Variation in the Diagnostic and Treatment Pathway in Peritoneal Mesothelioma: A Mixed-Methods Study in the United Kingdom
Abstract
Peritoneal mesothelioma (PM) is a rare and aggressive cancer with a significant impact on the patient quality of life. This study aimed to explore variability in the care pathway of people with PM and to explore the patient experience of the care pathway. A mixed-methods, longitudinal approach was employed. The three stages of the study were (1) cross-sectional survey of PM patients and family members exploring demographic characteristics, patient pathway, experiences of treatment and care; (2) qualitative, case study series of individual patients, their family member/carer/friend and professionals; and (3) case note review of case study patients living with PM. Forty-seven patients (30 women and 17 men) responded to the survey. We recruited seven case studies comprising seven patient participants, eight carers and six professionals. Findings revealed a significant delay in diagnosis due to nonspecific symptoms and challenges in differential diagnosis. The study highlights the need for improved timely diagnosis, enhanced communication between healthcare providers and patients, and referral to specialist mesothelioma multidisciplinary teams. Recommendations include the need to implement smoother treatment and management pathways, to increase referrals to specialist multi-disciplinary teams and to engage patients in decision-making throughout the treatment pathway.
1. Introduction
Recommendations from the National Mesothelioma Audit 2020 [4] are that all patients diagnosed with PM ‘…should be referred for discussion at a mesothelioma multidisciplinary team (MDT) meeting, be signposted to Mesothelioma UK resources, and in patients with an ECOG (Eastern Cooperative Oncology Group) good performance score consider referral to the National Peritoneal Mesothelioma Multidisciplinary Team (NMDT)’ [4]. However, the extent to which these referrals are made appropriately, and whether access is uniform across the UK, remains unknown.
Audit data suggest variation in access to, and experiences of, care in patients living with PM in the United Kingdom [4]. This study aimed to explore the variability in the care pathway of people with PM and to explore the patient experience of the care pathway. This paper will report experiences in gaining a diagnosis of PM and the treatment pathway.
2. Materials and Methods
2.1. Study Design
The study used a longitudinal mixed-methods design.
- 1.
Cross-sectional survey of PM patients and family members exploring demographic characteristics, patient pathway, experiences of treatment and care.
- 2.
Seven qualitative case study series, each case consisting of one patient, one family member/informal carer/friend and up to three professionals.
- 3.
Case note review of case study patients living with PM.
This research was reviewed by the NHS research ethics committee (REC reference 21/PR/1486, IRAS ID 300947). Informed consent to participate was received from all participants.
2.2. Participants and Procedures
Participants for the cross-sectional survey were recruited using a multi-pronged sampling strategy incorporating convenience and snowball sampling. Information about the questionnaire was disseminated via The Peritoneal Malignancy Institute Basingstoke (PMI), HASAG (a national asbestos support group charity), mesothelioma patient support groups, Mesothelioma UK and the Mesothelioma UK Research Centre (MURC). The study was shared via social media to gain wider participation.
The longitudinal case study used a convenience sampling method. Patient participants were identified via the survey, which asked respondents to provide their email address if they were interested in participating in the case study. Asbestos support workers, and HCPs such as oncologists and clinical nurse specialists (CNS), were referred to as ‘professionals’. Family/informal carers/friends were referred to as ‘carers’.
Based on the existing literature and previous studies conducted by the research team [8–10], a sample size of seven case studies was considered sufficient to ensure data saturation [11]. Carers and professionals were identified by the patient. This method of identifying carers and professionals allowed recruitment to take place in a timely fashion [12] and for patients to self-select those people who they felt had most involvement in their care pathway. Purposive sampling was used to select a range of participant demographics and variation in experience such as age, time since diagnosis and gender with a focus on positive deviance. Patients were invited to participate and were provided with written and verbal information prior to consenting to the interview. Professionals and carers identified by the participant were invited to participate.
2.3. Stage 1: Data Collection: Cross-Sectional Survey
Survey content was informed by previous studies conducted by the Mesothelioma Outcomes Research and Experience (MORE) survey [13], Mesothelioma UK Research Centre Research Prioritisation Exercise [14] and a rapid evidence review [15].
The survey collected data on demographic characteristics: pathway of treatment and care (including details of diagnosis, referrals, number of specialties seen, time to diagnosis, onward referral or not) to NMDT, CNS involvement and treatments offered and received and experiences of care. Potential participants were asked to complete the survey via Google Forms or a hard paper copy, depending on participant preference. At the beginning of the online survey, there was a link to the Patient Information Sheet (PIS). By completing the survey, the participant implied consent. On request, hard paper copy PIS and surveys were posted.
Data analysis included the use of descriptive statistics and exploration of the relationship between patient variability of treatment and care using bivariate correlations when appropriate. SPSS Version 29 was used for the analysis.
2.4. Stage 2: Data Collection: Longitudinal Case Study Series
We recruited seven case studies comprising seven patient participants, eight carers and six professionals (Table 1). Patients were asked to take part in serial interviews (up to three) over a course of 12 months. Carers and professionals were interviewed once. Each interview took place online or over the phone, depending on patient preference, and lasted up to 1 hour. Patient and carer interviews were undertaken between February and December 2022. Professional interviews were conducted between August and October 2022.
| Survey: patient participants | ||
|---|---|---|
| Number (%) | ||
| Gender | Female | 30 (63.8%) |
| Male | 17 (36.2%) | |
| Ethnicity | White British | 41 (87.2%) |
| White other | 4 (8.5%) | |
| Other/did not say | 2 (4.2%) | |
| Age (years) | < 30 | 2 (4.3%) |
| 30–39 | 5 (10.6%) | |
| 40–49 | 3 (6.4%) | |
| 50–59 | 10 (21.3%) | |
| 60–69 | 11 (23.4%) | |
| 70+ | 16 (34.0%) | |
| Highest level of education completed | Secondary school | 12 (25.5%) |
| Further education (A levels, etc.) | 7 (14.9%) | |
| Higher education (degree) | 15 (31.9%) | |
| Postgraduate | 12 (25.5%) | |
| Other/did not say | 1 (2.1%) | |
| Case study: patient participants | ||
| Gender | Female | 5 (71.4%) |
| Male | 2 (28.6%) | |
| Age | 50–75 years | 7 (100%) |
| Case study: carer participants | ||
| Gender | Female | 3 (42.9%) |
| Male | 4 (57.1%) | |
| Age | 50–75 years | 7 (100%) |
| Case study: professional participants | ||
| Gender | Female | 4 (66.7%) |
| Male | 2 (33.3%) | |
| Age range | 50–58 years | 6 (100%) |
| Role | Mesothelioma CNS ∗ | 2 (33.3%) |
| Consultant oncologist | 2 (33.3%) | |
| Asbestos support charity worker | 2 (33.3%) | |
| Years in mesothelioma services | 5–10 years | 2 (33.3%) |
| 10–15 years | 1 (16.7%) | |
| 20–25 years | 3 (50%) | |
- ∗Clinical nurse specialist.
The interview topics were generated through the exploration of existing literature and previous studies conducted by a research team [8,10]. The semi-structured interview schedule focussed on patients/carers’ experiences of living with PM (from before diagnosis to current), experiences of the care pathway, diagnosis, referral, treatment; barriers and facilitators to a consistent care pathway; and satisfaction with care. The interview schedule (Appendix 1) for professionals asked about perceived variability in the PM patient pathway and any implications of this. Interviews were recorded, transcribed verbatim and imported into Quirkos© software for analysis.
Given the lack of evidence in patient experience and mesothelioma, it was decided to organise the case studies according to a descriptive framework of the pathway [16,17]. Once the framework was developed, findings were plotted onto this framework. Four steps in data analysis were followed: (1) the patient and carer transcripts were re-read through for accuracy, and any patterns were noted; (2) the descriptive framework based on key experiences in the peritoneal pathway was developed. VS, CG, SEM and SW developed separate frameworks and then discussed them and developed an amalgamated framework. They each then coded one patient and one carer interview and amended the coding framework. Iterative revisions to the coding framework were made following further team discussions; (3) data for all patients, carers and professionals was coded and (4) the framework was then populated with data from patients, carers and professionals. Developed codes were then organised into potential themes, which were arranged in tables and revised. Rigour was supported by data immersion, iteration, team discussions, reflexive analysis and audit trail. To ensure that the themes were grounded in the data, they were supplemented by direct quotes from the participants. All participant names are pseudonyms.
2.5. Stage 3: Case Note Review
For the case note review, data were collected from hospital medical records, using a standard proforma, which collected information on the date first presenting to an HCP, presenting symptoms, any alternative diagnoses documented, hospital specialist/s referred to, date diagnosis received, who gave diagnosis, number of hospital admissions, treating speciality oncologist and did the patient have a named CNS allocated to them. The case notes of seven patients were those of the seven interviewed patients, and the data obtained from the case notes were entered onto a pseudonymised case report form and given a unique study number.
3. Results
Patient, carer and professional quotes are included within the results section, and further key quotes are located in Appendix 2.
3.1. Demographics of Study Participants
Forty-seven patients (30 women and 17 men) responded to the survey between February 2022 and December 2022 (Table 1). Overall, most patients had epithelioid mesothelioma (46%); 6% had sarcomatoid, 4% had a well-differentiated papillary mesothelial tumour, 23% had multicystic mesothelioma and 21% did not know which type of mesothelioma they had. All patients had primary PM and did not have pleural mesothelioma upon diagnosis. Half (51%) of the respondents had a spouse as a carer. Twenty-five percent of the respondents had a high level of education (postgraduate).
3.2. Pathway to Diagnosis
The survey and interview data revealed considerable variation in the symptoms that patients experienced prior to receiving their diagnosis. The most reported presenting symptoms were abdominal pain, tiredness, change of bowel habit, shortness of breath, sweating and weight loss. Interviews with patients reported that the nature of the PM symptoms had led to some delaying going to their GP as they had not recognised them as serious or requiring urgent attention.
‘There was one antibiotic after another, thinking, you know, they’d sent in people asking him if he’d ever had TB, if he’d ever had liver problems and, you know, they were just, they hadn’t got a clue’ (Carer, case study 4).
‘Patient clearly has had a long and frustrating route to diagnosis as often occurs in patients with rare tumours’ (Professional, case note 1).
| Survey results | Number of patients | |
|---|---|---|
| Initial contact with HCP ∗ | GP ∗∗ | n = 35 (87.5%) |
| Nurse at GP ∗∗ surgery | n = 2 (5%) | |
| Emergency department | n = 3 (7.5%) | |
| Time to diagnosis | n = 32 | |
| 183 days, median ∗∗∗ | ||
| Informed of an alternative diagnosis | n = 18 (38%) | |
| Oncology management team | Lung oncologist | n = 25 (69%) |
| Gynaecologist | n = 5 (14%) | |
| Colorectal oncologist | n = 3 (8%) | |
| Cancer of unknown primary | n = 3 (8%) | |
| Referral to NMDT ∗∗∗∗ | n = 16 (34%) | |
| Patient request for second opinion | n = 4 (8.5%) | |
| Treatments offered | Chemotherapy | n = 30 (70%) |
| Immunotherapy | n = 9 (21%) | |
| Clinical trial | n = 4 (9%) | |
| Received CRS and HIPEC ∗∗∗∗∗ | n = 16 (38%) | |
| Patient experience of investigation and diagnosis process | Satisfied | 64% |
| Not satisfied | 36% | |
- ∗Healthcare professional.
- ∗∗General practitioner.
- ∗∗∗Outlier removed from median. Median was skewed by a very large outlier who had to wait a very long time. The mean average with the outlier is 330 days; the mean without the outlier is 242 days.
- ∗∗∗∗National Peritoneal Mesothelioma Multidisciplinary Team.
- ∗∗∗∗∗Cytoreduction surgery and heated intraperitoneal chemotherapy.
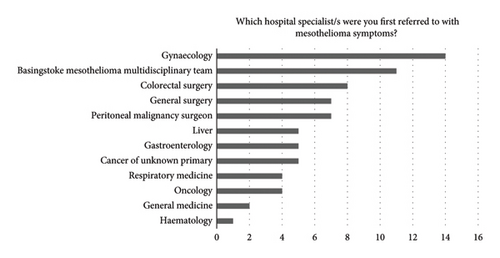
The survey revealed a median of 183 days to receive a diagnosis of PM (Table 2), and 45% of surveyed patients perceived avoidable delays in their diagnosis. Survey results and case note review data indicated that extended diagnostic pathways were partly explained by the broad range and multiple specialities patients were referred to (Figure 1) and show that multiple alternative diagnoses were given (Table 3). Interviews and case note review data reflected the complex routes to receiving a diagnosis of this rare cancer.
| Patient case | Number of days between first presentation to a HCP ∗ and receiving a diagnosis | Specialist giving diagnosis | Number of differential diagnoses (other than PM ∗∗) | Number of specialists referred to | Treatment under which oncology speciality | Referral to NMDT ∗∗∗ | First treatment |
|---|---|---|---|---|---|---|---|
| C1 (F) | 210 | Sarcoma consultant | 3 | 5 | Lung | ✓ | Chemotherapy |
| C2 (F) | 30 | Gynaecologist | 0 | 1 | Lung | ✓ | CRS & HIPEC ∗∗∗∗ |
| C3 (M) | 180 | Lung oncologist | 1 | 3 | Lung | ✗ | Diagnostic surgery at a local hospital resulted as treatment |
| C4 (M) | 90 | Respiratory medicine | 3 | 3 | Lung | ✗ | Chemotherapy |
| C5 (F) | — | Gynaecologist | 0 | 2 | Lung | ✓ | Immunotherapy |
| C6 (F) | 120 | Lung oncologist | 0 | 0 | Lung | ✓ | Chemotherapy |
| C7 (M) | 30 | Cancer of unknown primary team | 1 | 2 | Lung | ✓ | Chemotherapy |
- ∗Healthcare professional.
- ∗∗Peritoneal mesothelioma.
- ∗∗∗National Peritoneal Mesothelioma Multidisciplinary Team.
- ∗∗∗∗Cytoreduction surgery and heated intraperitoneal chemotherapy.
Eighteen surveyed patients and four case note patients were informed of an alternative diagnosis before PM diagnosis (Tables 2 and 3): ovarian cancer (n = 5), endometriosis (n = 3), cancer of unknown primary (n = 3), pseudomyxoma peritonei (PMP) (n = 3), no cancer found (n = 2), irritable bowel syndrome, appendicitis, sarcoma, paraganglioma, peritonitis, mullerian tumour and pancreatic cancer (n = 1). Forty patients were given a PM diagnosis by a consultant. Seventy percent said the diagnosis was understandable, 19% said the diagnosis was not understandable and 62% said it was given in a sensitive way.
3.3. Management
Survey results demonstrate variation in oncology speciality for ongoing management and treatment for PM patients (Table 2). Sixteen patients were referred to the NMDT and/or a peritoneal malignancy surgeon. Six patients sought a second opinion from the NMDT for diagnosis confirmation and surgical opinion for CRS and HIPEC. Two patients had a second opinion with a mesothelioma oncology expert, and two patients sought a second opinion overseas. Case note data showed that one patient asked their GP to refer them to another oncologist to explore the option of an immunotherapy clinical trial. Professionals interviewed demonstrated a recognition that much of their (HCP) experience came from pleural mesothelioma as this formed the largest part of their clinical caseload.
‘I think when we went to [NMDT centre], they were obviously the centre of expertise. I really felt in safe hands when I went there and we were, even before the operation, we were linked up with [name] who’s the specialist nurse who was brilliant. If we had any questions you could phone up, I mean she wasn’t always on the end of the phone, but she′d always ring back and have a chat with you and reassure you. And she was just that intermediary really between the doctors and us, and it was really good’ (Carer, case study 7).
‘For peritoneal cancers in general we often involve the (national) peritoneal cancer unit. But that’s certainly in my mind not about primary management of the disease, but we consult them for the specific aspects of surgical intervention if us as a mesothelioma MDT and them as the peritoneal cancer MDT think that surgery may have something to offer’ (Professional 3).
However, there was evidence that this may not be a consistent practice across England as some oncologists may not be aware of the NMDT; others were more likely to refer only if surgery was felt to be a treatment option. Factors influencing referral to the NMDT were awareness of the NMDT, the local MDT decision to refer, for example, deciding not to refer due to the delays in waiting for NMDT meeting outcomes (NDMT being monthly), and individual perspectives of the local team on benefits of surgery versus SACT in PM.
3.4. Treatment
Of the surveyed patients, 87% were satisfied with their treatment. Seeing a gynaecological oncologist was associated with being less satisfied with treatment (Pearson statistic −0.395, p = 0.006, p ≤ 0.01 n = 47), though these numbers are small. Patients were asked if they were offered various treatments, such as chemotherapy, immunotherapy and clinical trials (Table 2). No patients received radiotherapy. Most patients referred to the NMDT were recommended SACT. Sixteen patients received CRS and HIPEC at the PMI surgical centre. Review of case note data revealed one patient, and their family enquired with the oncology clinic registrar about CRS and HIPEC offered at the NMDT, but the professional explained there was significant peritoneal involvement and therefore first-line treatment would be chemotherapy. The case notes continued, with the doctor explaining that if there was a suitable response to chemotherapy, referral to the NMDT would be appropriate.
3.5. Factors Influencing Variability
Survey data showed vast variability of the pathway but no correlations. There was no significant correlation between satisfaction (in investigation or treatment) and time to referral or time to diagnosis (Table 4). There was no significant correlation between avoidable delays (Pearson 0.269, p = 0.11, n = 47). There were no relationships between presenting symptoms and the first specialist the patient was referred to (Table 5). There was no significant correlation between avoidable delays and patients’ perception of how delays affected their quality of life (Pearson 0.269, p = 0.11, n = 47), with patients who had experienced delays more likely to have an effect on the quality of life. Lack of correlation is likely due to the small sample size or because indeed the PM pathway is hugely varied between patients.
| Time to first referral | Time to diagnosis | |
|---|---|---|
| Satisfaction with investigation | Pearson −0.030 | Person 0.049 |
| p value 0.868 | p value 0.788 | |
| n = 33 | n = 32 | |
| Satisfaction with treatment | Person 0.104 | Person −0.111 |
| p value 0.565 | p value 0.544 | |
| n = 33 | n = 32 | |
| First specialist seen | |||
|---|---|---|---|
| n | Pearson correlational statistic | p value | |
| Abdominal symptoms | 47 | 0.123 | 0.411 |
| Appetite/nausea | 47 | 0.283 | 0.054 |
| Back pain | 47 | 0.213 | 0.150 |
| Sweating | 47 | −0.219 | 0.139 |
| Shortness of breath | 47 | −0.259 | 0.079 |
| Accidental discovery | 47 | 0.026 | 0.861 |
| Other | 47 | 0.132 | 0.375 |
3.6. Overall Experience of the Diagnostic and Treatment Pathway
‘It was very useful that [CNS] at [city] knew straight away to direct me to [national MDT centre], I think that was really helpful. So, I don’t know if in other hospitals they’d be quite so, that link would be quite so strong, so that was really good’ (Patient 2).
‘She [consultant] said it with, again, such compassion… I felt she was totally looking after me, my interests, not anything else’ (Patient 7).
4. Discussion
This study provides valuable insight into the patient experience of the diagnosis and treatment pathway for PM. It builds on existing evidence that PM is difficult to diagnose, with patients presenting with nonspecific symptoms that were often concluded to be more common illnesses. These nonspecific symptoms cause a long-drawn-out diagnostic phase before a final diagnosis was reached, and our study reports the average time between the first symptoms and diagnosis was half a year. Ideally, PM should be included in the differential diagnosis of patients with a peritoneal neoplasm; however, this is understandably challenging given its rarity. Patients are likely to have an enhanced experience of the care pathway when teams communicate well, and specialist MDTs are utilised [18]. Our findings highlight the value of the NMDT in centralising treatment recommendations for this rare cancer, with the aim of providing equity in care, although it appeared not to be a routine practice across the United Kingdom. Review at the NMDT also identified patients suitable or not suitable for CRS and HIPEC.
4.1. National Health Service (NHS) and the Management of Peritoneal Malignancy in the United Kingdom
The NHS was established in 1948 to provide free health services in the United Kingdom to everyone in the country based on need, not ability to pay. The NHS is funded by national taxation and is led by NHS England. The government sets the framework for the NHS and is accountable to Parliament for its operation. However, most decisions are made by the local NHS and by patients with their clinicians.
There are a limited number of centres in the United Kingdom providing peritoneal malignancy services (PMS). NHS England commissioned a service for the assessment and management of patients with peritoneal malignancy, and as such, PMP and colorectal peritoneal metastases have highly specialised commissioning at PMI Basingstoke Hospital and the Christie Hospital in Manchester. As part of this commissioning arrangement, the service must evaluate the assessment and management of this disease type and report on its effectiveness. An interim commissioning policy for PM has been superseded by NHS England’s decision not to routinely commission CRS and HIPEC for PM. Requests to date have been unsuccessful, and PMI Basingstoke currently offers this service at local tariff rates and continues to host the NMDT.
Surgical, gynaecological and colorectal specialities are likely to have the knowledge of PMS available in the United Kingdom; however, nonsurgical specialities could be less aware. The authors believe that specialities unaware of PMS could negatively impact the care pathway and suggest that PMS positivity impacts the patient care pathway and experiences through cohorting a rare cancer within centres of excellence and expert HCPs.
4.2. Multidisciplinary Team Working
While our findings show that referral to the NMDT for PM patients does not appear to be a routine practice in England, the wider literature shows that there may be benefits to MDT referral for mesothelioma patients. MDTs have been integrated in routine cancer care in the United Kingdom since 2000. Specifically, the NHS Cancer Plan recommends that all cancer patients should be discussed in cancer-specific MDT meetings [19]. To facilitate this, the UK Department of Health Mesothelioma Service Framework [20] recommends mesothelioma cases be discussed at a specialist mesothelioma MDT meeting to provide opportunity for discussion among experts. This is also the recommendation in the British Thoracic Society guidance [21]. The National Mesothelioma Audit [4] and the European Society for Medical Oncology pleural mesothelioma clinical guidelines recommend that treatment strategies should be discussed at an MDT with experience in mesothelioma management [22]. The benefits of a specialist mesothelioma MDT are enhanced patient satisfaction, staging, diagnostic accuracy, classification of subtype, treatment and increased recruitment in clinical trials [3,7,19,23,24]. Brandl et al. [1] suggest that centralising expert surgical opinion is effective at selecting patients appropriate for CRS and HIPEC and this expertise makes important contributions to the management of patients with PM.
4.3. Communicating a Diagnosis
Interviewed patients commented on the need for better communication and discussion around diagnostic tests and treatment options including CRS and HIPEC. They also reported the need for more information about what to expect from having PM and regular HCP–patient check-ins. Patients wished to understand different treatment options available as they could make more informed decisions. Well-coordinated, timely, thorough and compassionately delivered diagnoses were valued, but often HCPs lacked the depth of knowledge about PM.
When communicating a PM diagnosis, HCPs are faced with balancing the provision of accurate information while maintaining hope [25]. Taylor, Warnock and Tod [18] studied the challenges associated with communicating a mesothelioma diagnosis, which included the lack of time allocated to patients and carers at diagnosis, the lack of access to ongoing training for HCPs delivering diagnoses and the lack of suitable clinical environments in which to deliver information. Furthermore, Wittmann et al.’s [26] study of oesophagogastric cancer found that some patients wanted a great deal of information regarding their illness compared to the HCP’s perception. By identifying how much information a patient wishes to receive and the best way to deliver this information, patient experience can be positively impacted.
Patient participants also expressed they wanted their HCP to acknowledge the prognosis differences between pleural and PM. Most published studies including pleural and PM do not differentiate between the two, and many studies only include pleural patients [27]. There is a scarcity of information available for people living with PM and those that care for them in both personal and professional capacities.
5. Limitations
To our knowledge, this is the largest experience study of PM in the United Kingdom examining the diagnostic and treatment pathway. While this is the largest sample of PM patients surveyed, due to the rare nature of PM, our sample size was small and may not be representative, and interpretation should be considered cautiously.
6. Recommendations for Practice
Recommendations for practice have been designed to enhance the experience of the PM pathway. Moving forward, it is hoped that the study’s recommendations improve patient experience and provide equity of care across the United Kingdom (Table 6).
| Improve timely diagnosis and promote smoother treatment and management pathways | • Provide good partnership working and communication |
|---|---|
| Refer all PM patients to specialist MDTs | • All PM patients should be referred for a discussion at a mesothelioma MDT and should be considered for referral to the NMDT (recommendation 9 of the mesothelioma audit 2018 [4]) |
| Improve the delivery of diagnosis | • Forward planning before delivering a PM diagnosis |
| • Give accurate disease-specific information at the right time | |
| • Signpost to support services | |
| • Take care around prognosis and differentiate between pleural and PM prognoses | |
| Engage patients in decision-making throughout the treatment pathway | • Discuss treatment options available |
7. Conclusions
This study provides valuable insights into the care pathway experiences of PM patients, their carers and professionals. The experiences showed variation and uncertainty across the whole care pathway, from initial investigations, to diagnosis, to treatments and information and support. It supported the literature of diagnosis delays. Multiple specialities were involved in diagnosis and care, with lung oncology being favoured as the preferred speciality for treating PM. Specialist PM MDT services were highly valued and supported a more coordinated care pathway. Examples of excellent and positive experiences illustrate the possible foundations for improving care. We hope that our study recommendations will help improve the experience of the PM pathway.
7.1. Footnote
Since completing this study, the first-line SACT has been changed from chemotherapy using a platinum-based agent and pemetrexed to immunotherapy using ipilimumab and nivolumab. The Checkmate 743 clinical trial [27] included pleural mesothelioma only, and it is important to note that immunotherapy research is limited in PM. PM patients can receive first-line ipilimumab and nivolumab.
Disclosure
The views expressed are those of the author(s) and not necessarily those of Mesothelioma UK or University of Sheffield.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This project was funded by Mesothelioma UK as part of a portfolio of research conducted by the Mesothelioma UK Research Centre (MURC). The MURC is an alliance between Mesothelioma UK and the University of Sheffield.
Appendix 1: Interview Schedule: Professional Interview
[The Interview Schedule will be amended depending on the professional background of the participant]
- •
Age
- •
Job role
- •
Length of time working with people living with peritoneal mesothelioma
- •
Based on your experience, what helps people living with peritoneal mesothelioma before receiving a diagnosis? Prompts: Awareness of symptoms, help-seeking, access, referrals
- •
What are some of the challenges that people living with peritoneal mesothelioma experience before receiving a diagnosis? Prompts: Awareness of symptoms, help-seeking, access, referrals
- •
Based on your experience, what helps people living with peritoneal mesothelioma at diagnosis?
- •
What are some of the challenges that people living with peritoneal mesothelioma experience at diagnosis?
- •
What could be done to improve the diagnostic experience of people living with peritoneal mesothelioma? Prompts: Is there anything that could be done differently to improve the way that information/support/explanations are given at diagnosis? Timing different?
- •
Can you think of any things that make it easier or more difficult for people living with peritoneal mesothelioma to access treatment and care? Prompts: What would make things easier for them? What makes things more difficult?
- •
Are there particular treatments or aspects of care that you think should be more easily accessible? Prompt: Why? Practically, how could this be done?
- •
Who or what helps people living with peritoneal mesothelioma make decisions about treatment and trials for mesothelioma? Prompt: Hospital healthcare professionals, GPs and practice nurses, partners, other family members or friends, helplines, support groups?
- •
Which sources of support are particularly useful while living with peritoneal mesothelioma? Prompt: Any particular people, organisations, websites? What role have they played? What is it about them that you feel is supportive? Are there any specialist teams, treatments, support groups, etc., that you know of but are underused by patients and their families? And why is that?
- •
Where do people living with peritoneal mesothelioma receive their information about claiming benefits and making legal claims? Prompts: What were the circumstances? Who informed you? What were your initial thoughts?
- •
In your experience, what encourages or discourages people living with peritoneal mesothelioma to claim benefits or make legal claims?
- •
Which factors impact on the quality of life of those living with mesothelioma?
- •
What are the things that make patient and their families’ lives more challenging when living with peritoneal mesothelioma?
- •
Do some people living with peritoneal mesothelioma face more challenges than others? What types of challenges? And, why is this?
- •
What are the things that make patients and their families’ lives easier when living with peritoneal mesothelioma?
- •
Additional comments about the experience of people living with peritoneal mesothelioma?
- •
Questions about the study?
Ending
Appendix 2: Quotes
‘I was feeling a bit uncomfortable in my abdomen but nothing particularly, not really painful, not to such an extent that I was immediately concerned but just uncomfortable and not feeling quite right. And it went on for, sort of, a month to six weeks or so and I was thinking, well maybe I ought to get this checked out’ (Patient 2).
‘I suppose it’s a bit like other peritoneal malignancies and pelvic cancer in that quite often their presenting symptoms can be quite nonspecific and sometimes even subtle …. at the beginning’ (Professional 3).
‘It was very much, this is what we’ve decided you’re doing, consent forms are here, we’ll start in a couple of weeks, bye. And then I had to leave the room, still in shock. No discussion about the type of treatment, it was, you’re having chemotherapy’ (Patient 5).
‘The news was a shock, but it was always going to be, and I don’t think it could have been done any better or kinder’ (Carer, case study 6).
‘So, all my consultant career I guess, although they [PM patients] are very few and far between in, well everywhere really, but they’re a rare beast aren’t they’ (Professional 7).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




