Identifying Risk Factors for Central Venous Access Device Complications in Pediatric Patients With Cancer: A Scoping Review
Abstract
Background: To systematically review the risk factors for central venous access device (CVAD)–associated complications in pediatric patients with cancer.
Methods: A scoping review with systematic search criteria was conducted using PubMed, Embase, and CINAHL databases from 2012 to 2022. Cohort studies and the control arm of randomized controlled trials, which reported CVAD-associated complications in pediatric patients aged 0–18 years, were included.
Results: 381 studies were identified in initial screening, with 25 studies included in the review. Device-related factors were commonly reported as risk factors for central line–associated blood stream infection, CVAD-associated venous thromboembolism (VTE), and device failure. Tunneled central venous catheter (TCVC), multilumen devices, and larger diameter lumens were risk factors for CLABSI. TCVCs were a risk factor for device failure. PICCs were a risk factor for CVAD-associated VTE. Provider and patient-related risk factors were rarely reported. There is significant inconsistency in the quality of reporting of vascular access data.
Conclusion: External devices are associated with an increased risk of CVAD-associated complications. There is a limited good quality prospective evidence on risk factors for CVAD-associated complications in this cohort. Further research is needed to understand drivers of these complications, inform preventative strategies, and guide device-selection decisions. Improved research quality through adherence to benchmarking reporting standards for vascular access data is also needed.
1. Introduction
Globally, it is estimated that 400,000 children and adolescents aged 0–19 years develop cancer each year [1, 2]. Central venous access devices (CVADs) are essential in these patients to enable the delivery of both anticancer and supportive therapies [2, 3]. Their use facilitates the safe delivery of chemotherapy, blood products, parenteral nutrition, analgesia, antiemetics, and other supportive medications. CVADs also improve quality of life by avoiding pain and anxiety of venepuncture and cannulation [4].
While CVADs are essential to provide necessary treatment, one in four patients with these devices will have a significant complication and/or device failure prior to completion of therapy [5, 6]. Complications are varied and include central line–associated blood stream infection (CLABSI), thrombosis, local infection, mechanical complications, and dehiscence [6, 7]. Each of these complications requires treatment and can result in delays to the delivery of anticancer therapy and has been shown to increase morbidity and mortality [4–8].
There is the literature documenting the incidence and risk factors for CVAD-associated complications in pediatric patients [6, 9, 10]; however, evidence for CVAD-associated complications in pediatric patients with cancer is largely limited to single-site experiences or isolated diagnoses. Pediatric patients with cancer are a unique cohort whose risk profile differs from the general pediatric population, secondary to factors relating to the cancer itself and the treatment administered. To date, no systematic review exists describing the risk factors for CVAD-associated complications in this patient population. CVAD-associated complications have significant impacts on both the patient/family as well as the healthcare system. Interventions aimed at preventing CVAD-associated complications will be more effective if driven by an understanding of their risk factors. The aim of this scoping review was to determine the existing evidence and identify gaps in knowledge regarding potential risk factors for CVAD-associated complications in pediatric patients with cancer.
2. Methods
The structure of this study was informed by the Joanna Briggs Institute Reviewer’s Manual [11] and is reported in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews.” [12] This study was registered with PROSPERO, and the protocol published on 22 September 2022 (CRD42022359467). The five phases of conducting a scoping review developed by Arksey and O’Malley [13] were used to guide the structure of this review and included the following: (1) identify the research question, (2) identify the relevant studies, (3) select studies, (4) collect data, and (5) collate, summarize, and report results.
2.1. Identify the Research Question
- 1.
What are the risk factors for CVAD-associated complications in pediatric patients with cancer?
- 2.
What evidence has been published on this topic?
2.2. Identify the Relevant Studies
The US National Library of Medicine National Institutes of Health (PubMed), Embase, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) were systematically searched. Medical subject headings and searches were developed in conjunction with a healthcare librarian (see Supporting file 1) and screened for inclusion independently by two reviewers (J.N. and E.W.J.) using Covidence [14]. References of full-text articles were reviewed to identify additional studies. Disagreements were resolved through review by a third reviewer (A.U.).
2.3. Select Studies
2.3.1. Inclusion Criteria
A systematic search (see Supporting file 1) was conducted to identify studies examining risk factors for CVAD-associated adverse events in pediatric patients with cancer. Procedural and acute insertion complications were not included. This review wants to understand the relationship between CVADs, the patient, their disease, and CVAD-associated complications. Procedural complications pertaining to the surgical procedure itself were, therefore, not included. Studies were eligible for inclusion if they met the following criteria: (1) cohort design (prospective or retrospective); (2) control arm of randomized controlled trials (RCTs); (3) failure and/or complications of CVADs included as an outcome measure; (4) pediatric patients aged 0–18 years; (5) patients with an oncological diagnosis; and (6) CVAD inserted for any length of time during their treatment. Studies were excluded if they were not written in English and/or if they were published prior to 2012 to best reflect current practices. Risk factors recorded were either explicitly reported by the study or the review authors identified them as potential risks. Studies in which risk factors were not reported were excluded.
2.3.2. Outcome Definitions
For the purpose of this study, CVAD-associated complications are defined as per Table 1.
| Overall | The cumulative total number of complications (defined above) within the study cohort |
| CVAD failure | Failure of the device prior to completion of planned therapy as a result of any of the defined complications [6, 15] |
| Central line– associated blood steam infection (CLABSI) | A laboratory confirmed bloodstream infection that is not secondary to an infection at another body site [16] |
| Local CVAD infection | Presence of erythema, swelling, tenderness at the insertion site ± fever [16] |
| Occlusion | Either complete occlusion (unable to aspirate and inject) or partial occlusion (unable to aspirate or inject) [6, 17] |
| CVAD-associated venous thromboembolism (VTE) | Ultrasound evidence and clinical symptoms (pain/erythema/swelling/line dysfunction) of CVAD-associated VTE [17] |
| Dislodgement or migration | Any movement resulting in the CVAD migrating out of a central vein (central veins include lower 1/3 SVC, RA, SVC/RA junction or IVC for lower limb insertion [18, 19] |
| Breakage and/or rupture | Visible split/break in the CVAD material resulting in external leakage and/or radiographic evidence of internal leak [17] |
| Dehiscence | Separation of the margins of surgical incision with/without protrusion of device [20] |
- Abbreviations: CLABSI, central line–associated blood stream infection; CVAD, central venous access devices; IVC, inferior vena cava; RA, right atrium; SVC, superior vena cava; VTE, venous thromboembolism.
2.3.3. Risk Factor Definitions
Potential risk factors for CVAD-associated complications were informed by a previously established conceptual risk factor model by Chopra et al. [21] and an adapted model by Gibson et al. [22] and are defined as per Table 2.
| Device Factors | Device type (PICC, TCVC, TCCVC, TNCCVC, TIVAD) Number of lumens Device location Timing of device placement |
|---|---|
| Provider factors |
|
| Patient factors |
|
- Abbreviations: CVAD = central venous access device, PICC = peripherally inserted central catheter, TCVC = tunneled central venous catheter, TCCVC = tunneled cuffed central venous catheter, TIVAD = totally implanted venous access device, TNCCVC = tunneled noncuffed central venous catheter.
2.4. Collect Data
Data extraction was performed by one reviewer using a standardized data extraction form, checked by a second reviewer. The data fields extracted included country, study design, population, cancer diagnosis, catheter type, frequency of CVAD failure and/or complications, catheter days, and CVAD risk factors. In the event of missing data, the corresponding study author/s were contacted to seek clarification about the missing data.
2.5. Collate, Summarize, and Report Results
Each study was organized in the data extraction tool in accordance with the outcome measures (Tables 1, 2). Risk factors identified in each study were thematically characterized into either device, patient, or provider-related factors as outlined above (Table 2).
2.5.1. Risk of Bias Assessment
The Mixed Methods Appraisal Tool (MMAT) [24] was used by two reviewers to assess the quality of evidence for the studies included. Disagreements in rating were discussed with a third reviewer. After meeting two preliminary screening questions, studies were critically appraised based on the methods used across 5 criteria. Each criterion is rated with a “yes,” “no,” or “cannot tell,” and the percentage of “yes” answers determines the overall quality rating. MMAT has no “minimum” criteria to consider a study adequate but encourages interpretation of evidence in the context of reduced study quality. Studies with low methodological quality were not excluded, but this was reflected in the synthesis and descriptive analysis.
3. Results
3.1. Systematic Search Results
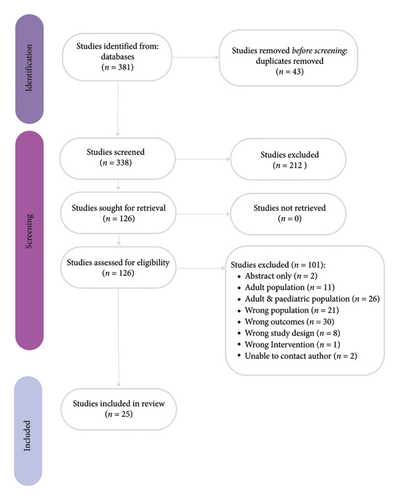
Figure 1 demonstrates the study selection process in accordance with the PRISMA guidelines [25]. A total of 381 studies were identified in initial screening from March 2012 to December 2022. After 43 duplicates were removed and 126 full text articles reviewed, 25 studies were identified as meeting the inclusion criteria (see Figure 1).
3.2. Characteristics of Included Studies
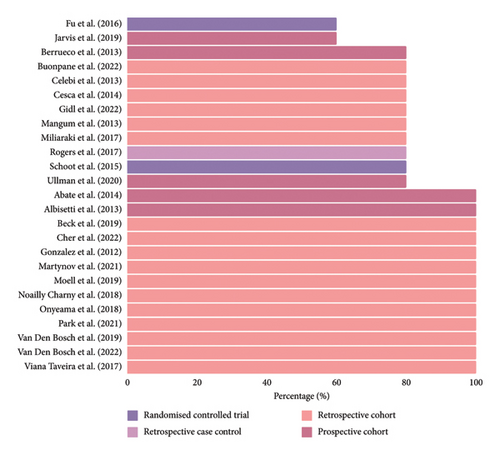
Study characteristics are summarized in Table 3. Of the 25 studies included, there were seventeen (65.4%) retrospective cohort studies, six (23.1%) prospective cohort studies, one (3.8%) retrospective case-control study, and two (7.7%) RCTs (control arm only). Ten (38.5%) studies included hematological malignancy only [26–28], one (3.8%) study included solid tumor malignancy only [29], and the remaining fifteen (57.7%) studies included all malignancies [7, 30–43]. Studies were conducted across Asia and Pacific [35, 40, 42], North America [10, 27, 33, 37, 44–46], South America [43], and Europe [7, 26, 28–32, 34, 38, 39, 41, 47–49]. Twelve studies (46.2%) were published prior to 2017, and the remaining 14 studies (53.8%) were published between 2018 and 2022.
| Citation | Year | Country | Design | Population | Patients (N) | CVAD complications | Studied risk factors |
|---|---|---|---|---|---|---|---|
| Buonpane | 2022 | USA | RC | All malignancy | 6553 | 1, 2, 3, 4 | Yes |
| Cher | 2022 | Singapore | RC | All malignancy | 243 | 3 | Yes |
| Gidl | 2022 | Austria | RCT | Hematological | 1026 | 6 | Yes |
| Van Den Bosch | 2022 | Netherlands | RC | Hematological | 98 | 1, 3, 4, 6, 7, 8 | Yes |
| Martynov | 2021 | USA | RC | Hematological | 238 | 1, 3, 4, 7 | Yes |
| Park | 2021 | South Korea | RC | All malignancy | 470 | 3 | Yes |
| Ullman | 2020 | Australia | PC | All malignancy | 56 | 5 | Yes |
| Beck | 2019 | Germany | RC | All malignancy | 296 | 1, 3, 4, 6, 7, 8 | Yes |
| Jarvis | 2019 | Norway | PC | Hematological | 47 | 6 | Yes |
| Moell | 2019 | Sweden | RC | All malignancy | 154 | 3 | Yes |
| Van Den Bosch | 2019 | Netherlands | RC | All malignancy | 201 | 1, 3, 4, 6, 7, 8 | Yes |
| Noailly Charny | 2018 | France | RC | Hematological | 192 | 6 | Yes |
| Onyeama | 2018 | USA | RC | Hematological | 198 | 6 | Yes |
| Miliaraki | 2017 | Greece | RC | All malignancy | 91 | 3 | Yes |
| Rogers | 2017 | USA | RCC | Hematological | 40 | 3 | Yes |
| Viana Taveira | 2017 | Brazil | RC | All malignancy | 188 | 3 | Yes |
| Fu | 2016 | USA | RC | Hematological | 198 | 1, 2, 3, 4, 6, 7 | Yes |
| Schoot | 2015 | Netherlands | RCT | All malignancy | 154 | 3 | Yes |
| Abate | 2014 | Italy | PC | Solid tumor | 155 | 1, 3, 4, 5, 6, 7 | Yes |
| Cesca | 2014 | Italy | RC | Hematological | 117 | 1, 2, 3, 4, 5, 6, 7, 8 | Yes |
| Albisetti | 2013 | Switzerland | PC | All malignancy | 114 | 6 | Yes |
| Berrueco | 2013 | Spain | PC | All malignancy | 73 | 3, 4 | Yes |
| Celebi | 2013 | Turkey | RC | All malignancy | 31 | 2, 3, 4 | Yes |
| Mangum | 2013 | USA | RC | All malignancy | 743 | 1, 2, 3 | Yes |
| Gonzalez | 2012 | USA | RC | Hematological | 172 | 1, 3, 4, 7 | Yes |
- Note: 1 = overall complications, 2 = failure, 3 = CLABSI, 4 = local infection, 5 = occlusion, 6 = CVAD-associated VTE, 7 = dislodgement/migration, 8 = breakage and/or rupture, 9 = dehiscence.
- Abbreviations: CVAD = central venous access device, PC = prospective cohort, RC = retrospective cohort, RCC = retrospective case-control, RCT = randomized control trial, USA = United States of America.
3.3. Study Quality
The MMAT tool [24] was used to assess the quality of the studies, and overall, the quality of the studies included was mixed, as summarized in Figure 2. There were several studies that did not provide adequate definitions for outcomes. Only device failure data were able to be included for Buonpane, Lautz and Langer [33], and Mangum et al. [37] as other definitions were unable to be clarified by the authors. Several studies were unable to clarify if patients with CVAD-associated VTE were symptomatic and thus were excluded [7, 27, 36, 49]. Six studies had study populations < 100 patients, which may not be representative of the target population [10, 32, 33, 38, 42, 47].
3.4. Outcomes: Risk Factors for CVAD-Associated Complications
3.4.1. Overall CVAD-Associated Complications
Possible risk factors for CVAD-associated complications are listed in Figure 3. Device type was the most reported factor, with several studies [7, 33, 37, 49] including a large retrospective cohort North American study (n = 6553 patients) by Buonpane et al. [33] reporting lower incidence of CVAD revision, replacement, or addition in those with TIVADs compared with TCVCs and PICCs (Odds ratio [OR] 0.25, 95% CI 0.21–0.29, p = <0.0001). Lumen number was assessed in one German study by Beck et al. [31] (n = 296 patients) and increased complications were seen in double lumen versus single lumen TCVCs (hazard ratio [HR] = 3.421, 95% CI 1.71–6.873, p = 0.001; HR 2.58, 95% CI 1.34–4.979, p = 0.005, respectively).
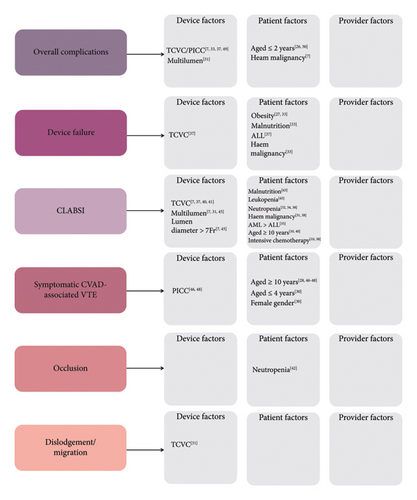
Demographic and clinical risk factors were less commonly reported. Limited data existed on diagnosis as a risk factor, with a single retrospective cohort study from the Netherlands (n = 201 patients) reporting increased complications with hematological malignancies compared with solid tumor malignancies (OR 2.20, 95% CI 1.09–4.47, p = 0.029) [7]. There was conflicting evidence surrounding age as a risk factor. Cesca et al. [26] found on univariate analysis age ≤ 2 years to be a risk factor for CVAD-associated complications and removal (p = <0.01). Similarly, Martynov et al. [50] reported that independent of the type of catheter, infants (< 1 year) had higher rates of complications (p = 0.01). In contrast to this, several studies [7, 27, 29] including a prospective cohort study by Abate et al. [29] (n = 155 patients) concluded that age ≤ 5 years was not a significant risk factor (p = 0.58), though this study did not include infants (< 1 year). There were no data on provider risk factors for overall complications.
3.4.2. Device Failure
A single retrospective cohort study by Mangum et al. [37] (n = 743 patients) identified device type as a risk factor, reporting that patients with TCVCs had an increased risk of device failure compared to those with TIVADs (OR 4.8, 95% CI 1.9–12, p = <0.001) [37]. Obesity [27, 33] and malnutrition [33] were also associated with device failure. Fu et al.‘s [27] large retrospective cohort study (n = 1026 patients) demonstrated obesity as a risk factor for premature catheter removal (OR = 6.90, 95% CI 1.62–29.43, p = 0.009). Similarly, Buonpane and Lautz and Langer [33] found malnutrition (OR = 2.34, 95% CI 1.91–2.87, p = <0.0001) and obesity (OR = 1.41, 95% CI 1.05–1.90, p = 0.02) to be risk factors.
Studies varied on malignancy type as a risk factor for device failure. A large retrospective cohort study by Mangum et al. [37] (n = 743 patients) reported higher device failure in patients with acute lymphoblastic leukemia (ALL) compared to those with acute myeloid leukemia (AML; OR 3.0, 95% CI 1.5–3.4, p = <0.0001; OR = 0.97, 95% CI 0.5–1.8, p = 0.91, respectively). Conversely, a smaller retrospective cohort study by Fu et al. [27] found no difference between ALL and AML (p = 0.86). One large retrospective cohort study by Buonpane et al. [33] (n = 6553 patients) found lower device failure in those with solid tumor malignancy compared with hematological malignancy (OR 0.87, 95% CI 0.76–0.99, p = 0.034). There were no data on provider risk factors for CVAD failure.
3.4.3. CLABSI
Device type was a risk factor with several RC studies [7, 37, 40] and one RCT [41] reporting increased CLABSI risk with TCVCs compared with TIVADs (HR 0.22, 95% CI 0.10–0.51, p = <0.001). Device size and lumen number were also identified as CLABSI risk factors [7, 31, 45]. Beck et al. [31] reported double lumen CVADs as a risk factor compared with single lumen CVADs (HR 3.465, 95% CI 1.38–8.7, p = 0.008). Van Den Bosch et al. [7] reported double lumen CVADs and increased lumen diameter (> 7 Fr) to both be risk factors (OR 3.31, 95% CI 1.68–6.54, p = 0.001 and OR 4.31, 95% CI 2.16–8.64, p = <0.001, respectively). Martynov et al. [45] reported double lumen CVADs and increased lumen diameter (> 7 Fr) as risk factors with univariate analysis (HR 2.68, 95% CI 1.346–5.319, p = 0.005; HR 2.23, 95% CI 1.148–4.329, p = 0.018, respectively); however, this was not replicated on multivariate analysis.
There are conflicting findings regarding neutropenia and white cell count (WCC) as risk factors for CLABSI. A retrospective cohort Brazilian study by Viana Taveira et al. [43] (n = 188 patients), found WCC < 1000 mm−3 to be a risk factor (RR 1.64, 95% CI 1.22–2.2, p = <0.01); however, a retrospective cohort Singaporian study by Cher at al [35]. (n = 243 patients) did not find WCC to be a risk factor (OR 0.88, 95% CI 0.28–2.73, p = 0.811). Some studies identified neutropenia as a risk factor [32, 34, 38]; however, this was not consistent with several other retrospective cohort studies and one retrospective case control study [10, 35, 39, 40, 45].
Other risk factors for CLABSI included factors relating to diagnosis and type of treatment. Hematological malignancy, compared with solid tumor malignancy, was reported as a risk factor in several retrospective cohort studies [31, 35, 38]. A Greek study by Miliaraki et al. [38] (n = 91 patients) reported hematological malignancy as a risk factor (OR 5.37, 95% CI 2.2–13, p = 0.005). Similarly, a study by Beck et al. [31] (n = 296 patients) found an increased risk of CLABSI in hematological malignancies versus solid tumor malignancies (HR 3.7, 95% CI 1.52–9.17, p = 0.004). Cher et al. [35] (n = 243 patients) studied risk factors associated with early CLABSI in the first 30 days post line insertion and found that AML compared with ALL and solid tumors was associated with higher rates of CLABSI (OR 5.09, 95% CI 1.27–0.3, p = 0.003). In contrast to this, one retrospective cohort study found no association between hematological malignancy diagnosis and CLABSI [45]. In relation to the type of treatment, two retrospective cohort studies reported a higher incidence of CLABSI in those receiving “high intensity” chemotherapy (the chemotherapy regimen was not defined) [10, 38].
Less frequently reported CLABSI risk factors included age (> 10 years in two studies [10, 40]) and body mass index (chronic malnutrition in one study [43]). Fever in the 24 h preceding CVAD insertion was assessed in one study and had no association with CLABSI [44]. Provider factors were only assessed in one retrospective cohort study [35] (n = 243 patients) with no association between surgeon rank, approach or number of attempts and CLABSI.
3.4.4. CVAD-Associated VTE
CVAD-associated VTE risk factors included device type. Two retrospective cohort studies by Noailly et al. [48] (n = 192 patients) and Onyeama et al. [46] (n = 198 patients) reported PICCs having a higher VTE risk than TCVCs and TIVADs (OR 5.6, 95% CI 1.2–26.5; OR = 7.88, 95% CI 1.76–35.25, p = 0.007, respectively).
Several demographic and clinical factors were reported as risk factors for VTE. Multiple studies [28, 46–48], including a large RCT by Gidl et al. [28] (n = 1026 patients), reported older age (> 10 years) as a risk factor (HR 2.156, 95% CI 1.04–4.469, p = 0.03). A single retrospective cohort study by Albisetti et al. [30], (114 patients) found younger age (< 4 years) (adjusted OR 0.89, 95% CI 0.81–0.97, p = 0.01) and female gender (adjusted OR 2.31, 95% CI 1.04–5.1, p = 0.03) to be independent risk factors for TIVAD-associated VTE.
3.4.5. Occlusion
One study reported risk factors for CVAD occlusion. A prospective pre-postimplementation study by Ullman et al. [42] (n = 56 patients) in their prospective preimplementation data reported neutropenia as a risk factor (incident rate ratio [IRR] 1.44, 95% CI 1.15–3.97, p = 0.02).
3.4.6. Dislodgement/Migration
Risk factors for dislodgement/migration were only assessed in one retrospective cohort study by Beck et al. [31], which found TCVC to be associated with higher rates of dislodgement than TIVADs (HR 6.22, 95% CI 1.412–27.392; p = 0.016).
3.4.7. Local Infection, Dehiscence, and Breakage/Rupture
None of the included studies reported risk factors for local infection, dehiscence, or breakage/rupture.
4. Discussion
This scoping review is the first to explore, summarize, and critique the existing literature on risk factors for CVAD-associated complications in pediatric patients with cancer and synthesizes findings from 25 included studies. The available literature on risk factors was focused on CLABSI, CVAD-associated VTE, and device failure, with little or no studies reporting risk factors in relation to occlusion, dislodgement/migration, breakage/rupture, local infection, or dehiscence. This review highlights that risk factors for CVAD-associated complications in this cohort are poorly understood, and further prospective data are needed to understand what drives these complications and inform preventative strategies. Establishing risk factors in this cohort will allow assessment of which factors are modifiable, and in those that are not modifiable, we can then consider how to reduce the risk of CVAD-associated complications.
Most prior research examining risk factors for CVAD-associated complications was focused on CLABSI (N = 20, 77%), with device-related factors the most commonly reported (n = 11, 55%). External devices (e.g., TCVCs and PICCs) and devices with multiple lumens were associated with an increased risk of CLABSI. This is likely related to increased handling during use and maintenance, while also providing several points of entry for potential microorganisms and thus infection [51]. When clinically appropriate, the literature supports the choice of TIVADs with the smallest diameter catheter and the minimal number of lumens, in accordance with current guidelines [51, 52]. However, device-selection in this population is complex and often driven by treatment-related factors (i.e., intensive chemotherapy and high supportive care demands) or clinical deterioration, which often is not compatible with TIVADs or single lumens. Device-type may not be a modifiable risk factor due to clinical requirements, and to address the issue of CLABSI, we also need to consider how we can mitigate modifiable patient and provider factors to reduce a patient’s risk.
Clinical factors for CLABSI were minimally reported and often had contradictory results. Several disease-related factors were reported including increased CLABSI risk in those with hematological malignancy, particularly AML, and those receiving intensive chemotherapy. The treatment of hematological malignancies, especially AML, involves intensive blocks of chemotherapy ± hematopoietic stem cell transplantation (HSCT). This combination of treatment results in significant myelosuppression (prolonged neutropenia) and a high risk of infection [53]. While diagnosis is not modifiable, there are clinical trials being conducted to look at how we can reduce the risk of CLABSI in this population through altering our CVAD care [54] and the use of prophylactic antibiotics during intensive treatment blocks [55]. Two retrospective cohort studies and one prospective cohort study reported neutropenia to be a risk factor for CLABSI [32, 34, 38]; however, this was not consistent among several other studies [10, 35, 39, 40, 45]. Neutropenia itself is often not modifiable, but decisions around timing of device insertion could be altered to reduce a patient’s risk. Interestingly, provider factors were only examined in one retrospective cohort study by Cher at al [35] with no association between surgeon rank, approach or number of attempts, and CLABSI. Provider factors are potentially modifiable depending upon the institution, and further research in this area may help reduce CLABSI in situations where clinical and device factors are not modifiable.
The small number of studies reporting risk factors for CVAD-associated VTE focused on device and age. PICCs were associated with increased risk of CVAD-associated VTE compared with TCVCs and TIVADs. These results are consistent with a recent systematic review and meta-analysis of 11,940 adult patients with cancer which found that TIVADs had a lower risk of VTE compared with PICCs (OR 0.38, 95% CI 0.25–0.58) [56]. Similarly, a recent systematic review and meta-analysis by Jaffray et al. [57] found that PICCs resulted in an increased risk of CVAD-associated VTE in the general pediatric population. Due to ease of access, wider provider availability, and option for insertion with minimal sedation, PICCs have become increasingly utilized in this cohort of patients, but with time, we have seen the potential complications of this device, particularly CVAD-associated VTE. It is thought that the increased VTE risk from PICCs is from partial intraluminal obstruction as PICCs are inserted into smaller vessels and as a result they occupy a greater proportion of the vessel lumen and thus increase VTE risk [58]. The previous literature has discussed increased VTE risk in patients with (ALL), secondary to the cancer itself and its treatment with asparaginase and steroids [57, 59]. This review did not identify these as significant risk factors, but the data are very limited due to small numbers (10 studies published on hematological malignancy cohorts with ALL making up a small proportion of these), and treatment regimen not being reported.
A bimodal distribution of VTE has been documented in pediatric patients, with higher incidence in infancy and adolescence [60], and this trend was also found in our review of pediatric patients with cancer [61]. Virchow’s triad postulates three factors predisposing a patient to a vascular thrombosis: hypercoagulability of blood, venous stasis, and endothelial damage [62]. As catheter to vessel ratio appears to play a role in VTE risk [58], this may be further exacerbated in infants who physiologically have small caliber vessels, with further endothelial damage from difficulty with insertion, increased hypercoagulability due to malignancy, and its associated treatment. Adolescents may have increased risk of VTE due to the type of malignancy and its associated complications. Adolescents are most commonly diagnosed with lymphomas, which often present with mediastinal masses (resulting in altered venous flow), and these patients frequently receive PICCs to commence therapy [63]. In addition to their malignancy-associated risk, adolescents are also susceptible to endothelial damage from treatment complications such as dyslipidemia and diabetes, are often immobile for prolonged periods during treatment, and may receive treatment with estrogen therapy [64]. There is likely a complex interplay between device, provider, and patient factors contributing to VTE risk, and while better quality, prospective data are needed on VTE risk factors in this cohort, being mindful of the patient’s age, and device type should be considered when making device-selection decisions.
Overall, despite CVADs being the most common device used in pediatric patients with cancer, our review highlights how poorly we understand these devices and their complications in this cohort. Concerningly, there was a complete lack of the literature published on risk factors relating to local infection, dehiscence, dislodgement/migration, and breakage/rupture. Each of these complications has associated investigations (e.g., line position studies) and treatments (e.g., antibiotics and procedural manipulation) leading to unplanned hospital admissions and stress placed upon the patient, parent, and health system [4, 5, 7]. The consequence of many of these complications is also device failure, which results in treatment delays and has been linked with increased morbidity and mortality [4, 65]. There is insufficient research in this cohort to understand why and what is driving these complications. Part of the problem with capturing this data relates to the history of incomplete and inconsistent documentation of data when devices fail or complications occur [66]. As discussed in this review, several studies have to be excluded or only select data used as a result of inadequate outcome definitions or incomplete data, and multiple complications had no data at all. Schults et al. [66] developed an international consensus of 50 items that should be collected at minimum for vascular access research, covering patient demographics, device characteristics, insertion details, management details, and complications. If we are to tackle the significant issue of CVAD complications and device failure, then we need to understand why it is happening, and that requires prospective research to be conducted on the risk factors for these complications and for researchers to adhere to the minimum standard of reporting for vascular access data [66].
4.1. Limitations
This review was limited by several factors including heterogeneity in study methods and populations, incomplete definitions of outcomes, and incomplete reporting of data. All these factors resulted in a relatively small number of studies reporting risk factors for CVAD-associated complications in pediatric patients with cancer. While an attempt to keep the data relevant to contemporary CVAD care was made by limiting studies to the last 12 years, practices for CVAD care have evolved and there is a risk that the results may not accurately represent modern complication rates and risk factors. In addition, while our review reports associations between CVAD complications and various risk factors, these do not imply causation.
5. Conclusion
Overall, this scoping review provides an analysis of a broad range of potential risk factors for CVAD-associated complications in pediatric patients with cancer. This review identified multiple device-related risk factors for CVAD-associated complications, particularly the use of an external device, more than one lumen number and increased lumen size. Our understanding of provider factors (e.g., skill level, timing, and technique of insertion) and patient factors (e.g., age, cancer diagnosis, and treatment protocol) in this cohort and the relationship of these is poorly understood. Further research in these areas is needed to help us better understand what drives these complications and to inform preventative strategies. We also need to improve the quality of our research through encouraging researchers to adhere to benchmarking standards for vascular access data reporting.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jenna L. Nunn: conceptualization, methodology, software, validation, formal analysis, investigation, writing–original draft, review, and editing. Mari D. Takashima: methodology, writing–review, and editing. Erin M. Wray-Jones: methodology, investigation, writing–review, and editing. Trisha A. Soosay Raj: conceptualization, methodology, writing–review and editing, and supervision. Diane M. Hanna: conceptualization, writing–review and editing, and supervision. Amanda J. Ullman: conceptualization, methodology, writing–review and editing, and supervision.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Artificial intelligence, language models, machine learning, or similar technologies were not used for any aspect of the creation of this manuscript.
Supporting Information
Supporting file 1. Systematic Search Terms.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




