Development of a Nomogram for Predicting Overall Survival of Oral and Oropharyngeal Squamous Cell Carcinoma
Abstract
This study aimed to examine the correlation between Ki-67 expression and clinicopathological features of oral and oropharyngeal squamous cell carcinoma (OSCC/OPSCC), evaluate the prognostic value of Ki-67, and develop a prognostic model incorporating Ki-67 expression. A total of 469 patients diagnosed with OSCC/OPSCC, hospitalized between 2012 and 2022, were included. Patients’ surgical specimens were subjected to immunohistochemical staining to determine their Ki-67 expression. Clinicopathological data of patients were also collected. Patients exhibiting male sex, poor pathological differentiation, advanced TNM stage, and metastasis demonstrated elevated levels of Ki-67 expression. Age and recurrence did not affect that. According to the Cox proportional hazards model, Ki-67, age, recurrence, metastasis, and primary tumor site were identified as independent risk factors for patient prognosis. Kaplan–Meier survival analysis indicated that the higher the Ki-67 expression, the shorter the patients’ overall survival. A prognostic model using independent risk factors was developed. The model was presented as a nomogram with a c-index of 0.768. The calibration curve also showed a good predictive performance of the model. This study confirmed the prognostic significance of Ki-67 expression in OSCC/OPSCC. A prognostic prediction model that incorporated Ki-67 expression was developed, providing a new tool for predicting patient prognosis in clinical practice.
1. Introduction
Oral and oropharyngeal cancer are the most frequent malignant tumors affecting the oral and maxillofacial region. Due to their high mortality and detrimental functional consequences, these cancers have emerged as a pressing public health issue. An estimated 495,801 new cases and 240,498 deaths from oral squamous cell carcinoma (OSCC) and oropharyngeal squamous cell carcinoma (OPSCC) are predicted worldwide in 2022 [1]. Despite dedicated efforts from clinicians and researchers over the past 3 decades to refine the diagnosis and treatment of oral cancer, the incidence and mortality of oral cancer are continuously increasing, according to a Global Burden of Disease Study carried out in the 10 most populous nations [2]. Squamous cell carcinoma (SCC) is the most prevalent histological type of oral and oropharyngeal cancer [3]. Its highly invasive nature, coupled with its tendency to trigger cervical lymph node metastasis, leads to a critical challenge in therapy, ultimately limiting patient survival time. Numerous studies have searched for biomarkers for OSCC and OPSCC, such as p53, CD44, and VEGF [4–6]. However, due to limited sample sizes and other reasons, no definitive prognostic predictive biomarker has been established for clinical practice [7, 8].
A complicated, genetically encoded regulatory mechanism dictates the control of cell proliferation. In cancer, this regulatory mechanism breaks down and leads to uninhibited tumor cell growth. Ki-67, a macromolecular protein located within the cell nucleus, exhibits a presence during cell mitosis in the G1, S, G2, and M phases, reaching peak expression in the M phase. After completion of cell mitosis, Ki-67 diminishes rapidly and is absent in quiescent (G0) cells [9]. Therefore, Ki-67 is a valuable marker for rapidly and precisely identifying cell proliferation activity within both normal and tumor cell populations. Numerous studies have consistently demonstrated that the expression of Ki-67 is upregulated in cancerous tissues and is significantly associated with an unfavorable prognosis in various types of tumors, including but not limited to lung cancer, breast cancer, and bladder cancer [10–12]. Gadbail et al. [13] have revealed a significant correlation between elevated Ki-67 expression and the poor differentiation status of tumor cells in the context of oral submucosal fibrosis and OSCC. Moreover, high Ki-67 expression was associated with advanced clinical TNM stage, metastasis, and shorter survival duration (less than three years) in contrast to patients with early clinical TNM stage, no metastasis, and longer survival (more than three years). These findings suggest a potential association between Ki-67 and an unfavorable prognosis in OSCC and OPSCC [14].
Although several studies have suggested that Ki-67 is an adverse prognostic predictive marker for OSCC and OPSCC [14–16], conflicting perspectives have emerged, with some scholars suggesting no discernible correlation between Ki-67 expression and prognosis [17–19]. The conflict here may argue the limitations of sample size which may underscore the necessity for data validation from larger sample cohorts. To address this, the present study meticulously compiled clinical and pathological data from 469 OSCC/OPSCC patients hospitalized between 2012 and 2022. The patients were followed up until December 31, 2022, to evaluate the association between the expression of Ki-67 and the clinicopathological features of OSCC/OPSCC patients, as well as explore its potential impact on prognosis.
2. Materials and Methods
2.1. Patients and Specimens
Formalin-fixed and paraffin-embedded tissue blocks obtained from surgical specimens were evaluated. The evaluation involved 469 patients hospitalized between 2012 and 2022 diagnosed with OSCC or OPSCC. At the same time, we performed a thorough examination of the clinical records of all patients, which provided us with information on their age, sex, location of the primary tumor, pathological grade, clinical TNM stage, presence of recurrence and metastasis, duration of follow-up, and outcome. West China Hospital of Stomatology Institutional Review Board has approved this study (ethics approval number: WCHSIRB-D-2023-437). The Declaration of Helsinki protocols were followed throughout the study.
2.2. Immunohistochemistry
Paraffin-embedded tissue blocks were cut into 2-μm sections for immunohistochemical staining. For dewaxing and hydrating, the sections were sequentially incubated in xylene, 100% ethanol, 85% ethanol, and 75% ethanol. After rinsing in distilled water, the samples were immersed in citrate buffer and heated in a microwave oven at high power for 10 min, paused for 8 min, and then heated at medium power for another 10 min for antigen retrieval, which exposes the antigens and increases their affinity for the antibodies. Endogenous peroxidase was blocked by placing the sections in 3% hydrogen peroxide solution for 10 min at room temperature. Next, normal goat serum (product code: AR1009, 1:9 dilution, Boster Biological Technology Co., Ltd., California, USA) was added gradually to the sections and then left the sections at room temperature for 20 min. Then, the primary antibody (product code: GB121141-100, 1:100 dilution, Wuhan Servicebio Technology Co., Ltd, Wuhan, China) was slowly added to the sections and incubated at 4°C overnight. Finally, the secondary antibody (product code: SP9002, Beijing Zhong Shan Golden Bridge Biological Technology Co., Ltd., Beijing, China) was added and set for 30 min at 37°C. The freshly prepared diaminobenzidine (DAB) solution was used to treat the tissues, and this resulted in a brownish-yellow coloration of the cell nucleus. The sections were then washed with distilled water to terminate the reaction. Next, the units were counterstained with hematoxylin for 3 min, blued and rinsed under running water, and sealed with gum.
2.3. Quantification of Immunohistochemistry
DAB causes the nuclei of Ki-67-expressing cells to turn brownish-yellow, and cells are counted as positive if their nuclei are observed to be brownish-yellow, regardless of the intensity of the staining. The sites with the highest Ki-67 expression were identified in the samples. Then, the total number of Ki-67-positive cells was observed and recorded under high magnification, divided by the total number of tumor cells, and the ratio obtained was multiplied by 100% to determine the expression of Ki-67.
2.4. Statistical Analysis
This study used R Version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism Version 10.0.2 (GraphPad Software, San Diego, California, USA) for data analysis and graphics drawing. Wilcoxon rank sum test, Kruskal–Wallis H test, and Dunn’s multiple comparison test were used to compare the expression of Ki-67 in different age groups, sex, primary tumor sites, pathological grade, clinical TNM stage, recurrence, and metastasis. Univariate Cox regression analysis separately incorporates factors that impact patients’ prognosis and then eliminates mediators through stepwise regression. And then, multivariate Cox regression analysis is used to establish the final Cox proportional hazards model and obtain independent factors related to tumor prognosis. In addition, the expression of Ki-67 was grouped according to the median and the optimal cutoff obtained by the receiver operating characteristic (ROC) curve. Kaplan–Meier survival curve and the log-rank test were used to compare the estimated overall survival of the high-expression and low-expression groups. p < 0.05 means the difference is statistically significant. Finally, a prognostic model was established using the five independent risk factors validated by the Cox proportional hazards model, which was presented in the form of a nomogram. And, a validation of the predictive accuracy of this nomogram was performed. Ultimately, the model underwent external validation utilizing an external dataset encompassing 70 OSCC/OPSCC patients, and corresponding calibration curve and concordance index (C-index) were generated to assess its performance and evaluate its generalization capability.
3. Results
3.1. General Information
A total of 469 cases were included in the study. The population consisted of individuals aged 19 to 90, with a median age of 60 and an interquartile range of 15 years. Using the median age of 60 as the cutoff value, we divided the sample into two groups: 236 individuals (50.3%) aged 60 or younger and 233 individuals (49.7%) aged above 60. The sample included 305 males (65.0%) and 164 females (35.0%). There were 315 patients (67.2%) diagnosed with moderately differentiated SCC (MDSCC), 78 patients (16.6%) diagnosed with well-differentiated SCC (WDSCC), and 76 patients (16.2%) diagnosed with poorly differentiated SCC (PDSCC). MDSCC is the most prevalent pathological grade. Of the patients, 206 (43.9%) were in the early clinical stage, while 263 (56.1%) were in the advanced clinical stage. Of the 469 cases analyzed, 56 (11.9%) experienced local recurrence, while 413 (88.1%) did not. Additionally, 185 (39.4%) exhibited cervical lymph node or distant metastasis, in contrast to 284 (60.6%) without metastasis. In all OSCC and OPSCC cases, the tongue was the most frequent location, accounting for 227 patients (48.4%). The buccal mucosa followed with 85 patients (18.1%), then the oropharynx with 58 patients (12.4%), the gingiva with 44 patients (9.4%), the floor of the mouth with 26 patients (5.5%), the palate with 19 patients (4.1%), and the lip with 10 patients (2.1%). The median Ki-67 expression for all cases was 40%, with an interquartile range of 30% and a mean of 40% (±22.165) (Table 1).
| Clinical characteristics | Cases, n (%) |
|---|---|
| All patients | 469 (100) |
| Age (years) | |
| ≤ 60 | 236 (50.3) |
| > 60 | 233 (49.7) |
| Median [IQR] | 60 [53–68] |
| Range | 19–90 |
| Sex | |
| Male | 305 (65.0) |
| Female | 164 (35.0) |
| Grade | |
| WDSCC | 78 (16.6) |
| MDSCC | 315 (67.2) |
| PDSCC | 76 (16.2) |
| Stage | |
| Early stage | 206 (43.9) |
| Advanced stage | 263 (56.1) |
| Recurrence | |
| Nonrec | 413 (88.1) |
| Recurrent | 56 (11.9) |
| Metastasis | |
| Nonmeta | 284 (60.6) |
| Metastatic | 185 (39.4) |
| Ki-67 (%) | |
| Median [IQR] | 40 [30–60] |
| Mean ± SD | 40 ± 22.165 |
| Site | |
| Tongue | 227 (48.4) |
| Buccal mucosa | 85 (18.1) |
| Lip | 10 (2.1) |
| Gingiva | 44 (9.4) |
| Floor of mouth | 26 (5.5) |
| Palate | 19 (4.1) |
| Oropharynx | 58 (12.4) |
3.2. Correlation Between Ki-67 Expression and Clinicopathological Features
In this study, the analysis of Ki-67 expression considered clinicopathological factors such as sex, age, primary tumor sites, pathological grade, clinical TNM stage, recurrence, and metastasis as grouping variables. The average Ki-67 expression level was higher in males than in females, and this difference was statistically significant (p < 0.0001). There was no statistically significant difference in the mean value of Ki-67 expression between the lower and upper age groups after grouping according to the median age of 60 years (p > 0.05). In terms of pathological grade, the expression of Ki-67 was increased with decreasing differentiation of tumor cells (p < 0.0001). A statistically significant difference exists in the mean expression of Ki-67 between early and advanced stages (p < 0.0001). As the tumor develops, Ki-67 expression increases. Metastatic OSCC and OPSCC patients had higher mean Ki-67 expression than their nonmetastatic counterparts (p < 0.0001). However, there was no significant difference in Ki-67 expression between recurrent and nonrecurrent OSCC and OPSCC patients (p > 0.05). Furthermore, the expression of Ki-67 appears to be correlated with the location of the SCC. The expression of Ki-67 in SCC originating from the tongue, buccal mucosa, and gingiva is lower than that originating from the oropharynx. Additionally, the expression of Ki-67 in the buccal mucosa is lower than that in the floor of the mouth (Table 2).
| Variable | n | Ki-67 expression | p value | |
|---|---|---|---|---|
| Mean | SD | |||
| Age | ||||
| ≤ 60 years | 236 | 43.831 | 21.141 | 0.6198 |
| > 60 years | 233 | 45.052 | 23.186 | |
| Sex | ||||
| Male | 305 | 46.905 | 22.482 | < 0.0010 |
| Female | 164 | 39.848 | 20.865 | |
| Grade | ||||
| WDSCC (A) | 78 | 30.846 | 17.878 | A < B < 0.0001 |
| MDSCC (B) | 315 | 44.635 | 21.796 | B < C < 0.0001 |
| PDSCC (C) | 76 | 57.566 | 19.536 | A < C < 0.0001 |
| Stage | ||||
| Early (I–II) | 206 | 39.801 | 20.238 | < 0.0001 |
| Advanced (III–IV) | 263 | 48.068 | 22.956 | |
| Recurrence | ||||
| Nonrec | 413 | 44.228 | 22.545 | 0.4884 |
| Recurrent | 56 | 45.982 | 19.245 | |
| Metastasis | ||||
| Nonmeta | 284 | 40.722 | 21.426 | < 0.0001 |
| Metastatic | 185 | 50.141 | 22.125 | |
| Site | ||||
| Tongue (A) | 227 | 43.546 | 21.412 |
|
| Buccal mucosa (B) | 58 | 40.235 | 20.643 | |
| Lip (C) | 10 | 47.000 | 20.028 | |
| Gingiva (D) | 44 | 40.455 | 22.845 | |
| Floor of mouth (E) | 26 | 56.346 | 19.878 | |
| Palate (F) | 19 | 36.105 | 24.841 | |
| Oropharynx (G) | 58 | 54.052 | 23.197 | |
3.3. Relationship Between Ki-67 Expression and Prognosis
As of December 31, 2022, patients’ overall survival and outcome were obtained through follow-up. The relationship between clinicopathological features and patient prognosis in OSCC and OPSCC was compared using univariate Cox regression analysis. All variables except sex were found to affect the prognosis. The Cox proportional hazard model was established after stepwise regression which eliminates the mediating variables, pathological grade, and clinical TNM stage, and it was finally determined that Ki-67 (p < 0.01), age (p < 0.05), recurrence (p < 0.001), metastasis (p < 0.001), and primary tumor site (p < 0.05) were independent indicators of patient’s prognosis. Patients with higher Ki-67 expression, older age, recurrence, metastasis, and tumor located in the oropharynx had a worse prognosis than patients with lower Ki-67 expression, younger age, no recurrence, no metastasis, and tumor located elsewhere in the oral cavity (HR > 1 for all five factors) (Table 3).
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.025 | 1.002–1.049 | 0.030 | 1.025 | 1.002–1.049 | 0.036 |
| Sex | ||||||
| Male | 1.000 | — | — | — | ||
| Female | 0.750 | 0.449–1.251 | 0.271 | — | — | — |
| Grade | ||||||
| WDSCC | 1.000 | — | — | — | ||
| MDSCC | 1.576 | 0.738–3.362 | 0.240 | — | — | — |
| PDSCC | 4.365 | 1.892–10.073 | 0.001 | — | — | — |
| Stage | ||||||
| Early stage | 1.000 | — | — | — | ||
| Advanced stage | 3.554 | 2.051–6.160 | < 0.001 | — | — | — |
| Ki-67 | 1.020 | 1.009–1.031 | < 0.001 | 1.015 | 1.004–1.026 | 0.007 |
| Recurrence | ||||||
| Nonrec | 1.000 | |||||
| Recurrent | 2.192 | 1.284–3.740 | 0.004 | 2.755 | 1.601–4.741 | < 0.001 |
| Metastasis | ||||||
| Nonmeta | 1.000 | |||||
| Metastatic | 3.939 | 2.401–6.465 | < 0.001 | 3.978 | 2.373–6.671 | < 0.001 |
| Site | ||||||
| Oral cavity | 1.000 | |||||
| Oropharynx | 2.137 | 1.288–3.546 | 0.003 | 1.709 | 1.020–2.862 | 0.042 |
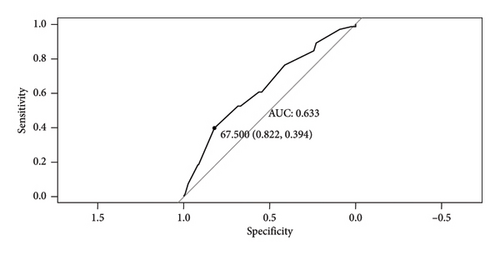
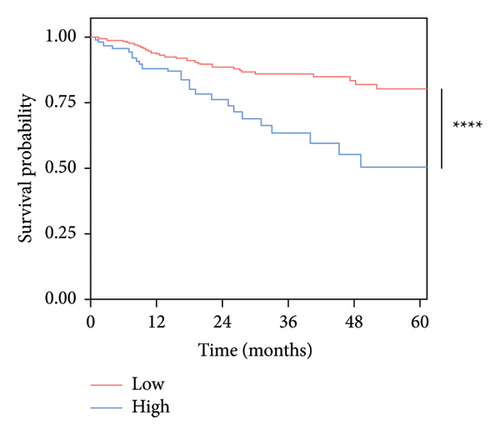
To obtain optimal Ki-67 grouping, this study implemented two different cutoff values of Ki-67 expression. Based on the data, these values were the most reasonable. One group utilized 40%, the median, as the cutoff value. The other group used the optimal cutoff value of 67.5%, as determined by the ROC curve (Figure 1). The threshold has a specificity of 0.822, a sensitivity of 0.394, and an area under the curve (AUC) of 0.633. Two Kaplan–Meier survival curves were drawn independently (Figure 2). The curves indicate that patients with high Ki-67 expression had significantly shorter survival time than those with low Ki-67 expression. Patients in the high-expression group exhibited a poorer prognosis than those in the low-expression group, with a statistically significant difference observed (p < 0.05). These findings suggest that Ki-67 is an adverse prognostic marker for OSCC and OPSCC. Furthermore, when comparing the two graphs, it has been found that grouping the expression of Ki-67 with the best cutoff value obtained from the ROC curve results in a more significant difference (p < 0.0001) in prognosis than the difference (p = 0.014) obtained by grouping the expression of Ki-67 with the median.

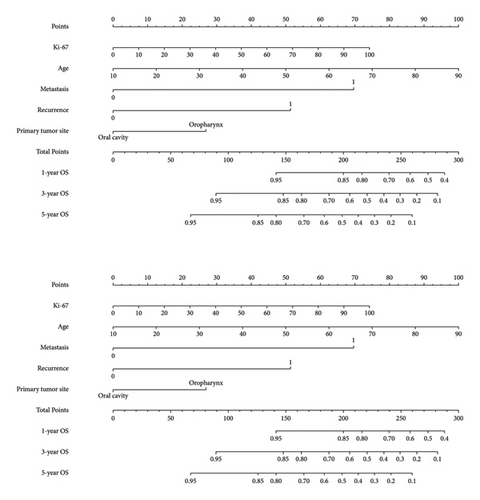
3.4. A Nomogram Incorporating Ki-67 Expression
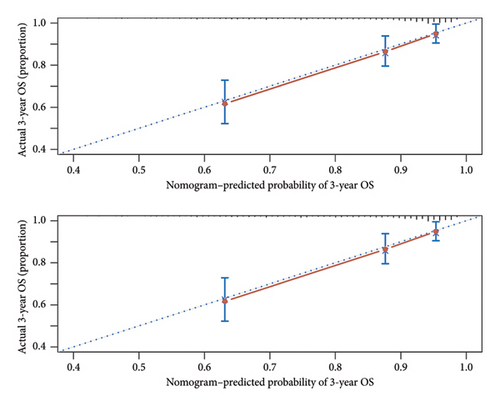
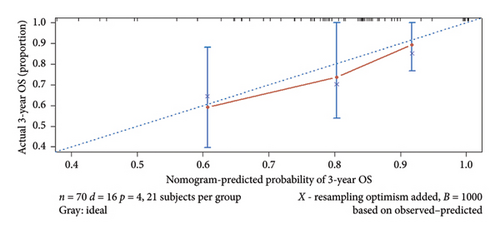
A prognostic model was developed using the five independent risk factors of Ki-67 expression, age, recurrence, metastasis, and primary tumor site, which is presented in a nomogram (Figure 3). The nomogram corresponded to the patient’s Ki-67 expression, recurrence, metastasis, age, and primary site of the tumor to the scores and accumulated the scores to get the total score, and finally, the total score was used to correspond to get the prognosis of 1-, 3-, and 5-year survival rates. For example, there was a 60-year-old patient with OSCC with cervical lymph node metastasis but no recurrence after treatment with Ki-67 expression of 80%. By corresponding the patient’s condition to the score in the nomogram, it can be calculated that his total score is approximately 190, corresponding to the 1-year survival rate of 86%, the 3-year survival rate of 69%, and the 5-year survival rate of 55%. Also, the prediction accuracy of the model was validated. The C-index of the model was 0.768. The model was validated by the bootstrap method, and the calibration curve was plotted (Figure 4). From the calibration curve, it could be seen that the 3-year survival rate predicted by the nomogram is very close to the actual 3-year survival rate of the patients, indicating that the model has a good prediction efficacy. The nomogram underwent external validation as well. Upon validation using an external dataset comprising 70 patients, the C-index was determined to be 0.714. Additionally, a calibration curve was generated (Figure 5), which demonstrates that the nomogram maintains a robust predictive performance during external validation. This finding underscores the model’s reliability and generalizability.
4. Discussion
OSCC and OPSCC stand as two of the most pervasive tumors worldwide. The conventional treatment involves comprehensive and sequential therapy, with surgical resection as the preferred method, accompanied by preoperative and postoperative chemotherapy and radiotherapy [20]. These cancers significantly impact patients’ quality of life by hampering vital functions, such as swallowing, pronunciation, and appearance [21]. Despite significant improvements in treatment by various researchers in recent years, the 5-year survival rate for patients with OSCC or OPSCC has not been significantly improved. Patients often die from local recurrence or metastasis after surgery. Therefore, there is an urgent need to identify a biomarker capable of accurately and efficiently predicting the risk of metastasis, recurrence, and prognosis in OSCC and OPSCC.
Ki-67 was initially detected in the nuclei of Hodgkin lymphoma cells 4 decades ago [22]. As a biological marker, the expression level of Ki-67 is intimately linked to tumor development and is extensively utilized as a prognostic and predictive indicator in cancer diagnosis and treatment [23]. To begin with, as a marker of cellular proliferation, elevated Ki-67 expression typically signifies an accelerated rate of tumor cell division, which directly fuels cancer progression. Furthermore, Ki-67 exerts significant influence within the tumor microenvironment. The expression level of Ki-67 is closely associated with the invasive phenotype of tumor cells. Specifically, higher expression levels of Ki-67 typically indicate a greater degree of invasiveness in tumor cells [24]. In addition, it is also correlated with the aggregation and functionality of tumor-associated macrophages (TAMs). TAMs are capable of secreting immunosuppressive factors that diminish the immune system’s antitumor capabilities, thereby fostering an environment conducive to tumor growth and advancement [25]. In OSCC and OPSCC, there are two conflicting perspectives, potentially attributed to sample size limitations or variations in tumor sampling sites. To address this, the proposal suggests that performing multisite tumor sampling could enhance the detection of intratumor heterogeneity in OSCC/OPSCC [26]. This study included 469 patients hospitalized between 2012 and 2022, and Ki-67 was obtained as an independent adverse prognostic marker for OSCC/OPSCC according to univariate and multivariate Cox regression analyses. Patients with high Ki-67 expression demonstrated a poor prognosis and short overall survival. Additionally, age, recurrence, metastasis, and primary tumor site were identified as independent prognostic factors. Old age and the presence of recurrence/metastasis were recognized as risk factors for tumor prognosis [27]. The prognosis for SCC in the oropharynx was significantly worse than oral cavity. We speculate that there is a potential link between this difference and human papillomavirus infection [28].
This study comparatively analyzed Ki-67 expression, revealing its correlation with various factors including sex, site, clinical stage, pathological grade, and metastasis. The Ki-67 expression was significantly higher in males compared to females, indicating a generally elevated cell proliferation activity in males with OSCC and OPSCC. Previous study has suggested that this may be related to risk factors such as smoking and alcohol abuse [29]. There was no difference in Ki-67 expression between the different age groups, corroborating findings from earlier studies [12, 30, 31]. Ki-67 expression increased with clinical stage progression, consistent with earlier findings [32–35]. This suggests that tumors advance more rapidly as Ki-67 expression increases. Moreover, the expression of Ki-67 in the metastatic group was significantly higher than that in the nonmetastatic group, indicating that the increased cell proliferative activity reflected by elevated Ki-67 also promotes cancer metastasis, the biological mechanism of which needs to be further clarified. However, the study did not establish a significant association between local recurrence, a pivotal biological behavior closely linked to prognosis, and Ki-67 expression (p < 0.05).
In this study, we further investigated the effect of different cutoff values of Ki-67 expression on prognostic prediction. Upon a comprehensive review of pertinent literature, it was observed that a precise grouping method for Ki-67 remains currently elusive. Some researchers utilized the median, a common grouping method, to categorize Ki-67 into two groups of high expression and low expression [16], while others have directly used 50% as the cutoff value [29]. In order to account for the frequency distribution of Ki-67 expression, some investigators establish their own cutoff values to avoid creating very small and too many groups [15]. For instance, Liu et al. [31] utilized 25%, 50%, and 75% as their cutoff values to divide Ki-67 into four groups. To find the cutoff value of the expression of the marker, some researchers performed the ROC curve analysis and obtained a Ki-67 cutoff value of 27.75 [17]. In this article, the study utilized both the median and the optimal cutoff value obtained by the ROC curve, respectively, classifying Ki-67 into two groups. The results indicate that the optimal cutoff value identified through the ROC curve had better grouping performance, and the difference in prognosis between the two groups was more significant. Xie’s meta-analysis [14] revealed that using different antibodies in immunohistochemistry and different cutoff values in data analysis may affect identifying the prognostic role of Ki-67 expression. Thus, future research is necessary to establish a comprehensive set of standardized procedures for Ki-67 immunohistochemical identification, and the setting of cutoff value to conduct in-depth research on Ki-67.
Immunohistochemical staining has become a routine method for tumor pathological examination, and most patients’ surgical specimens will be subjected to immunohistochemical staining. In this paper, we analyzed Ki-67 expression as a crucial component of the prognostic model. Specifically, we included the Ki-67 expression as a continuous variable in the nomogram. This approach enhanced the applicability of the nomogram and avoided the impact of varying samples and cutoff values. In clinical practice, the nomogram allows clinicians to predict a patient’s 1-, 3-, and 5-year survival rates based on essential clinical information and Ki-67 expression. The survival rates can be obtained through simple calculations and used as a reference for treatment planning.
Nevertheless, this study failed to adequately consider the impact of different treatment modalities on patient prognosis. On the one hand, with the gradual implementation of integrated sequential therapy, which emphasizes the necessity of postoperative complementary treatments such as radiotherapy and chemotherapy for patients with locally advanced disease to prolong their survival, the survival of patients with locally advanced disease and high Ki-67 expression has been extended. On the other hand, due to various factors, including intolerance to postoperative radiochemotherapy and refusal of such treatments, some patients did not receive treatment according to the standard treatment protocol. This deviation from the standard treatment plan may have had the opposite effect on the study conclusions. Therefore, the treatment modality may be a confounding factor in the correlation between Ki-67 and prognosis.
Our study confirmed a correlation between increased expression of Ki-67 and worse prognosis in patients with OSCC/OPSCC. Furthermore, the potential use of Ki-67 as a therapeutic target for OSCC/OPSCC treatment is also being investigated. Numerous studies have utilized diverse techniques to inhibit Ki-67 expression in tumor cells for treatment. For instance, one study highlighted the efficacy of trichosanthin, in combination with granzyme B, which effectively downregulated Ki-67 expression in tongue cancer, leading to the inhibition of tumor cell proliferation and the acceleration of tumor cell apoptosis, consequently achieving a therapeutic effect [36]. Another study by Listiyana et al. [37] employed an ethanolic extract from C. cinerariifolium leaf to decrease the expression of Ki-67 in tumor cells and the degree of oral epithelial dysplasia. Further comprehensive studies are needed to explore the potential of Ki-67 as a promising therapeutic target for OSCC/OPSCC.
In conclusion, our results strongly support that Ki-67 is a negative prognostic marker for OSCC and OPSCC. Our investigation also explored the association between Ki-67 and a range of clinicopathological features. A prognostic prediction model that incorporated Ki-67 expression was developed, providing a new tool for predicting patient prognosis in clinical practice.
Ethics Statement
This study was approved by the West China Hospital of Stomatology Institutional Review Board (ethics approval number: WCHSIRB-D-2023-437). And the Declaration of Helsinki protocols were followed throughout the study. All patients provided written consent for the use of their specimens for research purposes; none were identifiable.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tianyi Wang led the conceptualization, methodology, formal analysis, investigation, and data curation and wrote the original draft. Bing Yan collaborated with Tianyi Wang in conceptualization, methodology, formal analysis, and investigation and was responsible for validation, manuscript review and editing, supervision, and project administration. Haoran Ding and Shiyong Zhuang contributed to formal analysis, investigation, and data curation. Yi Li was responsible for validation, review and editing, and supervision. All authors participated in the manuscript review.
Funding
This study was funded by the Science and Technology Program of Tibet Autonomous Region (grant no. XZ202402ZY0004).
Acknowledgments
The authors would like to thank Dr. Ruowen Zhang for his kind help on the English editing of this article.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




