treatment were crucial. Each synthesis step differed based on the dopant. Table 4 offers a succinct overview of the sol–gel method’s application in preparing RE ion-doped ZnO, highlighting dopants, synthesis conditions, and associated challenges.
| Dopants | Chemical reagents/precursor | Doping concentration | Synthesis conditions | Reference |
|---|---|---|---|---|
| Ho:ZnO | Ethanol amine (EA) Zn(CH3COO)2·2H2O], and Ho(NO3)3·5H2O | 1%, 3%, and 5% | Three layers of the substrate were spin-coated at 3000 rpm for 30 s, and after each layer was deposited, For 5 min, the substrate was warmed to 300°C, heated for 2 h at 500°C in a furnace | [101] |
| Sm:ZnO | Zn(CH3COO)2·2H2O] and Sm(CH3COO)3·H2O | 1%, 1.5%, and 2% |
|
[102] |
| Tb:ZnO | Zn(CH3COO)2 and Tb(CH3COO)3 | 0%–30% | Stirred at 60°C | [103] |
| Tb3:ZnO | Zn(CH3COO)2,Tb(CH3COO)3, and ethanol | 0.045, 0.07 mol | Stirred at 60°C for 1 h at room temperature, dried in the air | [104] |
| La, Ce, and Eu:ZnO | Zn(CH3COO)2·2H2O], La(CH3COO)3·H2O, Ce(CH3COO)3, and Eu(CH3COO)3·H2O | 1.0 mol% | — | [97] |
| Er:ZnO | Zn(COOCH3)2·2H2O, ErCl3, and MEA | 1–5 mol% | After an hour of stirring at 60°C, the film was placed in a furnace and annealed at 500°C, and 600°C, respectively, for 1 h | [105] |
| Sm:ZnO | Zn(NO3)2·6H2O and Sm(NO3)3·6H2O | 1, 3, and 5 mol% | At 120°C, the gel was dry, 500°C, the powders were calcined | [106] |
| La and Y:ZnO | Zn(NO3)2·6H2O, Ag(NO3)2·6H2O, La(NO3O9)·6H2O, Y(NO3)3·6H2O, and glycine | — | Heated for 15 min before being moved to a 100°C mantle, annealed for 5 h at 1100°C | [107] |
| Yb:ZnO | Zn(COOCH3)2·2H2O and Yb(NO3)3·5H2O | 0.05%, 0.25%, 0.5%, 1.0%, and 2.0% | For 2 h, the solution was refluxed at 80°C while being agitated for 10 min | [108] |
2.4. The Hydrothermal Method
The hydrothermal process involves the formation of the desired material by reacting metal precursors, such as zinc salt and RE salt, in an aqueous solution at high temperatures and pressures. This method has successfully produced RE ion-doped ZnO NPs with good crystallinity and purity [109, 110]. It enables the creation of homogeneous, crystalline particles with precise control over size and morphology, as REMs integrate into the ZnO lattice during the reaction, forming a solid solution. This approach offers low-temperature synthesis, scalability, and high-quality, uniform NP synthesis [2]. Figure 5 illustrates the synthesis of Ce-doped ZnO NPs [111].
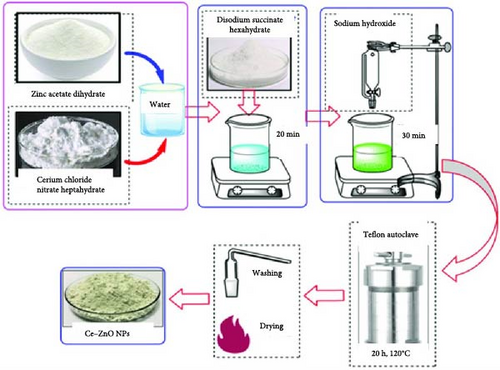
Table 5 summarizes the preparation of RE ion-doped ZnO using the hydrothermal method, detailing dopants such as Tb, Yb, Ce, Eu, Er, and their respective chemical reagents, doping concentrations, and synthesis conditions. For example, Ce doping involved stirring at 60°C for 12 h, vacuum desiccation at 80°C for 12 h, and calcination in N2 and air at specific temperatures and durations. The hydrothermal method is lauded for its ability to produce homogeneous, crystalline particles with controlled size and morphology at low temperatures, ensuring scalability and high-quality synthesis. Figure 5 likely illustrates the hydrothermal synthesis process of Ce-doped ZnO NPs, demonstrating its key steps. Both Figure 5 and Table 5 contribute to a comprehensive understanding of the hydrothermal approach for synthesizing RE ion-doped ZnO, emphasizing dopants, synthesis conditions, and its advantages in NP synthesis.
| Dopants | Chemical reagents | Doping concentration | Synthesis condition | Reference |
|---|---|---|---|---|
| Tb:ZnO | Zn(COOCH3)2·6H2O and Tb(NO3)3·6H2O | 1, 2, 3, 4, 5% mol | Stirred in a 70°C water bath for 1 h, then put in a Teflon-sealed autoclave and kept at 160°C and dried at 80°C for 12 h | [109] |
| Yb doped ZnO | Zn(NO3)2 and hexamethylenetetramine (CH12N4, HMT) | — | Stirred for 30 min and heated at 90°C for 24 h and then, deposited on a substrate and dried at 90°C for 2 h | [110] |
| Ce:ZnO | Ce(NO3)3·6H2O and Zn(CH3COO)2·2H2O | 0.5%, 0.75%, 1.0%, 1.25%, and 2.0% | Stirred at 60°C for 12 h, then vacuum desiccated at 80°C for 12 h. Calcined for 1 h at 100°C in N2 after 3 h of calcination in air at 220°C | [112] |
| Eu:ZnO | Zn(CH3COO)2·2H2O and Eu(CH3CO2)3·H2O | 1%, 3%, and 5%. | Hydrothermal treatment for 8 h at 150°C and dried at 80°C for 24 h | [113] |
| Er-ZnO | Zn(CH3COO)2·2H2O, Er2O3, (NH2)2CO, and concentrated hydrochloric acid (HCl) | 2%, 3%, and 4% in molarities | Mixed and placed in a 100 ml autoclave lined with Teflon for a full day at 155°C. 400°C for 2 h of annealing | [114] |
| Er and Yb:ZnO | Zn(CH3COO)2·2H2O, Er(CH3COO)2·4H2O, Yb(CH3COO)2·4H2O, and HMT | 5% | Stirred for 2 h, transferred into the Teflon-lined autoclave and heated at120°C for 18 h. Calcined at 600°C for 1 h | [63] |
| Eu:ZnO | Zn(NO3)2·6H2O and Eu(NO3)3·5H2O | 0.5% mol | Conducted by 20 min at 2, 4, 6, 8, and 1 MPa. The samples were dried at 40°C overnight | [115] |
| Ce:ZnO |
|
0.1%, 0.25%, 0.5%, 3%, and 5% | Stirred for 1 h before being moved to an autoclave lined with Teflon and heated for 7 h at 130°C | [116] |
2.5. The Chemical-Vapor Deposition (CVD) Method
CVD is another commonly used method for synthesizing RE ion-doped ZnO. In this method, precursor materials are deposited on a substrate, followed by thermal decomposition, resulting in the formation of the desired product. Several studies have reported successful synthesis of RE ion-doped ZnO using the CVD method. For example, the formation and optical properties characteristics of ZnO:Eu/ZnO NWs produced by sputtering aided MOCVD were described by Vinoditha et al. [113]. MOCVD creates ZnO NWs on the targets, and sputtering Eu2O3 targets on the NWs produces Eu-doped ZnO films. Using the NW configuration improves the crystal quality of ZnO host materials due to the strain relaxation effect. An amplification of Eu-related luminescence at 613 nm is observed at ambient temperature when ZnO:Eu is grown on the ZnO NWs instead of on film structures.
2.6. Spray Pyrolysis
Spray pyrolysis is a cost-effective method for synthesizing RE ion-doped ZnO, where precursor materials are sprayed onto a substrate and thermally decomposed to form the desired product. Studies have shown successful synthesis of doped ZnO using this method [117]. For instance, Thomas et al. [117] utilized spray pyrolysis to create FTO and Tb-doped FTO thin films, showcasing its effectiveness, affordability, and particle size control. Similarly, Devi et al. [118] observed changes in bandgap and crystalline size when studying Nd-doped ZnO thin films prepared via nebulized spray pyrolysis. Another study by Ayana et al. [119] synthesized Nd3+ doped ZnO thin films using chemical spray pyrolysis, highlighting the method’s ability to control crystalline orientation and dopant concentration.
2.7. Summary of Preparation Methods
Table 6 compares various preparation methods for RE ion-doped ZnO, including coprecipitation, solid-state reaction, sol–gel, hydrothermal synthesis, CVD, and spray pyrolysis. Each method offers specific advantages and challenges tailored to different applications. Coprecipitation excels in basic conditions and particle size control, while solid-state reaction enables diverse chemical synthesis. Sol–gel provides precise compositional control, albeit with higher precursor costs. Hydrothermal synthesis manages shape and size effectively but faces challenges in procedure control. CVD ensures high purity but requires high temperatures and volatile precursors. Spray pyrolysis is an affordable technique suitable for extensive deposition, despite challenges in achieving material homogeneity. The choice of method depends on application requirements, emphasizing the need to consider factors like cost, precision, and scalability in RE ion-doped ZnO synthesis for various technological applications.
| Preparation method | Advantages | Disadvantages | Nanostructure | Applications | References |
|---|---|---|---|---|---|
| Coprecipitation | Is basic, allows control of particle size | Inadequate control of shape. Poor stoichiometric phase accuracy | Flowers, particles | Sensors, catalysis, photovoltaic cell, piezoelectric nanogenerators, and optoelectronic devices | [82–85] |
| Solid state reaction | Allows the synthesis of a wide variety of chemicals | Bad control over shape. High temperatures are necessary | Particles | White LEDs and displays | [78, 95] |
| Sol–gel |
|
|
Crystals, particles | Photocatalysis and as an orange-red light-emitting substance in optoelectronics | [102, 103] |
| Hydrothermal | Good management of shape and size. Granulated powders can be produced | Challenging to manage the procedure, Issues with reproducibility and dependability | Rods, ellipsoids | Can be used as photocatalytic application and chemical sensors | [110, 116] |
| Chemical vapor deposition | Elevated purity comparatively high rates of deposition | Needs high temperatures and volatile precursors | Wires | Optical devices | [113] |
| Spray pyrolysis |
|
The initial droplet sizes vary, which may cause the material to become nonhomogeneous | Particles | Optoelectronics | [117–119] |
3. Characterization of REM-Doped ZnO
Characterization is vital for understanding the properties of RE ion-doped ZnO materials. Accurate techniques help assess synthesis success and doping effectiveness. Various methods offer insights into structure and performance, enhancing research understanding.
3.1. X-Ray Diffraction (XRD)
XRD is essential for analyzing the structural properties of REM-doped ZnO materials [120, 121]. XRD utilizes the diffraction of waves by atomic arrays to identify phases, crystallinity, and lattice parameters. The short electromagnetic wavelength and high energy of X-rays make them ideal for solid object diffraction. When X-rays interact with atoms, interference and scattering occur, resulting in a diffraction pattern with peaks corresponding to inter-atomic lengths. The observed peaks in both pure and RE-doped ZnO NPs align with the standard hexagonal ZnO, indicating highly crystalline and pure states [106]. The inclusion of RE metals induces a shift in XRD patterns toward lower theta values due to unit cell enlargement resulting from ionic radii mismatch. As gadolinium doping concentration increases, the unit cell volume shrinks, corroborated by XRD data [122]. Various studies confirm structural changes induced by RE doping, with some reporting secondary phases [116, 123]. Notably, La3+ and Gd3+ doping reduced diffraction peak strength, suggesting decreased crystallinity [82]. The drop in “D” values for doped ZnO indicates the formation of ZnO RE bonds, affecting crystallinity. Additionally, XRD validates findings of crystal size expansion due to RE element addition [124]. Surface analysis following Tb doping reveals hydrostatic strain-induced crystallographic flaws [103]. These findings underscore the significant impact of RE doping on structural properties, influencing crystal size, lattice characteristics, and surface structure. The XRD patterns of pure and RE-metal doped ZnO NPs are shown in Figure 6. The previously published standard sample of hexagonal ZnO (JCPDS-36-1451) is consistent with the peaks observed in both pure and REM-doped ZnO NPs. Since there are no other peaks that might suggest dopants or impurities, the samples are in a highly crystalline and pure form [100].
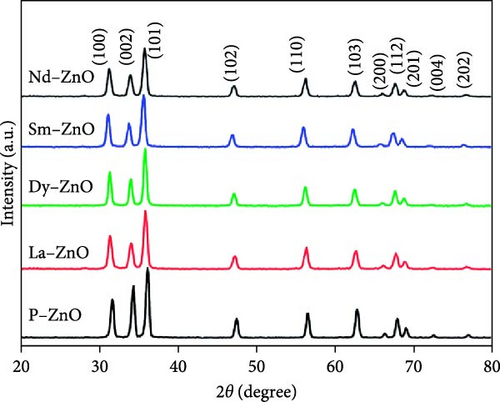
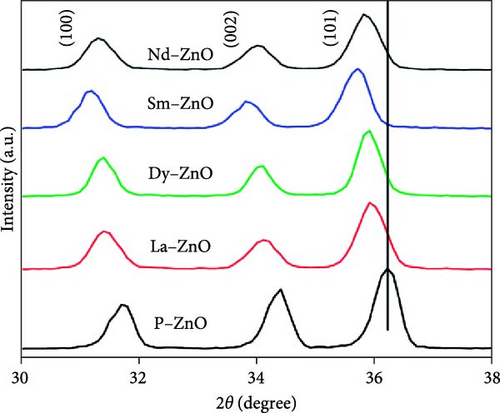
3.2. Scanning Electron Microscopy (SEM)
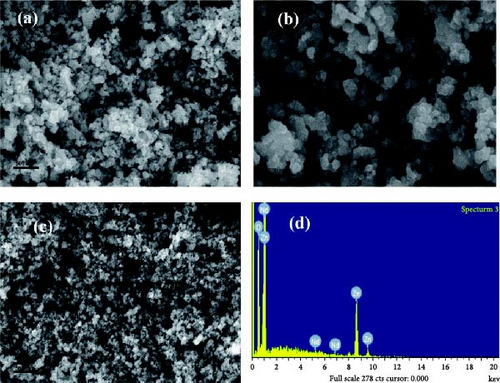
The SEM technique utilizes electrons to examine specimen surfaces, revealing topography and morphology details like texture, shape, size, and orientation of particles [125]. Coupled with energy dispersive X-ray spectrometry (EDS), SEM determines specimen chemical and elemental composition. SEM analysis of pure and Nd-doped ZnO NPs revealed irregularly sized particles with nearly spherical shapes for pure ZnO, while Nd doping resulted in roughened surfaces with dispersed Nd [100]. Figure 7 shows their SEM images. EDS analysis verified the existence of Zn, Nd, and O in Nd-doped ZnO NPs, indicating successful Nd incorporation [100]. SEM images of ZnO:Er3+, Yb3+ NPs, produced by Tsege et al. [85], depicted nearly spherical, linked particles with average diameters of 15–20 nm, showing homogeneity unaffected by doping. Divya and Pradyumnan [92] observed large, irregularly shaped ZnO morphology transformed into small, nanosized rods at optimal erbium doping, where particle size decreased with doping up to 0.6 weight percent but increased thereafter due to altered nucleation mechanisms. Erbium doping reduced the average crystallite size from 57.71 to 45.26 nm, as confirmed by EDS [96]. Ghrib et al. [95] noted uniform spherical undoped ZnO NPs becoming agglomerated forms upon doping, evident in SEM micrographs of undoped ZnO and ZnO doped with Tb at varying concentrations.
3.3. Transmission Electron Microscopy (TEM)
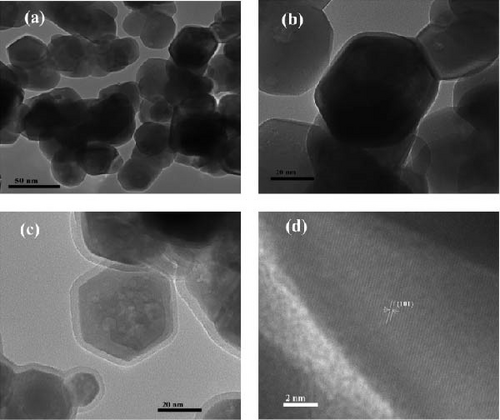
TEM examines the internal structure, crystallinity, and composition of RE ion-doped ZnO NRs at the nanoscale, offering insights into size, shape, and defects. TEM images (Figure 8) of undoped and Nd-doped ZnO NPs reveal spherical, hexagonal morphology without agglomeration for pure ZnO, consistent with an average crystallite size of 15–30 nm [100]. Figure 8a,b presents TEM images of the undoped P-ZnO NP structure at low and high magnifications, respectively. Nd modification preserves the NP structure, as seen in Figure 8c, where Nd particles cover the NPs, consistent with the XRD-determined crystallite size. Figure 8d shows the HRTEM image of Nd-ZnO [100]. Ghrib et al. [95] also observed TEM images pure and Tb-ZnO, confirming Tb-ZnO NP crystallization with an average particle size of 51.61 nm for Tb-ZnO and 25.8 nm of undoped ZnO, showing grain sizes ranges from 20 to 56 nm. Farhat, Rekaby, and Awad [123] found TEM-observed nano-like rod structures for 0.01 ≤ x ≥ 0.08 in ZnO, while pure ZnO and x = 0.10 samples lacked specific shapes. Hastir, Kohli, and Singh [84] noted TEM’s role in revealing Tb, Dy, and Er doping effects on ZnO morphology, transitioning from nearly spherical to dumbbell-shaped microstructures in undoped ZnO. Ben Haj Othmen et al. [102] observed nonhomogeneous grain sizes with a bimodal distribution in ZnO:Sm3+ nanocrystals via TEM, confirming successful doping. TEM characterization by Popa et al. [101] proposed a structural mechanism involving Ho ion agglomeration at ZnO grain surfaces, forming core–shell structures upon doping. Unusual emissions at ~662 nm were attributed to energy transfer from Ho ions to the ZnO matrix via structural defects. Ghrib et al. [95] confirmed Tb-ZnO crystallization via TEM, showing grain size variations from 20 to 56 nm, peaking at 5%wt Tb.
3.4. UV–Vis Spectroscopy
UV–Vis spectroscopy measures light absorption by electronic changes, typically in the UV–Vis range. Transmittance (T), the ratio of transmitted (I) to incident (I0) light intensity, characterizes how well a sample absorbs, reflects, or transmits light [126]. Farhat’s UV–Vis analysis showed optical band gap (Eg) variations: Eg increased for x = 0.08 and 0.10 but decreased for 0.00 < x > 0.06. Similarly, Popa et al. [101] observed a band gap decrease (3.28–3.22 eV) with increasing Ho content, maintaining UV–Vis transmission above 70%. This trend was consistent with findings by Soumahoro et al. [60]. Barsisa et al. [89] reported UV–Vis spectra of ZnO NPs doped and undoped with europium ions, noting slight wavelength changes with increasing europium concentration. Jihad et al. [127] validated these results, attributing absorption peaks to electron transitions from valence to conduction bands. The bandgap energy increased from 3.26 to 3.28 eV with rising Eu3+ concentrations, indicating bandgap enhancement [127]. Naik et al. [128] examined UV–Vis absorption spectra of undoped ZnO NPs with varying Eu3+ doping (1%, 3%, and 5%), observing a red shift in absorption spectrum with increasing Eu3+ concentration, indicative of band gap narrowing. Koao et al. [129] reported similar findings, attributing band gap modification to CT between ZnO bands and Eu3+ 4 f levels. UV–Vis absorption spectroscopy studies demonstrated enhanced Vis light absorption and a red shift in ZnO:Eu3+ NPs compared to pure ZnO, highlighting the effect of Eu3+ doping.
3.5. PL Spectroscopy
PL spectroscopy measures the excitation and emission of luminous materials, akin to UV–Vis spectroscopy but differing in the direction of electronic transition: PL transitions from higher to lower energy levels. Unlike absorption spectroscopy, PL detects no signal without PL emission [125]. PL spectra exhibit excitonic and trap/surface-related bands, notably deep-level emission (DLE) and near-band edge (NBE) around (550 and 380 nm), respectively [130]. Er, Gd, and Nd doping induce a “blue” shift in PL spectra compared to clean ZnO, attributed to conduction band to oxygen or donor–acceptor recombination antisites transition. The “red” shift in absorption edge indicates a narrowed bandgap due to oxygen vacancies and impurity atoms in RE-doped ZnO [130]. Increasing Gd-doping concentrations enhances PL intensity due to additional emitting centers [122]. The spectra of pristin ZnO and Gd doped ZnO display green emissions, with increased defect-related peak intensities correlating with higher Gd concentrations [122]. Ce4+-doped ZnO nanostructures exhibit strong optical properties with multi-emission peaks in the blue-green region [131]. Ce doping in ZnO NRs enhances defect emission and causes a red shift in UV emission, altering free carrier concentration as evidenced by Raman spectra shifts [132]. When excited at 465 nm, Eu3+ doped ZnO NPs show strong band emissions at 706, 652, 617,591, and 579 nm, with intensities increasing with Eu3+ concentration [128]. Similar emission bands are observed by other researchers [133, 134].
3.6. Electrical and Magnetic Characterization
Electrical and magnetic characterization techniques, including electrical conductivity measurements, Hall effect measurements, and magnetic measurements, are vital for assessing the magnetic and electrical properties of REM ion-doped ZnO materials, offering insights into charge transport mechanisms, carrier concentration, mobility, and magnetic behavior, crucial for potential applications in electronic and spintronic devices. Obied et al. [135] observed that the magnetic moment of 6% Gd-doped ZnO-NRs was twice that of 3% Gd doped samples, indicating higher Gd incorporation resulting in increased ferromagnetism and magnetization at room temperature. Similarly, Thangeeswari, Priya, and Velmurugan [136] reported on Gd codoping with (ZnO, Co), leading to room-temperature ferromagnetism with potential magnetization enhancement. Ghoul and Al-Harbi [137] demonstrated that (V, Gd) codoping in ZnO improves room-temperature ferromagnetism, suggesting that codoping enhances the ferromagnetic properties of doped ZnO systems. Raship et al. [138] found n-type conductivity in films with increasing Gd concentrations, with electrical resistance decreasing as Gd concentrations rose, attributed to the absorption of free electrons in the ZnO lattice through vacant 3d Zn states. Anand et al. obtained an electrical resistivity value of ~10−4 Ωcm, noting a decrease in electrical conductivity with increase in Gd concentration [139]. Similarly, 3% Gd-doped ZnO exhibited enhanced electrical conductivity compared to 3% (Al, Gd) codoped ZnO, indicating that elements like Gd can improve ZnO’s electrical conductivity.
4. Performance of PSCs With REM-Doped ZnO
PSCs have emerged as promising technology for efficient and low-cost photovoltaic devices, with their performance strongly influenced by the properties of charge transport layers and interfaces within the device. RE ion-doped ZnO materials have garnered significant attention as ETLs in PSCs because of their tunable potential and properties to improve device performance. By incorporating REMs such as Ce, neodymium (Nd), and La into the ZnO matrix, the optical, electronic, and surface properties can be modified. This doping improves electron mobility, enhances charge extraction, and reduces recombination losses in PSCs by passivating surface defects and trap states, leading to improved CT and reduced charge recombination at the interface with the perovskite absorber layer. Studies have demonstrated that RE ion-doped ZnO layers can improve the charge collection efficiency and overall device performance of PSCs. The enhanced electron transport properties contribute to higher fill factor (FF), open-circuit voltage (Voc), and PCE of the solar cells. Reduced charge recombination and improved charge extraction at the interface enhance photocurrent generation and device stability. Furthermore, RE ion doping can influence the optical properties of the ETL, modifying light absorption and transmission characteristics, leading to enhanced light harvesting and reduced reflection losses, ultimately increasing short-circuit current density (Jsc) and overall device performance. However, the performance of RE ion-doped ZnO in PSCs is dependent on various factors including doping concentration, dopant type, film morphology, and device architecture. Optimizing these parameters is crucial for achieving desired device performance. Additionally, careful consideration of the choice of RE ion and its compatibility with the perovskite layer is necessary to avoid adverse effects on the stability and long-term performance of PSCs.
4.1. Impact on Doping Concentration
The doping concentration of REMs in ZnO significantly impacts the performance of PSCs, affecting charge transport properties, extraction efficiency, and overall device performance. Optimizing this concentration is crucial to strike a balance between improving charge transport and minimizing detrimental effects. At low concentrations, REMs can act as shallow trap states, hindering charge carrier mobility and charge extraction, leading to decreased device performance, including lower open-circuit voltage (Voc) and FF. Conversely, higher doping concentrations can improve charge transport properties and enhance charge extraction by forming a higher density of shallow trap states, facilitating electron transfer and reducing charge recombination losses at the interface between the perovskite absorber layer and the doped ZnO ETL. Consequently, higher doping concentrations can increase short-circuit current density (Jsc) and PCE of PSCs. However, there exists a critical doping concentration beyond which PSC performance may deteriorate. Very high doping concentrations can lead to increased trap state density, causing nonradiative recombination and carrier trapping, reducing charge collection efficiency and overall device performance, including Voc, FF, and PCE. The optimal doping concentration varies depending on the specific REM, as each dopant ion interacts uniquely with the ZnO lattice. Hence, extensive experimentation and optimization are necessary to determine the ideal doping concentration for each specific dopant-ion-ZnO system. The doping concentration significantly influences various aspects of RE ion-doped ZnO’s properties in PSCs, including its electrical, optical, and structural characteristics. For instance, doping ZnO with REMs can improve charge extraction efficiency, increase charge carrier mobility, and reduce recombination losses, thereby enhancing overall device performance [140]. Doping concentration also affects the energy levels of REMs in ZnO, influencing energy band alignment at the perovskite/ZnO interface, which in turn impacts CT and injection processes [141]. Additionally, doping concentration can modify optical properties, such as absorption and emission spectra, affecting light absorption and conversion efficiency [142]. Furthermore, it can influence structural stability and crystalline quality, enhancing long-term stability and reliability [143]. Studies on Yb-doped ZnO nanostructures by Senol [63] and thin films of Yb-doped ZnO by Soumahoro et al. [60] and López-Mena et al. [144] demonstrate variations in bandgap, electrical resistivity, and optical properties with increasing Yb concentration. Similarly, Shabbir et al. [145] reported decreased bandgap energy and increased carrier concentration with increasing La concentration in ZnO thin films, influencing their electrical conductivity and hydrophilicity. These findings underscore the importance of optimizing doping concentration for enhancing the performance of RE ion-doped ZnO in PSCs and related applications in renewable energy.
4.2. Impact of REM-Doping on Crystal Structure and Morphology
Zamiri et al. [146] examined the influence of RE ions on the shape of chemically produced ZnO nanostructures and observed significant morphological changes. They observed that the morphology of the nanostructures changed from NR-like to nanoplate-like when ZnO was doped with Er and Yb. Only the dopants, which might serve as structure-driving agents that stick to the ZnO crystallographic planes, were blamed for these alterations. The shift from NR-like to nanoplate-like structures was consistent across various growth methods, indicating the dopants’ role in shaping the nanostructures. Al-Otaibi, Howsawi, and Ghrib [78] investigated the effect of doping ZnO with 5% Y, Tb, and Eu on crystallinity and surface morphology. XRD analysis revealed a predominant hexagonal wurtzite ZnO structure in all samples, with improved crystallinity in doped samples. However, doped ZnO samples exhibited rough surfaces due to grain aggregation, along with high microstrain along the c-axis. FTIR analysis confirmed higher crystallinity and the hexagonal wurtzite structure in all doped samples. The structural, morphological, optical, and magnetic characteristics of Yb-doped ZnO produced by coprecipitation were investigated by Sharma et al. [147]. Doping resulted in room-temperature ferromagnetism and a reduction of the band gap. After Yb ion insertion, spherical structures changed into agglomerated nonspherical structures, according to HR-SEM examination. Magnetic measurements indicated a transition from diamagnetic to ferromagnetic ordering with Yb doping. Er-doped ZnO NRs were examined for their structural characteristics by Achehboune et al. [148], who found that both pure and doped samples had a hexagonal (wurtzite) structure, with the doped sample also exhibiting a cubic phase of Er2O3. XRD results showed an increase in particle size and lattice strain after doping with Erbium, indicating Er incorporation into the ZnO lattice. The effect of La doping in ZnO for photoanode materials was examined by Shabbir et al. [145]. In line with XRD studies, they discovered that particle size reduced as La doping increased up to 5 weight percent, suggesting little effect on crystal structure. SEM study showed granular and sphere-like morphologies in both pure and La-doped ZnO thin films, which displayed a hexagonal wurtzite structure as confirmed by XRD analysis. The chemical analysis verified the successful incorporation of La without the presence of other impurities.
4.3. Impact of REM-Doping on the Optical Properties
Since the involved optical characteristic of the PSCs impacts light absorption and photocarrier production, which significantly determines the final photocurrent and output power, it also has a significant impact on the device performance [149]. Perovskite’s composition and bandgap can also be adjusted to achieve complete coverage of Vis-wavelength light absorption. Due to the UV light with high-energy photons in the incident solar spectrum, the optical quality also has a significant impact on the device stability [150]. Al-Otaibi, Howsawi, and Ghrib [78] UV research showed that the addition of RE ions to the ZnO system results in a decrease in the energy gap. The experiment demonstrated red shifting and violet and blue emission because of lattice interstitial deformation following RE doping. Pure and doped ZnO systems’ emission in the Vis range suggests that they are viable options for the creation of white light-emitting diodes and display panels. Singh, Kumar, and Singh [151] UV–Vis and PL spectroscopy methods were used to the optical characteristics. In the PL spectrum, two distinct peaks that are almost at 510 and 612 nm were detected. The emission at 510 nm might be attributed to deep level emission in the trap state, whereas the emission at 612 nm was likely caused by a charge transfer (CT) transition between O2− and Ce4+ ions. Up to 2% doping concentration did not affect these two emission peaks. The outcomes demonstrate that using this technique yields precisely codoped ZnO NPs with crisp emission spectra, allowing for the use of these materials in photodetectors and LEDs. Wang et al. [152] found that Tb-doped ZnO exhibits a decrease in band gap and an upward shift in the Fermi level. The material’s absorption peak and reflectance peak both show a red-shift and decline as the doping concentration rises (3.13%–6.25%). In the near-UV range of 240–380 nm, the reflectivity and absorption impact of Tb doped ZnO decrease as the concentration of Tb doping increases. Tb-doped ZnO has a greater absorption impact in the Vis spectrum than pure ZnO, while the reflectance is the opposite. Consequently, Tb doped ZnO can have improved photoelectric characteristics by choosing a higher doping level.
4.4. Impact of REM-Doping on PSC Stability and Durability
Incorporation REM-based DC materials were used in PSCs, the power output remained steady under typical operating conditions. Although Chen et al. [153] reported a photon downshifting layer SrAl2O4:Eu2,Dy3 (SAED)-incorporated long-term stability of 2600 h (more than 100 days) at ambient settings, long-term stability equivalent to traditional Si-based PV has not yet been proven. More study is required to gain a deeper understanding of the surface chemistry of RE-doped nanomaterials, specifically the kind of ligands that prevent forming an insulating barrier surrounding each DC/UC NC and obstructing the perovskite absorber’s direct access to the NC surface. Additionally, combining multiple stability-enhancing techniques at once, such as the use of appropriate protective coatings, encapsulation, the incorporation of down- and up-conversion materials, and the use of a combination of MA and FA in optimal concentration for the development of this technology [91]. Al-Otaibi, Howsawi, and Ghrib [78] examined, the structural, optical, and PL characteristics of pure and RE-doped ZnO in this work. The TGA data demonstrated that the various ZnO systems were rather stable. It was shown that stability remained even at temperatures higher than 450°C. Meng et al. [77] found that the related PSCs’ moisture, thermal, and UV stability all increased at the same time. This research suggests that adding CeOx to the ETM might effectively increase PSC stability and efficiency, opening new possibilities for ETM design in PSCs.
4.5. Comparison With Other ETLs Materials
In many PSCs that have been described so far, TiO2 materials have been utilized as ETLs. Despite the high electron recombination rates, which are also observed because of the low electron mobility and transportation capabilities, the electron injection rates between the perovskite absorber and TiO2 ETLs are extremely fast [154]. Furthermore, high-quality mesoscopic TiO2 layer needed a high-temperature procedure [155, 156]. Due to these properties of TiO2, it may be difficult to enhance device performance and expand their use in the creation of low-cost PSCs on a variety of flexible substrates [157, 158]. However, ZnO offers several advantageous characteristics, including large bandgap, high electron mobility, strong room-temperature luminescence, and great transparency. There are several different morphologies of ZnO, such as thin films, single crystals, NRs and NWs, all of which have been discovered and produced utilizing low-temperature solution techniques. In the native state of the semiconductor, N-type doping is caused by zinc interstitials or oxygen vacancies. Furthermore, ZnO is a well-known substance that has an energy level comparable to TiO2 but superior electron mobility (bulk mobility: 200–300 cm2/V·s) [159, 160] compared to TiO2. This makes ZnO a great option for a low-temperature processed electron-selective contact for light-emitting diodes, PSCs, transparent electrodes, and thin-film transistors.
5. Future Directions for REM-Doped ZnO Beyond PSCs
The REM-doped ZnO nanostructures have a wide range of industrial uses because of their special and adaptable characteristics and attributes [161]. Compared to undoped ZnO nanostructures, RE-doped ZnO nanostructures have significant applications in a variety of disciplines [162, 163]. Single electron transistors, photodetectors, fabricated LEDs, laser diodes, flat panel displays, transparent electrodes, nanogenerators, solar cells, optical waveguides, PZT transducers, surface acoustic wave devices, and optoelectronic devices are among the many applications for them in the semiconductor industry and electronics [161, 164–166]. They are also useful in photocatalysis, sensing applications, and spintronics for the development of solid-state memory storage devices and electromagnetic devices. They demonstrate the photocatalytic breakdown of a range of cationic and anionic dyes. As a result, they are employed for controlled and regulated drug release across locations and may have antibacterial and anticancer properties [161, 165, 167]. Zinc is a micronutrient that can be supplemented for both plants and animals. Printing inks, fire-resistant materials, synthetic fertilizer, fingerprint analysis, cigarette filters, and biosensors for the detection of different enzymes and other biomolecules are just a few more uses for REM-doped ZnO NPs.
5.1. Challenges and Limitations
Even while PSCs’ PCE has improved significantly in recent years, there is still a noticeable discrepancy between the PCE as it stands now and its theoretical maximum. Additionally, perovskite materials (ABX3) degrade quickly in humid environments due to their weak stability, which reduces solar activity. Enhancing PSCs’ PCE and stability is essential to their commercial viability and mass manufacturing. Moreover, as photoactive materials in solar cells, perovskites are limited in their capacity to absorb UV and near-infrared (NIR) light, which leads to notable losses throughout the solar spectrum. The absorption efficiency of solar light in the NIR region can be increased while safely utilizing the UV range by integrating REM ions into perovskite frameworks or using them as independent functional up-converting (UC)/DC layers. The PCE and UV stability of PSCs could both be enhanced by this strategy. Additionally, the band gap of perovskite materials can be optimized and the band alignment between the active layer and ETLs adjusted by doping them with REM ions. This reduces recombination events and facilitates charge extraction [91].
5.2. Further Research and Development
To advance the development and application of RE doped metal oxides, significant improvements in efficiency and brightness are essential. This can be achieved through refining the composition and fabrication conditions. Recent research has revealed how physical factors like temperature, strain, and electric fields can be utilized to modify luminous characteristics in real-time, offering insights into mechanisms and potential enhancements in luminescent efficiency. Furthermore, efforts have been directed toward gaining optical control over piezoelectric polarization, which could offer greater flexibility for developers of perovskite-based systems. In terms of applications, thin film structures are particularly advantageous for integration with diverse substrates, enabling the creation of multifunctional optoelectronic devices. The development of REM doped ZnO thin films on flexible substrates is therefore essential for the realization of flexible optoelectronics. Recent research has demonstrated that when compared to undoped ZnO and bulk ZnO crystals, RE (Ce, Er)-doped ZnO nanostructures show improved optical, structural, and PL capabilities. Numerous applications across numerous technological domains are made possible by these enhanced qualities [167]. It has been demonstrated that the degree of impurity atom doping greatly affects the band gap energies of the resultant doped ZnO, underscoring the potential of RE-doped ZnO nanostructures in thin-film manufacturing, spintronic applications, optoelectronic devices, and the creation of advanced devices.
6. Conclusions
Over the past decade, the advancement of PSCs has been extraordinary, marked by transformative shifts from lead-based to Pb-free perovskites, and from conventional mesoscopic topologies to planar structures. The advent of fabrication strategies under ambient conditions has significantly bolstered the stability and PCE of PSCs. However, substantial challenges persist on the path to commercialization. Among the myriad approaches for enhancing PSC performance, down-conversion, and up-conversion strategies have emerged as particularly promising and impactful for real-world applications. This review article highlights the significant potential of REM-doped ZnO in enhancing the performance of PSCs. RE dopants effectively improve the electron mobility of ZnO, reducing charge transport resistance and enhancing the overall efficiency of solar cells. These dopants also contribute to the stability of PSCs by mitigating degradation mechanisms, such as moisture-induced and thermal instability, which are critical for commercial viability. Furthermore, RE doping has shown promise in minimizing photocarrier recombination by introducing controlled energy levels and passivating defects within the ZnO structure. This optimization improves the lifetime and density of photogenerated carriers, directly impacting the photovoltaic performance. In addressing challenges related to toxicity concerns, stability issues, and scalability considerations associated with RE ion-doped ZnO nanomaterials, this review offers invaluable insights for future research and development endeavors. Despite potential cost implications, REMs consistently deliver remarkable PCEs of up to 22.9% in ZnO-based devices. This synthesis of recent empirical studies on REM-doped ZnO not only focuses on enhancing PSC electron mobility and stability but also scrutinizes the transition from mesoscopic to planar PSC architectures, highlights synthesis/fabrication strategies, and explores REMs’ potential in ZnO for up/down conversion processes. Overall, this review consolidates current knowledge and advancements in the field, serving as a guiding beacon for researchers and engineers and propelling the advancement of next-generation photovoltaic technologies.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.




