“Tracking Health: How Wearable Technology Is Transforming Cancer Care”: A Systematic Review
Abstract
Introduction: Cancer is a major global health problem with a significant burden in both developed and developing countries. Given the rapid growth and high acceptance of wearable devices (WDs) in cancer management, this systematic review aims to explore the outcomes of WDs in the management of cancer patients.
Methods: A structured search of PubMed, Cochrane, Web of Science, and Scopus databases was conducted according to the PRISMA statement guidelines. The search was limited to studies published from December 2015 to December 2023, yielding a total of 3617 studies. After quality assessment using the CASP checklists version 2018, 45 articles were included in the final review. Data analysis was performed using thematic analysis.
Results: Of the 45 studies included in the review, 33 were randomized clinical trials. Notably, a significant proportion of these trials (n = 14, or 31.1%) were published in 2021, and the majority were conducted in the United States (51.1%). The findings revealed that WDs were most frequently used in breast cancer studies, accounting for 53% of the total. Fitbit devices were the most commonly used among the various types of WDs, appearing in 62.2% of the cases. The review also identified 75 concepts that were initially grouped into 21 themes, which were then consolidated into six categories: physical activity, mental wellness, quality of life, clinical outcomes, administrative outcomes, and technology acceptance.
Conclusions: Cancer care requires effective methods, and the use of WDs results in high adherence rates and the ability to provide valuable data at all stages of the patient journey. WDs help improve patient outcomes by measuring health metrics such as step count, heart rate, energy expenditure, and sleep regulation.
1. Introduction
Cancer is a major global health problem with a significant burden in both developed and developing countries. The global cancer burden is expected to reach 28.4 million cases in 2040, an increase of 47% from 2020 [1]. Cancer has physical, physiological side effects, including muscle wasting, weight changes, decreased lung capacity, decreased body flexibility, and depression [2]. People with cancer, especially those at risk for comorbidities, experience excessive physical fatigue and a severely reduced quality of life. Despite the fact that effective management of the condition in the two-year period following a cancer diagnosis has been demonstrated to result in increased life expectancy [3–5], a significant number of people diagnosed with cancer are not physically active enough, and their physical activity levels decrease after diagnosis [6]. In addition, chemotherapy often causes fatigue, anxiety, depression, and other side effects in many patients [6, 7].
In the context of cancer treatment, patients undergoing surgery, chemotherapy, or radiation therapy are typically advised to maintain their physical activity levels at pretreatment levels [8]. A plethora of studies have demonstrated that participation in physical activity can mitigate the deleterious effects of chemotherapy and enhance the quality of life for cancer survivors [9]. It has been demonstrated that an increase in the level of continuous physical activity may contribute to an increase in survival duration [10]. It is an established fact that individuals with a history of cancer, who generally seek to enhance their general well-being, are able to increase their life expectancy and improve their quality of life. In this context, professionals are exploring the use of various technologies, such as applications, social media, and wearable device (WD), with the aim of improving disease management in diverse populations [11]. The impact of these tools on the physical activity levels and behaviors of cancer patients has yielded encouraging results [12].
Wireless digital storage devices, such as smartwatches and fitness wristbands, represent a nascent technological field that has undergone substantial development [12, 13]. The popularity of these devices can be attributed to their capacity to monitor health metrics, including step count, heart rate, energy consumption, and sleep regulation. These devices have exerted a substantial influence on global markets. Wearable sensors have the potential to provide evidence-based behavior change techniques, such as goal setting, feedback, attitude monitoring, and social support. Furthermore, these tools enable individuals to objectively monitor their physical activity levels [14]. Furthermore, the capacity to communicate with smartphones and computers via incentive and tracking applications facilitates effective health monitoring for users [15].
Smart technologies have been employed in the domains of chronic disease management, cardiovascular disease management, and physical activity monitoring. These technologies hold considerable promise in promoting health by means of continuous and regular monitoring of an individual’s activities [16]. The aforementioned technologies can also be applied to patients suffering from musculoskeletal problems or diabetes [17] and breast and colorectal cancer survivors [18]. The utilization of these technologies enables patients to make adjustments to their lifestyle, thereby mitigating potential cancer risks [19].
As the prevalence of cancer rises, there is an increasing need for the implementation of intelligent, regular, and long-term monitoring of patients. The utilization of WDs holds promise in accelerating the processing and dissemination of raw patient data [14, 20]. The advent of fitness trackers, smartwatches, accelerometers, and pedometers has engendered novel methodologies for the precise evaluation of patient performance [21]. These devices are designed to monitor patients’ behavior, including their physical activity (intensity/volume), sleep, heart rate, and blood oxygen saturation percentage, depending on the device type. The measurement data are either recorded in real time or a continuous manner [22]. A systematic review is a methodical evaluation of existing research that facilitates the consolidation of evidence on the outcomes of WDs in cancer care, thereby guiding future research and clinical practice. The objective of this systematic review is to provide a comprehensive overview of the current literature on the outcomes of WDs in the management of cancer.
2. Methods
2.1. Study Design
This systematic review was conducted on studies related to WDs and cancer published between December 2015 and December 2023 (the beginning of this study). The initial search period was set at the beginning of 2015. This temporal window was chosen because WD has matured since then and its use has grown exponentially. The review process involved the following steps: identification and screening of studies, assessment of eligibility, selection of studies, evaluation of quality, and extraction of data using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [23].
2.2. Information Sources and Search Strategy
A comprehensive search of four major databases—Web of Science, Medline, Cochrane, and Scopus—was conducted to identify relevant articles published up to December 10, 2023, concerning the use of WD in cancer management. The Mesh protocol was used to enter the keywords from all search databases, and subsequently results were exported into EndNote Version 20 software. The software’s duplicate removal function was subsequently employed to eliminate duplicates. Any residual duplicates were manually removed by the first author.
The search terms employed are outlined below: “Wearable Electronic Device” OR “Wearable Technology” OR “Wearable Technologies” OR “Wearable Device” AND “Tumor” OR “Neoplasm” OR “Cancer” OR “Malignant Neoplasm” OR “Neoplasia” OR “Malignancy.” The search strategy is outlined in detail in Appendix 1.
2.3. Screening
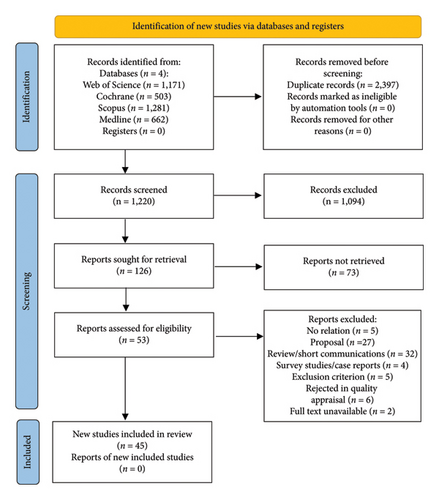
The titles and abstracts of the studies were then examined. Studies deemed irrelevant to the objective of the study were subsequently excluded. The analysis of each study was conducted by two reviewers (N Ch and HA) who independently reviewed the titles and abstracts. Studies that received approval from both reviewers were included, while those that received rejection were excluded. Studies that received approval from only one reviewer were referred to a third reviewer (MH), whose opinion was regarded as the gold standard in these cases. The articles that met the inclusion criteria were then reviewed according to the PRISMA 2020 standard (Figure 1).
2.4. Eligibility
This systematic review includes all clinical trial, cohort, and observational studies that describe the use of WD to manage cancer. However, case studies, surveys, qualitative studies, feasibility studies, and studies presenting models, proposals, and protocols were excluded.
2.5. Quality Assessment
The researchers assessed the quality of the studies using the CASP (Critical Appraisal Skills Program) checklist (https://casp-uk.net/). The purpose of this step was the exclusion of studies that would not be reliable. The checklist was divided into different types of studies. Each study was assessed separately. The checklist for cohort and observational studies comprised 12 questions, while the checklist for clinical trials contained 11 questions. The checklist’s scoring range was 0–100. Studies with a score above 60 were considered to be of sufficient quality and included in the review, while those with a score 60 and below were excluded (Appendixes 2 and 3).
2.6. Data Extraction
Subsequent to the quality assessment, a comprehensive review of the full text of each article was conducted, and the requisite data were extracted using predefined forms. The aforementioned forms encompass a comprehensive array of information, including the authors’ names, the year of publication, the country of publication, the study’s purpose, the interventions employed, the duration of the interventions, gender, age, the study’s type, the sample size, the cancer type, the cancer stage, and the setting (inpatient or outpatient). The extraction of data was carried out by N CH, H A and M H.
2.7. Synthesis
Thematic analysis was employed for data synthesis. The initial phase of the analysis entailed the independent extraction of data from the articles by N Ch and HA. Subsequent meetings were held to address any discrepancies. Author Hayavi Haghighi assessed the differences between the studies by examining their similarities and inconsistencies. The primary concepts were then classified with all three authors collaborating and participating. The initial themes were then extracted. A subsequent meeting was convened to discuss the initial themes, and the authors reached a consensus on the final themes.
3. Results
3.1. Data on Selection Process
A total of 3617 articles were retrieved from the database search, of which 2397 were replication studies. The remaining studies were screened for titles, abstracts, and full text. After the abstract reading phase, 126 studies were selected for full-text review. 45 studies were ultimately included in the analysis, and data were collected from these studies (Figure 1). Also, Table 1 shows the findings extracted from the eligible studies.
| Authors/year of publication/country | Purpose | Intervention(s) | Duration | Sex/age (range or mean) | Type of study/sample | Type of cancer/stage of cancer/setting | |
|---|---|---|---|---|---|---|---|
| (1) | Champ/2018/USA [24] | To analyze and quantify the sleep and activity patterns of women who are receiving radiation therapy for breast cancer | Assessing the change in activity levels and sleep using an activity tracking device before, during, and after radiation therapy (RT) for women with early stage breast cancer and ductal carcinoma in situ (DCIS) undergoing adjuvant RT | NS | Female (100%)/30–80 | Observational/10 | Breast/during NS/outpatient |
| (2) | Che Bakri/2021/NHS [25] | To assess the feasibility and validity of the use of wearable activity monitors (WAMs) to objectively measure the function of the upper limb and to monitor recovery after breast and axillary surgery | Wrist-worn sensors called AX3/wearable activity monitors (WAMs) to objectively monitor limb recovery after breast and axillary surgery | 1 year and 8 months | Female (100%)/55 ± 13.2 | Cohort/39 | Breast/NS/outpatient |
| (3) | Cheong/2018/South Korea [26] | To evaluate the effectiveness of a smartphone-based aftercare tailored rehabilitation exercise program in improving physical activity and quality of life in patients with colorectal cancer | Rehabilitation program using a mobile health care application on a wearable device | 12 weeks | NS/58.27 ± 11.74 | RCTa/75 | Colorectal cancer/NS/outpatient |
| (4) | De La Torre/2021/USA [27] | To examine relationships between motivation to exercise, WAT use, and adherence to physical activity recommendations in cancer patients | Wearable activity tracker (WAT) | 12 months |
|
Observational/608 | All cancer/stage III/outpatient |
| (5) | Dreher/2021/USA [9] |
|
Using Fitbits to track patient activity during and up to 6 months | 9 months | Female (100%)/50.4 | Observational/65 | Breast/Stage 1–3 cancer/outpatient |
| (6) | Ferrante/2020/USA [28] | To assess the feasibility and initial effectiveness of a web-based program for African American breast cancer survivors to lose weight | SparkPeople plus, a wrist-worn physical activity tracker (Fitbit), versus an active wait-list control group (tracker only) | 12 months | Female (100%)/21–75 (61.54 ± 8.83) | RCT/35 | Breast cancer/Stage I-III/outpatients |
| (7) | Ferrante/2022/USA [29] | To assess physical activity adherence and the association between adherence and physical activity outcomes among African American breast cancer survivors participating in a 12-month intervention using a physical activity tracker (Fitbit) | Follow-up (SparkPeople) plus, a wrist-worn physical activity tracker (Fitbit), versus an active wait-list control group (tracker only) | 12 months | Female/21–75 (61.54 ± 8.83) | RCT/34 | Breast cancer/Stage I-III/outpatient |
| (8) | Finley/2021/USA [30] | To assess feasibility and potential effectiveness of a gentle exercise intervention before surgery in patients with suspected or confirmed lung cancer. It also looked at how much exercise participants did before and after their operation | Activity tracker wrist-worn Garmin Vivo active | NS | NS/> 18 | RCT/18 | Lung cancer/clinical stage I-III/outpatient |
| (9) | Gandhi/2020/India [31] | To assess the effects of a pedometer-based exercise program on cancer-related fatigue and quality of life in breast cancer patients receiving chemotherapy | Pedometer | 7 weeks | Female (100%)/> 18 | RCT/44 | Breast/NS/outpatient |
| (10) | Haemmerli/2022/Switzerland [32] | To evaluate the possibility of continuous vital sign monitoring in pediatric cancer patients undergoing chemotherapy through the use of a wearable device | WDb Everion® to record nine different vital signs and health indicators/Augment Study App | 2 weeks | Female (45%), Male (55%)/< 18 years (mean = 6) | Observational/20 | Hemato/NS/outpatient |
| (11) | Hardcastle/2021/Western Australia [33] | To assess the lasting impact of a physical activity intervention on moderate to vigorous physical activity (MVPA), sedentary behavior, and cardiovascular risk factors in individuals who have survived cancer | Fitbit Alta™ | 12 weeks | Female: (58.6%), Male: (41.4%)/(65.0 ± 7.0) | RCT/68 | Adult colorectal or gynecologic cancer/stages 1 and 2)/outpatient |
| (12) | Hardcastle/2020/Australia [34] | To analyze the patterns of Fitbit wear-time and physical activity in cancer survivors over a 12-week intervention and follow-up period | Fitbit wear-time | 12 weeks |
|
RCT/29 | Colorectal and endometrial cancer/stage 1 or 2/outpatient |
| (13) | Hartman/2018/UC San Diego (USA) [35] |
|
Fitbit electronic activity device | 12 weeks | Female (100%)/21–85 | RCT/87 | Breast cancer survivors/NS/outpatient |
| (14) | Hartman/2022/San Diego (USA) [36] |
|
Fitbit | 12 weeks | Female (100%)/21–85 | RCT/75 | Breast/Stage 1/outpatient |
| (15) | Helbrich/2021/Germany [37] | To understand the relationship between self-reported and device-measured physical activity and determine if there were any differences in physical activity levels based on treatment type and duration | Garmin® vivo fit 3 wristbands | 6 months | Female (100%)/18–70 | RCT/99 | Breast/NS/outpatient |
| (16) | Koenig/2021/Switzerland [38] | To evaluate the possibility of continuous monitoring of vital signs in pediatric patients undergoing chemotherapy for cancer using a wearable device (Everion®) | Monitoring of vital signs (Everion®) | 2 weeks | NS/2–16 | Observational/20 | Pediatric patients/NS/outpatient |
| (17) | Kong/2021/Korea [39] | To compare effectiveness of a wearable activity tracker (WAT) plus advice (WAT + advice) versus advice alone on increasing leisure-time physical activity (LTPA) among breast cancer patients undergoing radiotherapy (RT) | Wearable activity tracker (WAT)/counseling (WAT + counseling) | 5 weeks | Female (100%)/19–65 (47.0) | RCT/143 | Breast/NS/outpatient |
| (18) | Le/2017/USA [40] | To evaluate the feasibility of a 6-month home-based exercise intervention for adolescent and young adult survivors of pediatric cancer, using a motivational electronic activity tracker | Motivational electronic activity | 6 months | Female (73.7%)/15–35 | RCT/19 | Childhood cancer/NS/outpatient |
| (19) | Li/2023/China [41] | To evaluate and compare the effects of a physical activity program and a behavioral activation program on psychological distress in adolescent and young adult cancer patients | Wearable device–based physical activity program | 8 weeks | Female (76/22%) Male: (23/78%)/15–39 (31.21 ± 5.539) | RCT/143 | All/NS/outpatient |
| (20) | Low/2018/China [42] | To develop a mobile health (mHealth) platform for the treatment of tumors | Fitbits | 2 years |
|
Cohort/71 | All/Stage IV/outpatients |
| (21) | Lynch/2019/Australia [14] | To evaluate the effectiveness of a 12-week intervention to increase moderate-to-vigorous physical activity (MVPA) and reduce sedentary behavior in postmenopausal breast cancer survivors | Wearable technology activity monitors (specifically the Garmin Vivo fit 2)/telephone-delivered behavioral counseling | 12 weeks | Female (100%)/≥ 50 (61.6) | RCT/83 | Breast/Stage I-III/outpatient |
| (22) | Maxwell-Smith/2019/Australia [43] | To examine the effectiveness and acceptability of a physical activity intervention for cancer survivors | Fitbit Alta TM/phone calls | 12 weeks |
|
RCT/68 | Colorectal and gynecologic cancer/Stage I-II/outpatient |
| (23) | McNeil/2019/Canada [10] | To examine the impact of various physical activity interventions on sedentary time and health-related fitness markers in cancer survivors | Polar A360® activity trackers | 12 weeks | Female/> 18 | RCT/45 | Breast cancer/NS/outpatient |
| (24) | McNeil/2022/Canada [44] | To assess the breast cancer survivors with prescribed physical activity (PA) levels using wearable activity levels by means of wearable activity tracker | Polar A360®/Polar Flow® application | 12 weeks | Female/> 18 | RCT/45 | Breast/NS/outpatient |
| (25) | Mendoza/2017/USA [45] | To assess the viability and acceptability of a 10-week intervention that utilizes Fitbit and Facebook to encourage physical activity among adolescents | Wearable PA/tracking device (Fitbit Flex) and Facebook group | 10 weeks |
|
RCT/52 | All cancers/NS/outpatient |
| (26) | Millstine/2019//USA [46] | To assess the feasibility of using the Muse wearable electroencephalography device to support meditation and reduce symptoms of fatigue, stress, and poor quality of life in recently diagnosed patients. This innovation presents a transformative opportunity in the landscape of breast cancer care, spanning early detection, prognostic precision, and personalized therapy, offering new hope for improved patient survival and long-term outcomes | EEGc device called muse | 3 months | Female (100%)/20–75 | RCT/30 | Breast/NS/outpatient |
| (27) | Nelson/2020/USA [47] | To evaluate physical activity patterns in breast cancer patients receiving chemotherapy | Wrist-worn activity tracker, the Fitbit/Charge HR | 3 years | Female (100%)/21–85 (49.6%) | RCT/32 | Breast/NS/outpatient |
| (28) | Nguyen/2021/Australia [12] | To investigate the impact of a wearable technology–based physical activity intervention on sleep outcomes in breast cancer survivors | Wrist-worn/ACTi Graph® GT3X | 12 weeks | Female (100%)/mean age = 62 | RCT/83 | Breast/NS/outpatient |
| (29) | Nilanon/2020/USA [48] | To assess the impact of physical activity on treatment-related symptoms, functional decline, and quality of life in breast cancer patients | Wearable activity tracker | 150 days | Female (56.1%) Male (46.9%)/≥ 18 | Observational/41 | NS/NS/outpatient |
| (30) | Nyrop/2018/USA [49] | To assess the feasibility and adherence of a home-based exercise intervention for women with early stage breast cancer undergoing chemotherapy | Fitbit ZipTM | 12 weeks | Female (100%)/24–64 (48.3 ± 9.4 | RCT/127 | Breast/stage I–III/outpatient |
| (31) | Pope/2018/USA [11] | To evaluate the effectiveness of a health education intervention delivered using social media combined with a smartwatch in promoting physical activity | Polar M400 smartwatch/Facebook | 10 weeks | Female/means of age = 52.6 ± 9.3 | RCT/30 | Breast/NS/outpatient |
| (32) | Rahimy/2021/USA [50] | To assess the effect of a walking program on physical activity levels in patients diagnosed with endometrial adenocarcinoma | Fitbit Alta | 6 months | Female (100%)/> 18 (means of age = 61) | RCT/46 | Endometrial adenocarcinoma/Stage I-III/outpatient |
| (33) | Robinson/2021/USA [51] | To assess the effectiveness of an intervention that combines a wearable UV sensor with behavior change strategies, such as daily text messages and goal setting, in reducing daily UV exposure in melanoma survivors | Text messages/wearable UV sensor | 21 days |
|
RCT/60 | Skin/NS/outpatient |
| (34) | Rogers/2015/USA [52] | To assess the impact of a behavioral exercise intervention on the physical activity levels, aerobic fitness, and quality of life of breast cancer survivors | Aerobic fitness | 6 months | Female/18–70 | RCT/194 | Breast/Stage I-III/outpatient |
| (35) | Smedley/2021/USA [53] | To investigate how self-reported data from wearable technology, specifically the Fitbit Alta HR, could improve patient-centered breast cancer care | Fitbit Alta HR fitness trackers | 28 days | Female (100%)/means of age = 62.5 | Observational/ | Breast/Stages 0-III/outpatient |
| (36) | Torrente/2021/Spain [54] | To determine the factors that predict poor health status and design personalized interventions to improve the quality of life (QoL) of lung cancer patients, clinical data, wearable devices, and QoL questionnaires will be integrated | Wearable device/artificial intelligence (AI) and knowledge discovery (KD) techniques | 1 week | NS/> 18 | Observational/140 | Lung (all stages)/outpatient |
| (37) | Valle/2017/USA [4] | To prevent weight gain in African American breast cancer patients, a distance and technology-based approach will be used | Tracker/email/app | 9 months | Female/> 18 | RCT/35 | Breast cancer/stage I-III/outpatient |
| (38) | Van Blarigan/2019/USA [55] | To promote physical activity in colorectal cancer survivors, an intervention using an interactive text message program and a Fitbit device was developed and evaluated | Fitbit Flex™/daily text messages | 12 weeks |
|
RCT/42 | Colorectal cancer/Stage II-III/outpatient |
| (39) | Van der Stam/2023/Netherlands [56] | To assess the effectiveness of wearable sensors in postoperative patients who have undergone major abdominal cancer surgery | Wireless accelerometer patch (Health Dot) | 1 year |
|
RCT/103 | Abdominal cancer/NS/inpatient |
| (40) | Wagoner/2019/USA [8] | To compare self-reported levels of physical activity with objective measures from activity trackers in women with early breast cancer who were starting chemotherapy |
|
28 days | Female/means of age: 56 | RCT/161 | Breast/Stage I–III/outpatient |
| (41) | Wu/2019/China [57] | To assess the feasibility and clinical value of using a wearable device connected to a mobile health (mHealth) platform to record physical activity (PA) in patients who have undergone gastrectomy for gastric cancer (GC) | Wearable device connected with mobile health (mHealth) | 28 days | Male (51%), Female (49%)/≥ 20 (68) | Observational/43 | Gastric cancer/NS/inpatient |
| (42) | Xu/2021/China [19] | To investigate the impact of exercise on the body composition and quality of life of breast cancer survivors | Smart bracelet monitoring/Mi Fit | 3 months | Female (100%)/18–65 (48.59 ± 8.04) | RCT/109 | Breast/Stage I–III/inpatient |
| (43) | Yurkiewicz/2018/USA [58] | To investigate the impact of wearable technology on the quality of life of young and adolescent cancer patients | Fitbits/iPads | 6 months |
|
Cohort/33 | Leukemia (ALL, AML) sarcoma lymphoma/NS/outpatient |
| (44) | Zeng/2020/China [59] | To investigate the effects of a 1-year combined fitness program, including the use of wristbands and individualized aerobics, on glycemic, agility, and balance in Chinese breast cancer survivors, focusing on the effects of the fitness program on the selected health parameters | Wristband | 12 months | Female (100%)/44.81 ± 7.94 | RCT/97 | Breast/NS/outpatient |
| (45) | Zhou/2020/China [18] | To describe the body composition profile of breast cancer survivors and investigate the impact of a short-term (3 months) wearable device–based lifestyle intervention on patients’ body weight and body composition | A wearable activity tracker (Mi Band 2 Amazfit health cloud data services) | 3 months | Female (100%)/18–70 (48.83 ± 8.44) | RCT/113 | Breast/stage I –III/outpatient |
- Abbreviation: NS = not specified.
- aRCT = randomized controlled trial.
- bWD = wearable device.
- cEEG = electroencephalography.
3.2. Distribution of Study Type/Setting
A total of 45 studies were included in the review, 33 of which were randomized clinical trials and 12 were observational cohort studies. Furthermore, the findings indicated that the majority of WDs were utilized in the outpatient setting (93.3%).
3.3. Distribution of Studies by Year and Country
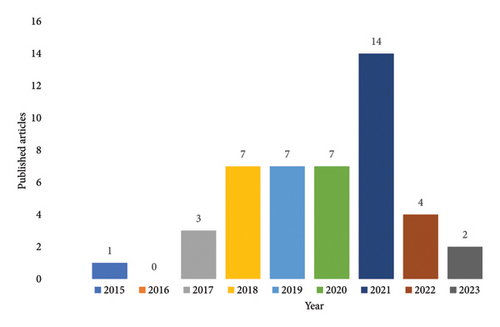
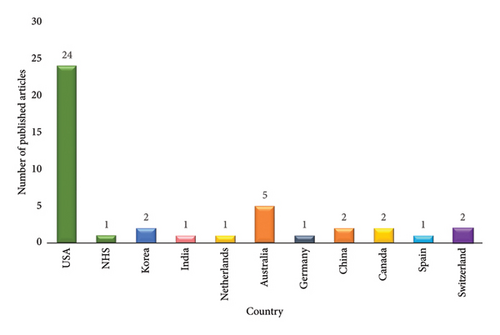
The results indicate that the majority of studies (n = 14, 31.1%) were published in 2021 (Figure 2), with the majority of these studies originating from the United States (51.1%), China (13.3%), and Australia (11.1%) (Figure 3). It is noteworthy that there has been an upward trend in the publication of studies since 2015, with a notable surge in the number of publications in 2021.
The World Bank classifies countries into four income groups: high-income, upper-middle-income, lower-middle-income, and lower-income (https://www.worldbank.org).
The classification identifies the United States, the United Kingdom, Korea, the Netherlands, Switzerland, Spain, Australia, Canada, and Germany as high-income countries, China as an upper-middle-income country, and India as a lower-middle-income country. Therefore, it can be concluded that from 2015 to 2023, an overwhelming majority of the studies, amounting to 87%, were conducted in affluent, high-income countries.
3.4. Distribution of Studies by Type of Cancer
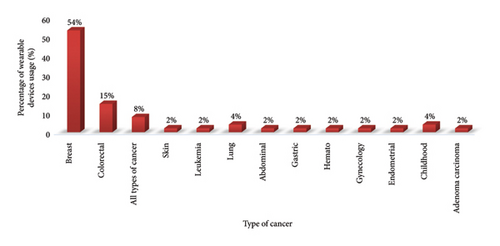
The study revealed that WDs are primarily utilized for managing breast cancer and colorectal cancer. Eight trials either had multiple cancer sites or lacked information on the site of cancer (Figure 4).
3.5. Distribution of Studies by Type of WDs
The survey results indicated that the most commonly reported WDs utilized for cancer management were Fitbits (62.22%), followed by Garmin (8.8%) and Everion (4.4%). These devices are utilized for a variety of purposes, including the monitoring of physical activity, the prediction of clinical deterioration, and the enhancement of patient outcomes.
3.6. Thematic Analysis
A thematic analysis was conducted to examine the effects of WD in cancer management. The analysis yielded 75 primary concepts, which were subsequently classified into 21 initial themes and 6 final themes. These themes encompassed domains such as physical health, mental well-being, quality of life, clinical outcomes, administrative outcomes, and technology acceptance. Table 2 presents the thematic analysis of outcomes of using WDs in cancer management.
| C | Primary concepts | Initial themes | Final themes |
|---|---|---|---|
| 1 | Increased physical activity [10–12, 14, 24, 26, 27, 33, 34, 41, 50, 54] | Physical activity promotion | Physical activity |
| 2 | Increased motivation to exercise [24, 55] | ||
| 3 | Enhanced enjoyment of exercising [27] | ||
| 4 | Increased steps taken [11, 30, 31, 34, 42, 57] | ||
| 5 | Greater flexibility [59] | ||
| 6 | Increased functional capacity [31] | ||
| 7 | Improved recovery of the upper limb [25] | Increased extremities strength | |
| 8 | Improved the strength of the lower limbs [26] | ||
| 9 | Improved lower body flexibility [59] | ||
| 10 | Decrease in BMI [10] | Better weight management | |
| 11 | Prevent losing weight [10, 18, 28, 31] | ||
| 12 | Body fat mass reduction [10, 18, 28, 31] | ||
| 13 | Reduced waist size [29] | ||
| 14 | Improved body composition [19] | Enhanced musculoskeletal indexes | |
| 15 | Increase in skeletal mass [31] | ||
| 16 | Reduced sarcopenia [18] | ||
| 17 | Increase in protein mass [13] | ||
| 18 | Enhanced motivation to diet [24] | Diet compliance | |
| 19 | Adherence to the diet [24] | ||
| 20 | Reduced anxiety [41, 46, 53] | Promoted mental health | Psychological well-being |
| 21 | Reduced depression [41] | ||
| 22 | Increased meditation [46] | ||
| 23 | Increased motivation [45] | ||
| 24 | Improved mental activity [58] | ||
| 25 | Increased self-control [29] | Cognitive improvement | |
| 26 | Increased goal setting [29] | ||
| 27 | Improving self-efficacy levels [29] | ||
| 28 | Increased self-reported cognition [35] | ||
| 29 | Lifestyle changes [24, 28, 31, 45, 50, 52, 58] | Improved lifestyle | QOL |
| 30 | Improved sleep quality [12, 24, 41] | ||
| 31 | Sun protection enhancement [51] | ||
| 32 | Improved social support [41] | Social support | |
| 33 | Monitor activities objectively [25, 43] | Monitoring | Clinical outcomes |
| 34 | Organ recovery and longitudinal monitoring [25] | ||
| 35 | Continuous recording of vital signs [32, 38] | ||
| 36 | Continuous monitoring [32] | ||
| 37 | Monitoring of vital signs [32] | ||
| 38 | Improved heart rate recording quality [38] | ||
| 39 | Tracking different aspects of health [58] | ||
| 40 | Self-weighing at home [4] | Self-measurement | |
| 41 | Reduced need for manual measurement [31] | ||
| 42 | Cardiac endurance [26] | Improved cardiorespiratory capacity | |
| 43 | Respiratory endurance [26] | ||
| 44 | Aerobic endurance [59] | ||
| 45 | Change in aerobic status [30] | ||
| 46 | Reduce side effects [32] | Reduction of adverse effects | |
| 47 | Relieving treatment-related toxicities [26] | ||
| 48 | Relieving cancer toxicities [26] | ||
| 49 | Reducing the death rate [32] | Mortality reduction | |
| 50 | Increased life expectancy [24] | ||
| 51 | Facilitating the treatment process [32] | Effective treatment | |
| 52 | Early detection of infection [32] | ||
| 53 | Early detection of injuries [56] | ||
| 54 | Predicting the risk of readmission [42] | ||
| 55 | Reducing the length of hospitalization [57] | ||
| 56 | Quick intervention in the care path [53] | ||
| 57 | Acceleration of the intervention process [32] | ||
| 58 | Provide real-time feedback [39, 51] | Decision support | |
| 59 | Provide real-time reminders [39, 51] | ||
| 60 | Decrease in blood glucose [59] | Improved clinical metrics | |
| 61 | Reduction of morning salivary cortisol [41] | ||
| 62 | Improved display of health status [32] | Data quality | Administrative outcomes |
| 63 | High quality vital signs [38] | ||
| 64 | Cross-population data comparison [32] | ||
| 65 | Receive patient information on time [53] | Time management | |
| 66 | Being on time [53] | ||
| 67 | Sedentary time reduction [42] | ||
| 68 | Reduction of self-report time [37] | ||
| 69 | Increase participant acceptance [41] | Technology usefulness | Technology acceptance |
| 70 | Adherence to the wearing of the device [9] | ||
| 71 | Increased adoption of technology [9] | ||
| 72 | Increased wearable device compliance [41] | ||
| 73 | Wristband versus other wearable device preference [51] | Technology ease of use | |
| 74 | Enjoy technology [58] | ||
| 75 | Improved patient interaction and experience [53] | ||
4. Discussion
This systematic review examined the outcomes and applications of WD in cancer management from 2015 to December 2023. A comprehensive review of 45 relevant articles revealed that these tools were predominantly utilized in high-income countries, particularly the United States, for the treatment of breast and colorectal cancers. The outcomes associated with the utilization of these devices were reported within six overarching themes.
The predominant utilization of WD has been in the management of breast cancer. Given its capacity to enhance patient outcomes, there has been a notable increase in the adoption of wearable technology within the realm of breast cancer care [60]. This innovation presents a transformative opportunity in the landscape of breast cancer care, spanning early detection, prognostic precision, and personalized therapy, offering new hope for improved patient survival and long-term outcomes [61]. A substantial body of research has demonstrated that breast cancer survivors have reported increased self-awareness, motivation, and comfort as a result of utilizing WDs [62]. It is imperative to acknowledge that the impact of these tools on clinical outcomes varies depending on the nature of the intervention [63]. The most widely used device is Fitbit. This device has been shown to measure steps taken, heart rate, and energy expenditure. In comparison to other devices on the market, such as those produced by Garmin, Fitbit has been demonstrated to exhibit a higher degree of reliability [64] and acceptability [65]. Fitbit’s popularity is due to its accuracy and user satisfaction [66]. The functionality of sleep tracking, motivation, accountability, and discretion are the characteristics that provide users with a valuable experience [67].
The next findings of this study indicate a positive correlation between high-income countries and the use of WD for cancer treatment and medical purposes in general, as well as a corresponding increase in public acceptance of this technology. The adoption of smart WD in high-income countries can be attributed to a number of factors, including trust [68] and other personal factors such as age, income, health status, and self-efficacy with technology [69]. The demand for these devices is driven by two factors. Firstly, there is a need to improve health and fitness outcomes. Secondly, advances in data analytics techniques have made it possible to collect and analyze data more efficiently [70]. The implementation of WD necessitates the formulation of a suitable data management plan, encompassing the collection, dissemination, and integration of data that is more feasible undertaking in high-income countries [63].
WDs are regarded as beneficial instruments for enhancing self-awareness, motivation, and physical activity, in addition to providing real-time feedback. The thematic analysis yielded 75 unique concepts, 21 primary concepts, and six final themes concerning the outcomes of using wearables in managing cancer. The subsequent section will provide an elucidation of these themes.
4.1. Physical Activity
This systematic review has shown that WDs are useful for categorizing levels of physical activity, weight management, diet compliance, and other physical activity outcomes. Physical activity refers to concepts related to increased movement, exercise, and activity. This section presents the results of an analysis of data on physical health, including levels of physical activity, motivation to exercise, enjoyment of exercise, number of steps taken, flexibility, and functional capacity. Cancer and cancer treatment can significantly reduce patients’ physical activity levels. Meanwhile, physical activity increases survival rates and reduces the risk of cancer recurrence. Overcoming some limitations of traditional physical activity and weight management programs, WDs are a motivational tool to increase physical activity awareness and are useful for measuring activity anywhere [61]. These devices are also supportive in determining the amount of minimum or maximum activity needed to improve health outcomes, either for patients who have completed chemotherapy or for those who are still receiving first-line therapy [71]. The present body of literature suggests a potential positive influence of WDs, such as Fitbits and pedometers, on the physical health and activity levels of survivors of breast, prostate, and colorectal cancer [61]. In their seminal work, Keats and Pan underscored the pivotal role of wearable technology in fostering physical activity and enhancing health outcomes among cancer patients, particularly those afflicted with breast cancer [72, 73]. The management of cancer treatment complications can also be greatly influenced by the right diet and adherence to it [74]. The tools have also been demonstrated to facilitate enhanced weight management by ensuring that patients maintain an appropriate level of physical activity and adhere to a diet that is specifically designed for individuals diagnosed with cancer.
4.2. Psychological Well-Being
Psychological well-being is defined as a state of mental equilibrium that is in harmony with the physical, mental, and spiritual aspects of the self. Digital health has demonstrated a general capacity to enhance psychological and mental health outcomes [75]. The concept of mental well-being encompasses a wide range of factors, including but not limited to anxiety, depression, meditation, motivation, mental activities, self-control, goal setting, self-efficacy, and self-evaluation. The emotional ramifications of therapeutic interventions, the psychological challenges associated with a cancer diagnosis, and the threat of the disease itself can all contribute to depression in patients diagnosed with breast cancer [76–78]. Recent studies have demonstrated that WD can exert a positive impact on mental activity and mental health in cancer patients. Blount’s research has demonstrated the efficacy of wearable physical activity trackers and health technology–based physical activity trackers in enhancing physical activity and promoting improved health outcomes in breast cancer patients [79]. Alhejaili and Alomainy also suggested that physical activity tracking can reduce stress and cognitive mental load, highlighting the potential of WD in monitoring mental wellness [80]. It is important to acknowledge that prior studies have documented the psychological implications of these tools, in conjunction with outcomes pertaining to physical activity. It can be deduced that an increase in physical activity would also result in a reduction in stress and anxiety [79]. Also, there is a demonstrable correlation between the intensity of physical activity and cognitive outcomes [35]. The regular checking of data from WD devices has been demonstrated to engender greater awareness of health status and cultivate a positive attitude toward such devices [35]. This, in turn, has been shown to result in enhanced overall psychological well-being. This systematic review suggests that WD could potentially enhance mental health and alleviate depression and anxiety in individuals with cancer.
4.3. QOL
The concept of quality of life encompasses factors that influence lifestyle choices and the capacity to access social support. These factors include lifestyle modifications, sleep quality, sun protection, and social support. Individuals diagnosed with cancer frequently undergo substantial alterations in their lifestyles, sleep quality, and social support networks [81]. Cancer patients who are undergoing chemotherapy may experience sleep disturbances [82]. A clinical trial demonstrated that the utilization of WDs resulted in a reduction in social participation limitations and an enhancement in sleep quality [11]. WDs have been shown to improve sleep quality in cancer survivors by reducing sleep disturbances and increasing physical activity [12]. The use of data from wearables can also lead to personalized recommendations for improving sleep [83]. Another study demonstrated that these devices can significantly improve the all domains of health-related quality of life in adolescent and young adult cancer patients [58].
4.4. Clinical Outcomes
Clinical outcomes are factors that are directly related to the care and monitoring of patients [84]. Improved clinical outcomes are largely due to the WDs’ ability to monitor and record vital data and provide proper and timely feedback, allowing for appropriate intervention. Several studies have reported that WD can facilitate the routine monitoring of vital signs of cancer patients, including heart rate, respiratory rate, and blood pressure [32, 38]. Continuous monitoring is imperative for the early detection of clinical deterioration and the subsequent adjustment of treatment. WDs have demonstrated efficacy in enhancing patient outcomes across various clinical settings, including general hospital and surgical wards, intensive care units, outpatient settings, and home care plans [85]. Incorporating data such as sleep patterns [24], activity levels [14], and heart rate variability [26] into clinical care can help healthcare providers identify and respond to their patients’ needs, ultimately improving patient safety and outcomes. WDs have been shown to improve clinical indicators, leading to increased survival rates among cancer patients and a reduction in cancer-related mortality [41].
4.5. Administrative Outcomes
Administrative outcomes are issues that facilitate the provision of care, yet they are not directly related to the bedside. This overarching theme encompasses two key aspects: data quality and time management. The advent of wearable activity monitors has enabled the collection of objective, real-time, and unobtrusive data, thereby facilitating the integration of technological advances into healthcare research and practice. WDs have the potential to influence the timeliness and quality of data in cancer care and have the capacity to streamline procedures and facilitate effective communication between patients and caregivers, thereby enhancing data quality and reducing time expenditures [32, 53]. This can enhance the efficiency of the health system in providing specialized oncology services.
4.6. Technology Acceptance
Technology acceptance is defined as the demonstration of the benefits of a technology to patients, thereby facilitating their acceptance of it [86]. The efficacy of the WDs is contingent upon patient acceptance and adherence to wearing the device [9]. A prevailing sentiment among cancer patients, including those receiving palliative care, has been expressed regarding their readiness to utilize WD for health monitoring purposes [87]. However, studies have reported variable adherence to these devices, with some showing high adherence and others noting lower use at certain times, such as at night [88]. Notwithstanding this heterogeneity, the prevailing tendency indicates a predilection for wrist-worn WD. Studies have reported adherence rates ranging from 60% to 100%, with higher rates observed in shorter studies [61]. Factors such as the type of cancer, the specific WD used, and the frequency of data synchronization may influence adherence.
4.7. Limitation
The present study is subject to two limitations. Firstly, a lack of statistical correlation exists between WD utilization and clinical outcomes. The review was precluded by the heterogeneity and differing methodologies of the studies. Secondly, the study did not aim to provide a detailed examination of the factors that affect the use of these tools, such as their level of complexity or connection with health information systems. Consequently, the development of future studies could offer a novel perspective on the utilization of these tools in the oncology field by means of a thorough investigation of these factors.
5. Conclusion
The management of cancer, encompassing prognosis, diagnosis, treatment, follow-up, and rehabilitation, necessitates efficacious methodologies. There is robust evidence supporting the utilization of WDs in cancer patients exhibiting high adherence rates and the capacity to furnish substantial data at all phases of a patient’s journey. These devices have been shown to enhance patient outcomes by quantifying health metrics such as step count, heart rate, energy expenditure, and sleep regulation. The most common outcomes of these technologies include improved physical health, characterized by increased physical mobility, and enhanced clinical outcomes, marked by enhanced vital sign monitoring, augmented cardiac and respiratory capacity, and optimized medication management. The expanding utilization of these devices, in conjunction with novel computational methodologies such as cloud computing, artificial intelligence, and machine learning, is anticipated to result in a substantial augmentation in their implementation within the domain of cancer care. Continued research in this domain is expected to enhance the evidence base for their effectiveness, facilitate precise measurement of their impact on patient outcomes, and ultimately contribute to the enhancement of public health.
Disclosure
No human subjects were involved in the project.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study did not use any funds.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




