Association of Dietary Intake With the Risk of Atopic Diseases: A Mendelian Randomization Study
Abstract
Introduction: Previous observational studies have shown an association between specific dietary intake and atopic diseases. However, few studies have analyzed the causal effects of dietary factors on risk of atopic diseases. Therefore, we conducted a Mendelian randomization (MR) study to explore these relationships.
Methods: In this study, we obtained summary statistics on dietary intake and atopic diseases including atopic dermatitis, allergic asthma, allergic conjunctivitis, and allergic rhinitis from large genome-wide association studies (GWASs) in European populations. MR analysis was performed using the inverse variance weighted (IVW) method, supplemented with MR Egger, weighted median, maximum likelihood, and weighted model analysis methods.
Results: Our study included 34 diet-related exposure factors. The results indicated that increased intake of filtered coffee could reduce the risk of developing atopic dermatitis. Conversely, higher average monthly intake of other alcoholic drinks was associated with an increased risk of atopic dermatitis. For allergic asthma, higher intake of filtered coffee was identified as a protective factor, while increased average weekly intake of spirits and cherry were considered risk factors. Furthermore, an increase in average weekly intake of beer plus cider was found to potentially lower the risk of allergic conjunctivitis. However, we did not discover any causal association between the risk of allergic rhinitis and the dietary intake factors.
Conclusion: This MR study validates the potential causal effects of specific dietary intake on different atopic diseases and provides strong support for the development of individualized prevention strategies and health interventions at the family level.
1. Introduction
Atopic diseases are genetic allergic conditions that primarily include atopic dermatitis, allergic asthma, allergic conjunctivitis, and allergic rhinitis [1]. The basis for these diseases is the tendency of individuals with an allergic constitution to produce Immunoglobulin E (IgE) antibodies in response to common environmental antigens [2, 3]. Over the past few decades, the incidence of atopic diseases has been steadily increasing [4, 5]. The chronic recurrent course, heavy economic burden, and the involvement of the entire family in the treatment process have greatly reduced the quality of life for patients and their families [6, 7]. Atopic diseases are rapidly becoming a serious global public health, medical, and economic concern [8, 9].
Multiple environmental factors are involved in the onset and development of atopic diseases, with diet being a particularly notable area of interest [10]. While extensive research has been conducted on this topic, the results have been inconsistent. For instance, a cross-sectional study found that children with atopic dermatitis experienced reduced itching and improved sleep after eliminating eggs and milk from their diet [11]. Conversely, another similar crossover study found no significant benefits in atopic dermatitis patients who excluded eggs and milk from their diet compared with the control group [12]. In the case of allergic rhinitis, a questionnaire survey found that a higher intake of vegetables may have a protective effect on mild and persistent symptoms [13]. The results of another questionnaire survey revealed that the intake of fish and omega-3 polyunsaturated fatty acids may increase the risk of allergic rhinitis, atopic dermatitis, and allergic conjunctivitis among the Japanese population [14]. In a Mendelian randomization (MR) study, an increased frequency of alcohol intake was associated with an elevated risk of asthma, whereas the consumption of fresh and dried fruits was found to be protective against asthma [15], although these results may not necessarily apply to patients with allergic asthma. Although certain dietary risk factors for atopic diseases have been identified in studies, cross-sectional studies may be influenced by confounding factors, and there is insufficient evidence to support the causal role of these dietary factors in the risk of atopic diseases.
Randomized controlled trial (RCT) has unique advantages in determining causal relationships, but they may not always be feasible due to ethical or practical limitations. MR analysis is a method that explores causality by using genetic variations such as single nucleotide polymorphisms (SNPs) related to the exposure factor of interest as instrumental variables (IVs). In comparison to RCT, MR analysis does not require intervention, making it a more efficient and cost-effective approach to exploring the causal relationships between various exposure factors and outcomes. In addition, it is less susceptible to reverse causality and confounding factors, unlike observational studies [16]. Understanding the exact role of dietary intake in the risk of atopic diseases can provide valuable guidance in their prevention, management, and treatment. Therefore, we utilized the MR method to analyze the causal effects of diet-related exposure factors on four common atopic diseases, including atopic dermatitis, allergic asthma, allergic conjunctivitis, and allergic rhinitis.
2. Method
2.1. Data Source
The flowchart of the study design is shown in Figure 1. The genome-wide association study (GWAS) data utilized for dietary exposure factors were sourced from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk). In the data list, we search for exposure containing the element “intake” in the “trait” column and selected dietary-related exposure data. We utilized data of atopic diseases from the FinnGen R9 database [17], which include atopic dermatitis (phenocode: L12_ATOPIC, 13,473 cases and 336,589 controls), allergic asthma (phenocode: ALLERG_ASTHMA, 9631 cases and 210,122 controls), allergic conjunctivitis (phenocode: H7_ALLERGICCONJUNCTIVITIS, 20,958 cases and 356,319 controls, and allergic rhinitis (phenocode: ALLERG_RHINITIS, 11,009 cases and 359,149 controls)). To minimize ancestry mismatches, our analysis only included data from individuals of European population. Since the data used in this study are anonymized and publicly available, there was no requirement for ethical approval.
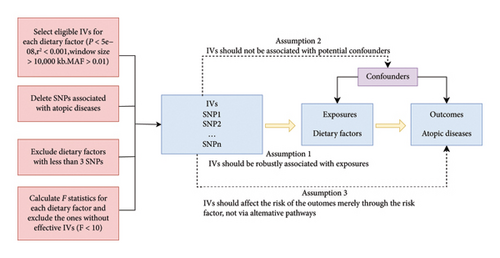
2.2. The Selection of IVs
We selected eligible genetic IVs based on a series of quality control standards. First, we selected SNPs that are significantly correlated with each exposure (p < 5 × 10−8). Next, we performed a clumping process (R2 < 0.001, window size = 10,000 kb) to avoid linkage disequilibrium (LD) and eliminate the impact of highly correlated SNPs. Third, we excluded SNPs with minor allele frequencies (MAFs) less than 0.01. Fourth, in order to prevent potential pleiotropic effects of the IVs, we searched for each SNP included as an instrument in our analysis in Phenoscanner, a database of human genotype–phenotype associations, and removed SNPs significantly associated with atopic dermatitis, allergic asthma, allergic conjunctivitis, allergic rhinitis, atopic disease, and allergic disease. In addition, dietary factors with fewer than 3 SNPs were excluded from the study as some MR sensitivity analyses require at least 3 SNPs for each exposure factor as IVs. To assess the strength of IVs for each dietary exposure factor, we calculated the F-statistic. Exposures with an F-statistic less than 11 were excluded to reduce bias caused by weaker genetic tools [18].
2.3. Pleiotropy, Heterogeneity, and Sensitivity Analysis
MR Egger regression was used to assess the possibility of pleiotropy. In the presence of pleiotropic effect (p ≤ 0.05), we performed MR pleiotropic residual and outlier tests using the MR-PRESSO package and removed the SNPs with the smallest pleiotropic p value. In addition, we used the inverse variance weighted (IVW) method and MR Egger regression to identify heterogeneity, with quantification performed using Cochran’s Q statistic. If the heterogeneity test indicated the presence of heterogeneity in the analysis (p < 0.05), we employed the random-effects model as the main method to address heterogeneity. Furthermore, we conducted a leave-one-out sensitivity analysis to evaluate whether the removal of a single SNP significantly affected the results.
2.4. MR Analysis
We used the IVW method as the main analysis to estimate the causal effects of various dietary exposures on four atopic diseases. The IVW method combines the effect estimate values of individual genetic variants using inverse variance as weights to improve the accuracy and reliability of the estimates. Given the number of tests (136 exposure-outcome pairs) performed, we used a Benjamini–Hochberg false discovery rate (FDR) procedure with an FDR of 0.1 to define the evidence of a significant association. In addition, we employed MR Egger, weighted median, maximum likelihood, and weighted mode analysis methods as supplementary methods. The MR analysis was conducted using the TwoSampleMR package in R (Version 4.3.0) software. Finally, a reverse MR analysis was performed to assess the possibility of reverse causality between genetically predicted dietary factors which showed significant associations with atopic diseases in the forward MR analysis (FDR < 0.1) and atopic diseases.
3. Results
After a series of quality screening steps, our study included 34 dietary-related exposure factors, including beverage intake (average weekly spirits intake, alcohol intake frequency, alcohol intake vs. 10 years previously, average weekly red wine intake, average weekly fortified wine intake, average weekly champagne plus white wine intake, average weekly beer plus cider intake, average monthly intake of other alcoholic drinks, tea intake, herbal tea intake, green tea intake, coffee intake, filtered coffee intake, and water intake), meat intake (processed meat intake, bacon intake, poultry intake, beef intake, nonoily fish intake, oily fish intake, pork intake, and lamb/mutton intake), fruit intake (dried fruit intake, fresh fruit intake, and cherry intake), vegetable intake (salad/raw vegetable intake and cooked vegetable intake), staple food intake (bread intake, sushi intake, and cereal intake), cheese intake, snackpot intake, scotch egg intake, and unsalted peanuts intake. Some dietary-related exposure factors (such as lobster/crab intake, mushroom intake, and milk intake) that did not have enough valid IVs were ultimately not included. The SNP number of each dietary intake ranged from 3 to 88 (Tables S1-S2), and the F-statistics were all greater than 11 (ranging from 30.64849 to 74.96291), indicating no bias caused by weak IVs.
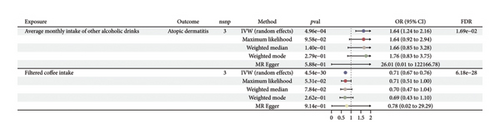
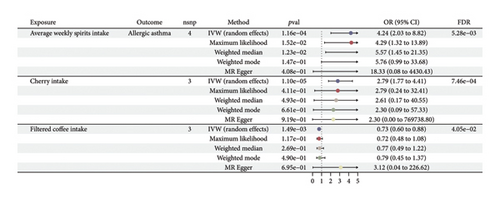
MR estimates of different methods are presented in Figure 2 and Table S3. In the IVW MR analysis, no significant associations were observed between 34 dietary intakes and the risk of allergic rhinitis. However, potential associations were identified for the risk of atopic dermatitis (2 dietary factors), allergic asthma (3 dietary factors), and allergic conjunctivitis (1 dietary factor). We found that an increased average monthly intake of other alcoholic drinks (OR, 1.64; 95% CI, 1.24–2.16; p = 4.96e − 04; and FDR = 1.69e − 02) was associated with an increased risk of atopic dermatitis. On the other hand, filtered coffee intake (OR, 0.71; 95% CI, 0.67–0.76; p = 4.54e − 30; and FDR = 6.18e − 28) was a protective factor for atopic dermatitis. An increase in filtered coffee intake (OR, 0.73; 95% CI, 0.6–0.88; p = 1.49e − 03; and FDR = 4.05e − 02) was found to be associated with a reduced risk of allergic asthma, whereas average weekly spirits intake (OR, 4.24; 95% CI, 2.03–8.82; p = 1.16e − 04; and FDR = 5.28e − 03) and cherry intake (OR, 2.79; 95% CI, 1.77–4.41; p = 1.10e − 05; and FDR = 7.46e − 04) increased the risk of allergic asthma (Figure 3). A higher average weekly beer plus cider intake (OR, 0.5; 95% CI, 0.31–0.79; p = 3.11e − 03; and FDR = 7.05–02) reduced the risk of allergic conjunctivitis (Figure 4). No significant evidence of horizontal pleiotropy or heterogeneity was detected, as indicated by the pleiotropy and heterogeneity tests in abovementioned results. Scatter plots, forest plots, funnel plots, and leave-one-out plots were shown in the supporting figures for atopic dermatitis (Figures S1–S4), allergic asthma (Figures S5–S8), allergic conjunctivitis (Figures S9–S12), and allergic rhinitis (Figures S13–S16). In the reverse MR analysis, no significant causal effects were observed between atopic diseases and the dietary factors mentioned above (all p values > 0.05) (Figure S17).

4. Discussion
MR analysis was conducted to evaluate the potential causality between dietary intakes and the risk of atopic disease in this study, which uses random allocation of alleles to replicate the randomization process in double-blind clinical trials. We identified specific dietary intake that may be associated with the risk of atopic dermatitis, allergic asthma, and allergic conjunctivitis.
Alcohol can affect the innate and adaptive immune systems [19]. Long-term alcohol abuse can damage the immune system, while moderate alcohol consumption may have beneficial effects on the immune system [20]. Studies have found that heavy drinkers have elevated serum IgE levels [21]. A prospective study in Denmark involving 19,349 participants found a U-shaped relationship between alcohol consumption and asthma risk, with the lowest asthma risk associated with moderate alcohol consumption [22]. Our MR analysis suggests that a high average monthly intake of other alcoholic drinks may increase the risk of atopic dermatitis, and a high average weekly spirits intake may increase the risk of allergic asthma. For allergic rhinitis, a prospective cohort study involving 1354 Danish females found an increased risk of perennial allergic rhinitis associated with alcohol consumption. In contrast, our study found that a high average weekly beer plus cider intake may have a significant protective effect against allergic conjunctivitis. This study found no association between other alcohol-related exposure factors and the risk of atopic diseases, such as alcohol intake frequency, alcohol intake versus 10 years previously, average weekly red wine intake, average weekly fortified wine intake, and average weekly champagne plus white wine intake. The results of the MR studies differ from those of the observational studies regarding the relationship between alcohol intake and the risk of atopic disease. Observational studies might be susceptible to the influence of other confounding factors, and it is possible that different types of alcoholic beverages may have different effects on atopic diseases. In addition, most observational studies have not taken into account the classification of different types of alcoholic beverages for their analysis. Consequently, further research involving both observational studies and MR studies is needed in the future to better elucidate the association between different patterns of alcohol consumption and atopic diseases.
Coffee rank among one of the most popular beverages consumed globally. A study using UK Biobank data explored the association between self-reported coffee consumption and the risk of asthma in 424,725 participants, cox proportional hazards regression analysis showed that moderate coffee consumption was associated with a reduced risk of adult asthma [23]. Coffee contains polyphenolic compounds such as chlorogenic acid and caffeic acid, which are powerful antioxidants that can effectively scavenge free radicals [24]. The results of a randomized double-blind placebo-controlled clinical trial suggested that the green coffee extract can balance the production of Th1/Th2 cytokines in human T cells [25]. Our MR study results showed that increased filtered coffee intake is a protective factor against atopic dermatitis and allergic asthma in Western countries. More research is needed to explore the causal relationship between various beverage intakes and the risk of atopic diseases.
Fruits and vegetables contain many potentially important antioxidants. A prospective cohort study involving 2011 participants found that higher fruit intake can help prevent respiratory allergies in schoolchildren [26]. Another study found a negative correlation between high dietary fiber intake in children and the risk of allergic rhinitis and sensitivity to specific allergens before adulthood [27]. However, in this study, we found that high cherry intake may have a promoting effect on allergic asthma. Our study did not find any causal effects of other dietary intake on atopic diseases.
This study has certain advantages and limitations. This is the first study to assess the impact of 34 dietary factors on the risk of atopic diseases using a two-sample MR analysis. Using genetic variations as IVs to infer causal relationships, the MR method can effectively overcome biases caused by reverse causality and confounding. To ensure the accuracy of the MR analysis, we performed pleiotropy, heterogeneity, and sensitivity analyses. To avoid unnecessary biases, we used data from European populations for exposure and outcomes. However, this MR study has some limitations. First, the sample size of the dietary intake GWAS data is small, and the limited number of IVs weakens the explanatory power of the phenotype variance, which may reduce the statistical power. Therefore, some nonsignificant results should not be interpreted as no effect of dietary intake on the outcomes. Second, we only considered 34 types of food, as other foods (such as lobster/crab intake, mushroom intake, and milk intake) lack sufficient IVs for evaluation. In addition, it should be noted that elements within a food category may have synergistic or antagonistic effects, and our study mainly investigated the overall effect of a food category. Third, since the study data samples are from European populations, the results may not be generalizable to other populations. Fourth, there may be some differences in the age distributions between the exposure and outcome GWAS populations, but the median age of first events for atopic diseases in the FinnGen cohort indicates substantial overlap with the adult population represented in the UK Biobank dietary GWAS. We acknowledge this limitation and encourage future studies to use age-matched cohorts to strengthen the robustness of causal inferences. In addition, further research is needed to explore potential age-related biological mechanisms that may underlie these associations. Finally, while MR analysis reduces confounding by using genetic variants as IVs, it cannot fully account for other unmeasured factors that may influence the association between dietary intake and atopic diseases. For example, lifestyle factors, including physical activity and smoking, as well as environmental exposures, such as air pollution or allergens, could interact with dietary factors in complex ways. These interactions may bias the observed associations or obscure causal relationships. In addition, the socioeconomic status may correlate with dietary patterns and environmental risks, introducing residual confounding. Although our study provides valuable insights, it is crucial to consider these limitations when understanding the findings. Future research should aim to address these limitations by integrating genetic data with comprehensive environmental and behavioral datasets. Methods like multivariable MR may help explain these complex links and give a clearer picture of the factors contributing to atopic diseases.
5. Conclusion
In this study, we found differences in dietary risk factors for different atopic diseases, highlighting the importance of personalized interventions. Developing personalized prevention plans in daily life will help better meet the needs of different individuals and provide targeted recommendations to reduce the risk of atopic diseases. In addition, our findings have important implications for parents with atopic diseases. Based on our findings, they can try to intervene at the family level to reduce the likelihood of their children developing the diseases. By adjusting the intake of specific diets, parents can provide a healthier living environment for the next generation. This health education and prevention measure can be easily implemented within the family and important public health implications.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Li Lei, Jing Chen, Chuhan Fu, and Qinghai Zeng contributed to the conception and design of the study; Xixia Dai, Ling Jiang, Yibo Hu, Songjiang Wu, Menglu Chen, and Yixuan Liang contributed to the analysis of data, visualization, and drafting of the text; and all authors read and approved the final version of the paper. Xixia Dai and Li Lei contributed equally to this work.
Funding
This study was supported by the Natural Science Foundation of Hunan Province (2024JJ6615 and 2023JJ20091), the “co-PI” project from the Third Xiangya Hospital of Central South University (202412), and the Scientific Research Program of Hunan Provincial Health Commission (B202304127273).
Acknowledgments
The authors extend sincere gratitude to the researchers and participants involved in the initial GWAS for their efforts in gathering and organizing extensive data reservoirs, along with those who enthusiastically engaged in this study. We express our gratitude to the original data contributors who facilitated this analysis, without whom this study would not have been possible.
Supporting Information
Table S1: Information of the exposures for atopic dermatitis, allergic rhinitis, and allergic conjunctivitis. Table S2: Information of the exposures for allergic asthma. Table S3: Results of the MR study testing the causal effects of dietary factors on atopic diseases. Figure S1: Scatter plot of the MR analysis of the effect of dietary factors on atopic dermatitis. Figure S2: Forest plot of the MR analysis of the effect of dietary factors on atopic dermatitis. Figure S3: Funnel plot of the MR analysis of the effect of dietary factors on atopic dermatitis. Figure S4: Leave-one-out plot of the MR analysis of the effect of dietary factors on atopic dermatitis. Figure S5: Scatter plot of the MR analysis of the effect of dietary factors on allergic asthma. Figure S6: Forest plot of the MR analysis of the effect of dietary factors on allergic asthma. Figure S7: Funnel plot of the MR analysis of the effect of dietary factors on allergic asthma. Figure S8: Leave-one-out plot of the MR analysis of the effect of dietary factors on allergic asthma. Figure S9: Scatter plot of the MR analysis of the effect of dietary factors on allergic conjunctivitis. Figure S10: Forest plot of the MR analysis of the effect of dietary factors on allergic conjunctivitis. Figure S11: Funnel plot of the MR analysis of the effect of dietary factors on allergic conjunctivitis. Figure S12: Leave-one-out plot of the MR analysis of the effect of dietary factors on allergic conjunctivitis. Figure S13: Scatter plot of the MR analysis of the effect of dietary factors on allergic rhinitis. Figure S14: Forest plot of the MR analysis of the effect of dietary factors on allergic rhinitis. Figure S15: Funnel plot of the MR analysis of the effect of dietary factors on allergic rhinitis. Figure S16: Leave-one-out plot of the MR analysis of the effect of dietary factors on allergic rhinitis. Figure S17: The MR analysis of the effect of atopic diseases on dietary factors.
Open Research
Data Availability Statement
The GWAS data utilized in our analysis originate from publicly accessible databases. Detailed links or websites pertaining to the data utilized in this paper can be found in the method section of our study.




