Efficacy and Safety of Topical Famotidine Combined With Thulium 1927 nm Fractional Laser in the Treatment of Melasma: A Split-Face Randomized, Single-Blind, Vehicle-Controlled Clinical Trial With Long-Term Follow-Up
Abstract
Background: Melasma is a common hyperpigmentation skin disorder with a high recurrence rate. Mast cell activation plays a role in its pathogenesis, with melanocyte activation via histamine receptor 2 (H2R) considered a potential mechanism. This study aims to evaluate the efficacy of topical famotidine combined with 1927 nm thulium fractional laser in treating melasma and reducing recurrence.
Methods: The study was designed as a split-face, randomized-controlled, single-blind trial. Participants underwent four full-face 1927 nm fractional laser treatments at 4-week intervals and applied 2% famotidine solution on a randomly assigned side and control solution on the opposite side of the face twice daily for 16 weeks. Skin assessments including VISIA imaging, modified melasma area severity index (mMASI) score, and DermaLab skin color detection were conducted by blinded dermatologists at Weeks 0, 4, 8, 12, and 16. Self-assessment scores and the melasma quality of life (MELASQoL) index were collected at baseline and Week 16. Long-term follow-up was performed at Week 64. All side effects were recorded. Statistical analyses included paired t-tests, repeated measures ANOVA, and Wilcoxon and Friedman tests.
Results: A total of 16 patients were enrolled in the study. At Week 16, the famotidine-treated side showed significant reductions in mMASI (p = 0.019, = 0.598) and melanin index (MI) (p = 0.006, = 0.672), with a slight improvement in erythema index (EI). ∆MI was significantly lower on the famotidine-treated side than the control (p = 0.012, Cohen’s d = 0.710). Both MELASQoL scores and patient self-assessments improved, with no obvious adverse effects observed. Long-term evaluation at Week 64 revealed sustained improvement in mMASI on the famotidine-treated side compared to the control side (p = 0.029, Cohen’s d = 0.686).
Conclusions: This study provides clinical evidence supporting H2R blockade as a potential melasma treatment. Famotidine may enhance laser efficacy and modulate histamine-mediated melanogenesis, offering long-term benefits in reducing recurrence.
Trial Registration: ClinicalTrials.gov identifier: NCT06313307
- •
Why was the study undertaken?
- ∘
Melasma is a challenging facial hyperpigmentation disorder, difficult to treat and easy recurrence placing a significant psychological and social burden on patients. Current treatments primarily focus on melanin reduction, with limited options targeting the upstream causes of the condition. Melanocyte activation via H2R may be a potential mechanism of treatment refractoriness and recurrence in melasma patients.
- •
What does this study add?
- ∘
In the present study, we show that
- ∘
Topical famotidine significantly improves melasma by reducing mMASI, MI, as well as MELASQoL scores and patient self-assessments after 16 weeks of treatment.
- ∘
Long-term follow-up upon 1 year after the last treatment showed that topical famotidine may reduce the recurrence of melasma.
- ∘
No severe adverse effect related to topical famotidine treatment was observed.
- •
What are the implications of this study for disease understanding and/or clinical care?
- ∘
Topical famotidine is a safe and effective treatment for melasma, improves lesions during treatment, and helps to reduce recurrence. Its effectiveness aligns with the role of mast cell activation in the upstream pathogenesis of melasma.
1. Introduction
Melasma is a prevalent hyperpigmented dermatologic condition characterized by symmetrical brown macules mainly on the facial area, notably on the malar and forehead regions. It predominantly affects individuals with Fitzpatrick skin types III-IV, with a higher incidence among females. Histologically, melasma is typified by increased melanocyte activity, enhanced solar elastosis in the upper dermal layers, disruption of the basement membrane facilitating melanocyte and melanin dispersion into the dermis, heightened vascularization, and an elevated mast cell (MC) count relative to unaffected skin [1]. The pathophysiology of melasma is complex and has not been fully elucidated. Possible pathogenesis of melasma includes ultraviolet (UV) exposure, genetic factors, and sex hormones [2]. Chronic skin inflammation and skin barrier dysfunction also contribute to the onset of melasma [1]. Albeit melasma represents as melanin overproduction, multiple cell types are implicated in the pathogenesis of melasma.
MC numbers are elevated in the dermis of melasma-affected skin, particularly in areas with solar elastosis [3]. Studies have indicated a potential correlation between the abundance of MCs and the resistance to treatment and recurrence of melasma [4]. While the exact role of MCs in melasma development remains unclear, previous research indicates that histamine, among the bioactive mediators released by MCs, plays a crucial role in the UV-induced activation of melanocytes [5]. Recent clinical trials have demonstrated the benefits of combining the oral histamine receptor 2 (H2R) blocker famotidine with the H1R blocker ketotifen for the treatment of melasma [6]. However, while the combined therapy of H1R and H2R antagonists has shown efficacy in treating melasma, further investigation is needed to determine whether the treatment effect is primarily attributable to the H1 or H2 receptor. Further studies indicated that the beneficial effect may arise from the inhibition of the H2 receptor.
Histamine receptors comprise four subtypes (H1–H4), with histamine stimulating melanocyte activation primarily through H2Rs [7, 8]. The role of H2R activation has been validated in vivo through UV-induced pigmentation in guinea pigs [5]. In addition, considering the superficial nature of melasma skin lesions, topical treatments may offer greater benefits than systemic application. Topical administration of famotidine may provide superior benefits over oral medication, although its effects warrant further evaluation.
Fractional laser–induced microscopic treatment zones can enhance local drug absorption and bioavailability while minimizing systemic absorption–associated adverse effects [9]. The nonablative 1927 nm fractional thulium fiber laser targets water instead of melanocytes and confers a greater ability to target superficial layers of the skin [10] and thus has less risk of post-inflammatory hyperpigmentation than melanosome-targeting lasers.
In the present study, we attempted to verify the role of H2R antagonist (H2RA) in the treatment of melasma by combining H2RA with 1927 nm fractional laser–assisted drug delivery [11]. We conducted a split-face, single-blinded study to assess the efficacy and safety of a 1927 nm fractional laser with topical famotidine for treating facial melasma. Given the high recurrence rate of melasma, the long-term effect was assessed 1 year after the final treatment session to evaluate lesion recurrence.
2. Materials and Methods
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiao Tong University (protocol code: 2022273). All the patients in this manuscript have given written informed consent to the publication of their case details. The study followed the principles of the Declaration of Helsinki and the regulations for Good Clinical Practice.
2.1. Patients
Patients with facial symmetrical melasma lesions from outpatient visits were screened. While those allergic to famotidine, pregnant or lactating, having received melasma treatment within the past month, or taking long-term contraceptive pills were excluded. Additional exclusion criteria included the presence of skin cancer, systemic diseases, mental illness, active facial inflammation or viral infections, recent facial injections or surgery within 2 months, immunodeficiency disorders, or occupations requiring prolonged sun exposure without adequate protection.
2.2. Study Design
The study was designed as a split-face randomized, single-blind, vehicle-controlled trial. Patients were randomly assigned with computer-based simple randomization in a 1:1 ratio to receive H2RA (2% famotidine (Shanghai Sine Wanxiang Pharmaceutical Co., Ltd., Shanghai, China) dissolved in double-distilled water containing poloxamer 407, glycerol, lauryl alcohol polyether-4, polyethylene glycol-8, and propylene glycol (Shanghai Ruizhi Medical Technology Co., Ltd., Shanghai, China) on either the left or right side of the face. A control solution contained the same excipients as the active formulation, excluding famotidine, to control for potential vehicle effects on the other side. H2RA and control solution were applied topically twice daily for 16 weeks on each side separately. All the patients received full-face 1927 nm fractional laser (Lavieen, WONTECH, Daejeon, South Korea) to enhance drug penetration with 4-week intervals for four sessions.
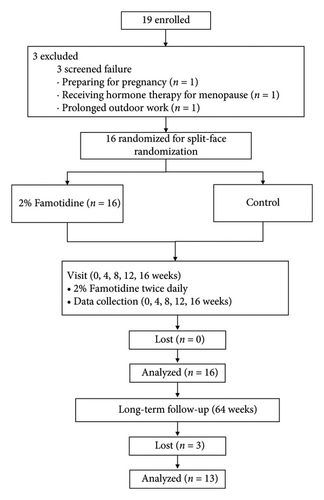
Before laser treatment, the compound lidocaine cream (Ziguang, Beijing Ziguang Medication Manufacture Corporation Ltd., Beijing, China) was applied for 40 min. The laser parameters were set at a duration of 600–800 μs, 10 W, and 0.8 mm density, with one pass, targeting only melasma lesions. Immediately posttreatment, 2% famotidine solution was applied to the designated side. Patients were instructed to use only moisturizers and sunscreen, while other skin-lightening products and medications were prohibited. The flowchart of the experiment is shown in Figure 1.
2.3. Clinical Evaluation
Secondary outcomes included skin color indicators such as melanin index (MI), erythema index (EI), and chromaticity values (L∗, a∗, b∗), assessed using a DermaLab system (Cortex Technology, Hadsund, Denmark). To minimize external variability, measurements were taken at five fixed points on the symmetrical areas of melasma lesions and adjacent unaffected skin, with differences represented as ∆MI, ∆EI, ∆L∗, ∆a∗, and ∆b∗
Self-assessment scores (0–10, 0 for no discoloration and 10 for very severe discoloration) and the melasma quality of life (MELASQoL) index [15] were recorded at Week 0 and Week 16. Higher MELASQoL scores indicated greater negative impacts on quality of life (QoL). To reduce observer bias, dermatologists assessing mMASI scores and skin color indicators were blinded to treatment allocation.
A long-term follow-up, including VISIA photography and mMASI assessment, was conducted at Week 64. All side reactions that occurred during the treatment and follow-up were recorded.
2.4. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA), released in 2016. Normally distributed data were analyzed using paired t-tests or two-way repeated measures ANOVA, while non-normally distributed data were assessed using the Wilcoxon signed-rank test or the Friedman test. The chi-square test was used for categorical variables, with statistical significance set at p < 0.05. Sample size determination was based on a priori power analysis using G∗Power, with an effect size of 0.8, a significance level of 0.05, and a power of 80% [16, 17], yielding a required sample of 16 participants after adjusting for a 10% dropout rate.
3. Results
3.1. Patients
Melasma patients attending the outpatient clinic from January 2023 to March 2023 were recruited. The study was performed from March 2023 to August 2023 at the Clinical Trials Center of the Dermatology Department of The Second Affiliated Hospital of Xi’an Jiaotong University. Long-term follow-up continued until August 2024.
A total of 19 patients were initially screened. Three patients were excluded from the study: one due to pregnancy preparation, another due to prolonged outdoor occupational exposure, and the third due to hormone therapy for menopause. Eventually, 16 patients were enrolled and completed all treatment sessions, and 13 patients completed the long-term follow-up at Week 64. The ratio of patients assigned to use famotidine on the left or right side of the face was 1:1. The baseline demographic characteristics of patients are shown in Table 1.
| Characteristics | Value |
|---|---|
| Female, n (%) | 16 (100) |
| Age (years), mean ± SD | 40.19 ± 6.221 |
| Family history of melasma, n (%) | 9 (56.25) |
| Fitzpatrick skin type, n (%) | |
| III | 5 (31.25) |
| IV | 11 (68.75) |
| Duration of melasma (years), mean ± SD | 7.375 ± 3.181 |
3.2. Efficacy
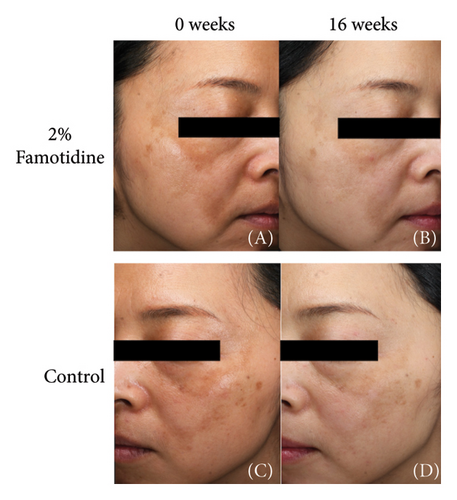
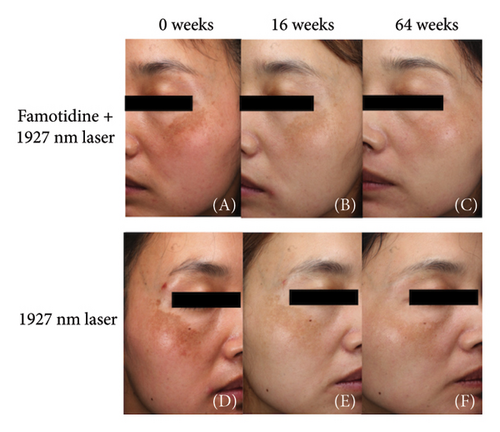
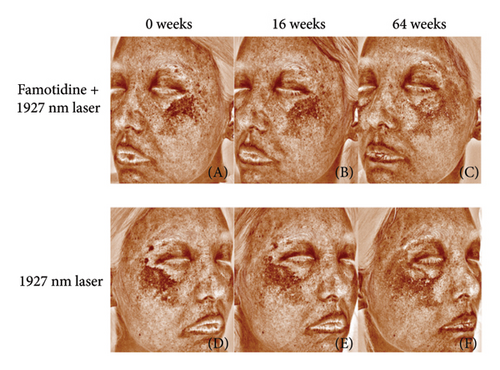
Both the famotidine-treated side and the control side exhibited marked reductions in the mMASI score (Table 2). To reduce individual variance by calculating the percentage of reduction of mMASI score, only the famotidine-treated side has a significant reduction (p = 0.019, = 0.598, 95% CI = [0.343, 0.856]) with a large effect size (Figure 2(a), Table 3). Clinical photographs depicting patient outcomes before and after treatment are shown in Figure 2(b).
| 2% Famotidine (median with IQR) | Control (median with IQR) | p | r | 95% CI | |
|---|---|---|---|---|---|
| 0 weeks | 2.10 (1.350, 2.400) | 1.80 (0.900, 2.250) | 0.348 | 0.283 | (0.015, 0.820) |
| 4 weeks | 1.80 (0.975, 2.625) | 1.50 (0.900, 2.250) | 0.759 | 0.097 | (0.016, 0.800) |
| 8 weeks | 1.80 (0.975, 1.800) | 1.50 (0.900, 1.800) | 0.877 | 0.049 | (0.011, 0.696) |
| 12 weeks | 1.20 (0.900, 1.800) | 1.20 (0.900, 1.800) | 0.589 | 0.163 | (0.012, 0.692) |
| 16 weeks | 1.20 (0.900, 1.800)a | 1.20 (0.900, 1.800) | 0.440 | 0.244 | (0.016, 0.762) |
| p | < 0.001 | 0.018 | |||
| Kendall’s W | 0.359 | 0.186 | |||
| 95% CI | (0.162, 0.614) | (0.026, 0.533) |
- Note: mMASI: modified melasma area severity index score. Comparison between groups: Wilcoxon rank-sum test, r for effect size. Comparison within group at different time points: Friedman’s test, Kendall’s W for effect size.
- Abbreviation: IQR, interquartile range.
- ap < 0.05 compared to Week 0.
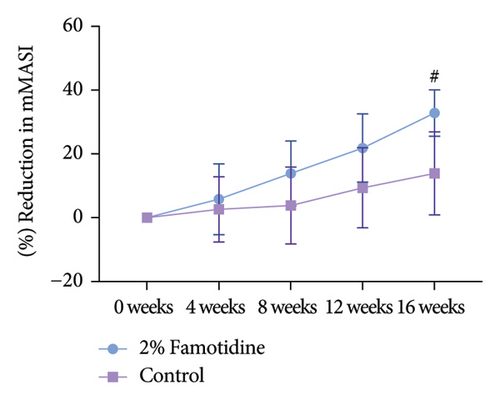
| 2% Famotidine (mean ± SEM, %) | Control (mean ± SEM, %) | p | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
| 0 weeks | 0.000 ± 0.000 | 0.000 ± 0.000 | — | — | — |
| 4 weeks | 5.801 ± 11.101 | 2.604 ± 10.255 | 0.629 | 0.123 | (−0.367, 0.613) |
| 8 weeks | 13.874 ± 10.150 | 3.819 ± 12.081 | 0.496 | 0.175 | (−0.316, 0.665) |
| 12 weeks | 21.857 ± 10.791 | 9.375 ± 12.566 | 0.405 | 0.214 | (−0.276, 0.704) |
| 16 weeks | 32.830 ± 7.270 | 13.889 ± 12.999 | 0.152 | 0.378 | (−0.112, 0.868) |
| p | 0.019 | 0.276 | |||
| 0.598 | 0.326 | ||||
| 95% CI | (0.343, 0.856) | (0.119, 0.751) |
- Note: mMASI: modified melasma area severity index score. Data shown are from 16 patients. Comparison between groups: paired t-test, Cohen’s d for effect size. Comparison within group at different time points: repeated measures analysis of variance (ANOVA), for effect size.
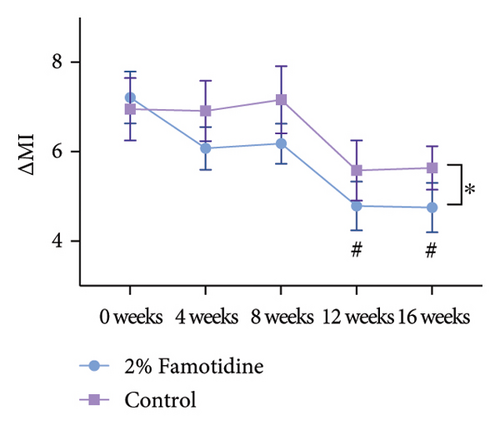
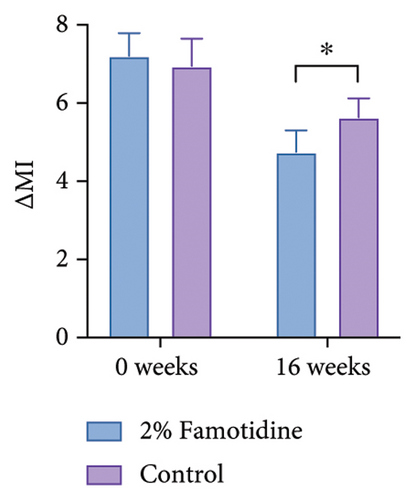
Due to the 4-week interval between visits, although measurements were taken under consistent conditions, to mitigate potential variance attributable to UV exposure and other confounding factors, we calculated the MI between melasma-afflicted skin and adjacent normal skin, denoted as ∆MI. Consistent with the mMASI findings, only the famotidine-treated side exhibited a significant decrease in ∆MI from baseline after 16 weeks, which declined from 7.21 ± 0.58 to 4.75 ± 0.55 (p = 0.006, = 0.672, 95% CI = [0.438, 0.883]), reflecting a large effect. The control group reduced from 6.95 ± 0.70 to 5.64 ± 0.48 (p > 0.05) (Figures 3(a) and 3(b) and Table 4). Furthermore, the ∆MI at 2% famotidine-treated side was significantly lower than the control side at Week 16 (4.75 ± 0.55 vs. 5.64 ± 0.49, p = 0.012, Cohen’s d = 0.710, 95% CI = [−1.200, 0.220]), showing a large effect.
| 2% Famotidine (mean ± SEM) | Control (mean ± SEM) | p | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
| 0 weeks | 7.21 ± 0.580 | 6.95 ± 0.697 | 0.558 | 0.150 | (−0.340, 0.640) |
| 4 weeks | 6.08 ± 0.478 | 6.91 ± 0.675 | 0.218 | 0.322 | (−0.812, 0.168) |
| 8 weeks | 6.18 ± 0.449 | 7.16 ± 0.752 | 0.092 | 0.450 | (−0.940, 0.040) |
| 12 weeks | 4.79 ± 0.544ab | 5.58 ± 0.672 | 0.158 | 0.372 | (−0.862, 0.118) |
| 16 weeks | 4.75 ± 0.553 | 5.64 ± 0.485 | 0.012 | 0.710 | (−1.200, 0.220) |
| p | 0.006 | 0.197 | |||
| 0.672 | 0.373 | ||||
| 95% CI | (0.438, 0.883) | (0.145, 0.771) |
- Note: Data shown are from 16 patients. Comparison between groups: paired t-test, Cohen’s d for effect size. Comparison within group at different time points: repeated measures analysis of variance (ANOVA), for effect size.
- Abbreviation: MI, melanin index.
- ap < 0.05 compared to Week 0.
- bp < 0.05 compared to Week 8.
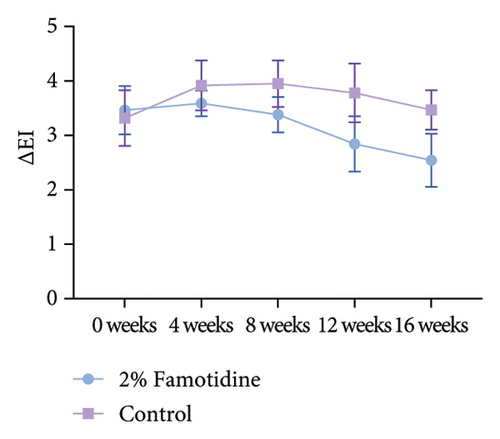
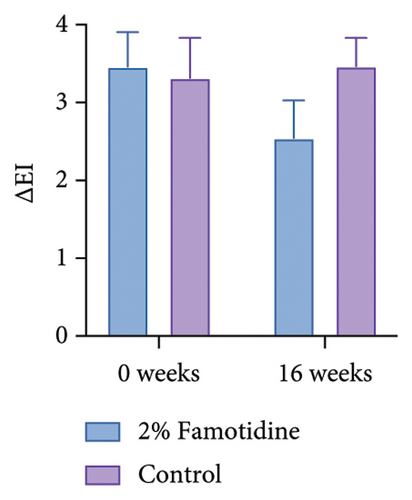
∆EI was calculated in the same way as MI, which represents the difference of erythema between melasma skin and adjacent normal skin. Only 2% of the famotidine-treated side slightly ameliorated ∆EI compared to the baseline, while the erythema on the control side was fluctuant (Figures 3(c) and 3(d) and Table 5). The skin chromaticity is defined by the value of L∗a∗b∗. ∆L∗ represents the difference in skin luminosity between melasma skin and adjacent normal skin. The a∗ value represents color hues ranging from red to green, and the b∗ value presents color hues ranging from yellow to blue. Both sides showed the skin brightness enhancement, and the 2% famotidine side had a more pronounced improvement than the control side (−7.13 ± 0.70 to −4.74 ± 0.48 vs. 6.71 ± 0.78 to −5.05 ± 0.36) (Figure S1 and Table S1). ∆a∗ demonstrated a minor decline on the 2% famotidine + 1927 nm laser side and an increase on the control side compared to the baseline, ∆b∗ did not show much difference between groups (Figure S1 and Tables S2 and S3).
| 2% Famotidine (mean ± SEM) | Control (mean ± SEM) | p | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
| 0 weeks | 3.463 ± 0.442 | 3.319 ± 0.514 | 0.839 | 0.052 | (−0.438, 0.542) |
| 4 weeks | 3.588 ± 0.237 | 3.919 ± 0.457 | 0.531 | 0.160 | (−0.650, 0.330) |
| 8 weeks | 3.381 ± 0.326 | 3.950 ± 0.426 | 0.250 | 0.299 | (−0.789, 0.191) |
| 12 weeks | 2.843 ± 0.510 | 3.781 ± 0.539 | 0.089 | 0.454 | (−0.944, 0.036) |
| 16 weeks | 2.544 ± 0.486 | 3.469 ± 0.632 | 0.112 | 0.422 | (−0.912, 0.068) |
| p | 0.561 | 0.841 | |||
| 0.206 | 0.104 | ||||
| 95% CI | (0.074, 0.698) | (0.051, 0.645) |
- Note: Data shown are from 16 patients. Comparison between groups: paired t-test, Cohen’s d for effect size. Comparison within group at different time points: repeated measures analysis of variance (ANOVA), for effect size.
- Abbreviation: EI, erythema index.
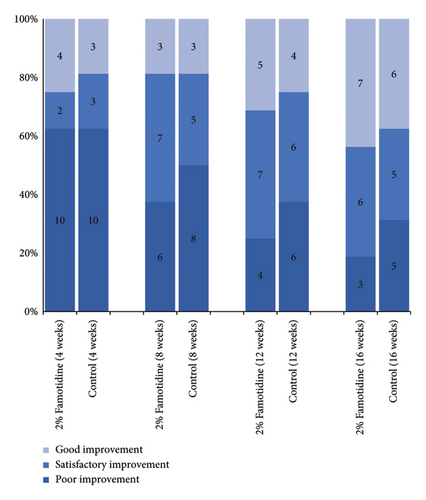
After all, the effectiveness of the 2% famotidine group was 43.8%, which was superior to the control side, 37.5% (p > 0.05, Figure 4 and Table 6).
| 2% Famotidine | Control | χ2 | p | Phi (φ) | 95% CI | |
|---|---|---|---|---|---|---|
| Marked improvement | 0 | 0 | ||||
| Good improvement | 7 | 6 | ||||
| Satisfactory improvement | 6 | 5 | ||||
| Poor improvement | 3 | 5 | ||||
| Effectiveness rate (16 weeks) | 0.438 | 0.375 | 0.130 | 0.719 | 0.064 | (−0.445, 0.301) |
- Note: Data shown are from 16 patients. Chi-square was used, phi (φ) for effect size.
Long-term follow-up was conducted 1 year after the Week 16 visit (Week 64), with 13 patients completing the study and 3 patients lost to follow-up. mMASI scores were collected, showing that the mMASI scores on both sides were slightly higher than at 16 weeks. However, the side treated with the 2% famotidine continued to exhibit a greater reduction in mMASI scores compared to the control side (Figure 5 and Table 7, 0.28 ± 0.06 vs. 0.05 ± 0.11, p = 0.029, Cohen’s d = 0.686, 95% CI = [−0.142, 1.230], n = 13), indicating a large effect size.
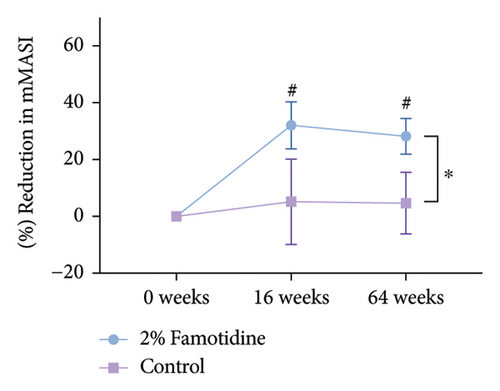
| 2% Famotidine (mean ± SEM) | Control (mean ± SEM) | p | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|
| 0 weeks | 0.000 ± 0.000 | 0.000 ± 0.000 | — | ||
| 16 weeks | 32.050 ± 8.258a | 5.127 ± 15.024 | 0.083 | 0.525 | (−0.018, 1.069) |
| 64 weeks | 28.204 ± 6.279a | 4.658 ± 10.823 | 0.029 | 0.686 | (−0.142, 1.230) |
| p | 0.003 | 0.906 | |||
| 0.654 | 0.018 | ||||
| 95% CI | (0.366, 0.877) | (0.005, 0.513) |
- Note: Data shown are from 13 patients. Comparison between groups: paired t-test, Cohen’s d for effect size. Comparison within group at different time points: repeated measures analysis of variance (ANOVA), for effect size.
- ap < 0.05 compared to Week 0.
3.3. QoL, Patient-Oriented Grading, and Side Effects
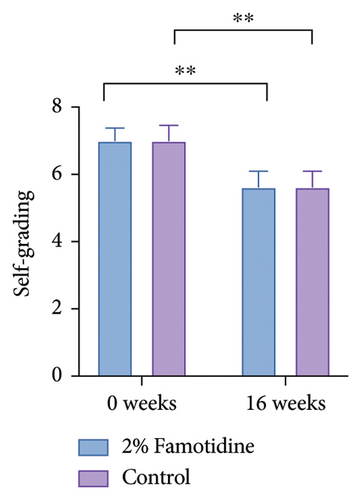
Both MELASQoL and patients’ self-grading of the melasma skin were improved after the treatment (Figure 6 and Table 8). Side effects include tolerable pain, mild erythema, mild edema, and exfoliation of scab within 10 days after the laser therapy, all of the side effects are tolerable. No patient complained of any skin irritation or other side effects from topical application of 2% famotidine (Table 9).
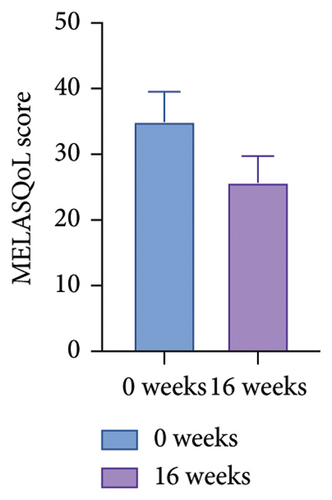
| MELASQoL | 0 Weeks (mean ± SEM) | 16 weeks (mean ± SEM) | p | Cohen’s d | 95% CI |
|---|---|---|---|---|---|
| 35.00 ± 4.534 | 25.75 ± 3.978 | 0.114 | 0.402 | (−0.070, 0.910) | |
| Self-grading | |||||
| 2% Famotidine (mean ± SEM) | Control (mean ± SEM) | ||||
| 0 weeks | 7.00 ± 0.376 | 7.00 ± 0.456 | 1 | — | — |
| 16 weeks | 5.63 ± 0.464 | 5.63 ± 0.464 | 1.000 | — | — |
| p | 0.005 | 0.008 | |||
| Cohen’s d | 0.824 | 0.771 | |||
| 95% CI | (0.334, 1.314) | (0.281.1.261) | |||
- Note: Data shown are from 16 patients. Paired t-test was used, Cohen’s d for effect size.
| 2% Famotidine | Control | |
|---|---|---|
| Mild erythema (hours/median) | 24 | 24 |
| Duration of pain (hours/median) | 4 | 4 |
| Mild edema (hours/median) | 3 | 3 |
| Exfoliation of scab (days/median) | 8 | 8 |
| Hyperpigmentation (n) | 1 | 2 |
- Note: Data shown are from 16 patients.
4. Discussion
Melasma is a common hyperpigmentary disorder primarily affecting facial skin, presenting a significant challenge in clinical management due to its chronic and relapsing nature. Various treatment modalities have been utilized, including depigmenting agents such as hydroquinone, retinoids, corticosteroids, chemical peels, and systemic medications such as tranexamic acid [18]. Some patients react poorly to those already established treatments. The pathogenesis of melasma that has been demonstrated so far involves UV radiation and hormonal influences under the genetic background [2, 19, 20]. The abovementioned factors lead to hyperreactive melanocytes in melasma skin with a range of pathological changes characterized by the damage in the basal membrane [21], solar elastosis, MC degranulation [22], increased vascularization [23], and defective barrier function [24]. Histopathological examination of melasma lesions reveals a significantly higher density of MCs compared to perilesional skin, particularly prominent in dermal elastotic areas as demonstrated by immunohistochemical staining [22]. Patients with melasma treated with oral tranexamic acid have shown a marked reduction in MC counts in the upper dermis [3]. Although several laser and light-based devices, including intense pulsed light and low-fluence Q-switched 1064 nm Nd: YAG laser, have shown promise in melasma treatment, recurrence posttreatment remains a concern [18, 25], especially in darker skin tones [4, 26–28]. Nd: YAG laser treatment increases dendritic cell density at the dermal–epidermal junction in melasma lesions, suggesting laser-induced melanocyte activation as a recurrence mechanism [29]. MC granules play a pivotal role in these pathological pathways, with histamine activation via H2R-mediated growth differentiation factor-15 (GDF-15) expression contributing to melanin synthesis [8, 30].
In this study, we conducted a split-face clinical trial to evaluate the efficacy and safety of topical H2RA application and 1927 nm laser in melasma treatment.
Nonablative fractional lasers (1550 nm Er glass and 1927 nm thulium) offer effective photoaging and dyspigmentation treatment with faster recovery and fewer complications in darker skin compared to other ablative lasers [31–33]. The combined use of topical famotidine with fractional laser therapy leverages laser-induced microscopic treatment zones to enhance transepidermal drug delivery [11], enabling targeted famotidine deposition with minimized systemic exposure. Though recently, melasma patients treated with oral ketotifen and famotidine have shown superior clinical improvement compared to placebo [6], standalone famotidine’s therapeutic contribution remains ambiguous due to unpredictable lesional drug concentrations following systemic administration. In contrast, localized delivery via fractional laser circumvents these pharmacokinetic limitations, theoretically maximizing intralesional drug levels and therapeutic precision. By concurrently augmenting famotidine absorption and evaluating H2R-mediated melanocyte modulation postlaser exposure, this dual mechanism may address both laser-enhanced drug delivery and recurrence prevention pathways.
Full-face treatment with the 1927 nm fractional thulium laser (FTL) demonstrated accelerated clinical efficacy in melasma pigmentation areas, with mMASI scores decreasing notably from Week 4 onward, though statistical significance (p < 0.05) was achieved only at 16-week follow-up. These findings align with prior studies reporting the therapeutic effect of 1927 nm FTL in melasma management [9]. However, fractional laser therapy may exacerbate melanocyte activation in melasma lesions, where MC hyperplasia and inherent melanocyte hyperactivity may coexist [29, 34].
Famotidine adjunctive therapy augmented mMASI score reductions compared to laser monotherapy, though statistical significance was not achieved (p > 0.05). Noninvasive skin analysis revealed significantly greater MI improvements in combination-treated lesions (p < 0.05), paralleled by increased skin brightness (L-value), suggesting famotidine-mediated melanin reduction. However, previous studies have shown that nonablative laser resurfacing treatment has been associated with an increased number of degranulated MCs compared to untreated groups [34], indicating MC involvement in the inflammatory response following laser treatment. Thus, the underlying mechanisms responsible for the greater improvement in melasma lesions observed with famotidine in this study remain ambiguous, whether through direct melanolysis or suppression of laser-induced melanocyte reactivation. In the meantime, the erythema scores showed limited improvement in both groups. It seems that famotidine’s inhibitory effect on postlaser erythema appears less pronounced than its melanin-suppressive effects, consistent with prior animal studies [5].
At the 64-week follow-up, bilateral mMASI scores remained superior to baseline despite mild recurrence. Notably, combination therapy demonstrated significantly greater mMASI reduction versus monotherapy (p < 0.05), supporting fractional laser’s sustained efficacy and supports the hypothesis that localized H2R inhibition may disrupt MC-derived histamine signaling melanogenic cascades, thus offering a pharmacologic suppression of recurrence pathways.
However, MCs contribute to melasma pathogenesis through multifactorial mechanisms beyond H2R-mediated pathways, including the synthesis of angiogenic factors such as VEGF and the release of tryptase and matrix metalloproteinases (MMPs) [22]. This mechanistic complexity suggests that famotidine’s therapeutic effects in melasma are likely constrained to H2R signaling modulation, rather than comprehensively addressing MC-derived inflammatory cascades.
We are unable to conclude that the efficacy of the treatment is definitive in men, and specific studies of treatment response in men are warranted.
We acknowledge that there are some potential limitations in design that synergistic effects between laser and famotidine may obscure independent drug efficacy, necessitating controlled studies without laser assistance. The single-blinded design may introduce potential bias, as participants’ awareness of treatment assignment might affect application compliance or introduce bias in self-reported outcomes. A double-blinded approach, with both evaluators and participants blinded, would strengthen methodological rigor and reduce participant bias in future trials. Besides, the modest sample size (n = 16) limits the statistical power to detect subtle differences. Despite this limitation, the split-face design enhanced internal validity by reducing interindividual variability. This study exclusively enrolled Fitzpatrick III-IV female participants, reflecting melasma’s higher prevalence in Asian female populations. However, recruitment from a single-center Chinese female population may show potential outcome variations across the Fitzpatrick spectrum and restrict broader applicability in people with different skin types and gender.
5. Conclusion
In conclusion, this split-face study provides clinical evidence supporting the targeting of H2R as a potential new strategy for treating melasma. The fractional laser may keep a sustained efficacy on melasma and the adjunctive H2R blockade famotidine may improve melasma and interference with laser-triggered histamine-mediated melanogenic pathways while suggesting potential long-term therapeutic benefits and safety. However, large-scale, multicenter, long-term, double-blind clinical trials are needed to validate these findings more comprehensively. Genetic, environmental, and cultural variations in diverse populations necessitate further investigation. The mechanistic ambiguity, whether famotidine directly inhibits melanogenesis or suppresses laser-induced MC activation, warrants further study via in vitro co-cultures and H2R knockout models.
Ethics Statement
This clinical trial was reviewed and approved by the Second Affiliated Hospital of Xi’an Jiao Tong University IRB; approval #2022273.
Consent
The patients in this manuscript have given written informed consent to the publication of their case details.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Shujuan He: methodology, validation, investigation, formal analysis, and writing–original draft. Dan Ye: data curation, visualization, and writing–original draft. Simeng Qiao: investigation and data curation. Luyue Zhang: data curation and writing–original draft. Xi Zhao, Yuxin Zhang, and Jing Liu: supervision. Weihui Zeng: supervision, project administration, writing , review, and editing; Zhao Wang: conceptualization, funding acquisition, resources, supervision, writing, review, and editing.
Shujuan He and Dan Ye are shared first authors.
Weihui Zeng and Zhao Wang are shared senior authors.
Funding
This research was supported by the National Natural Science Foundation of China ∗ 82201966 and the Natural Science Basic Research Program of Shaanxi Province ∗ 2023JC-QN-0924 ∗ 2023-JC-YB-787.
Supporting Information
Figure S1. The change of skin chromaticity along the treatment. (A) ∆L∗ at every 4 weeks on both sides. (B) ∆a∗ at every 4 weeks on both sides. (C) ∆b∗ at every 4 weeks on both sides. Data represent the mean of 16 patients. ∗p < 0.05, comparison between two groups.
Table S1. ∆L∗ of patients.
Table S2. ∆a∗ of patients.
Table S3. ∆b∗ of patients.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




