Effect of Oral Tranexamic Acid on Hair Melanin in Asian Women
Abstract
Introduction: Tranexamic acid (TXA) is widely used to treat melasma, but its potential effects on hair pigmentation remain unexplored. Concerns about hair whitening during TXA treatment have been raised, as it is often perceived as a sign of aging and may elicit negative emotional responses. This study aimed to evaluate the effects of oral TXA on hair melanin content and color.
Methods and Results: Seven middle-aged East Asian women completed a 3-month prospective observational study, taking 500-mg oral TXA daily, excluding menstruation periods. Hair samples were collected from 10 scalp regions before and after treatment. Melanin content was measured using liquid chromatography–tandem mass spectrometry, and hair color changes were assessed with a colorimeter. One participant was excluded due to hair dyeing during the study. After 3 months of TXA treatment, no statistically significant changes in hair melanin content or hair color were observed, even after accounting for individual differences.
Discussion: Oral TXA administered at 500 mg daily for 3 months did not significantly affect hair melanin content or color in middle-aged East Asian women. These findings provide reassurance for patients and clinicians regarding hair pigmentation during TXA treatment. Further research with diverse populations is recommended.
Trial Registration: Chinese Registry of Clinical Trials: ChiCTR2400092219
Summary
- •
Significance
- ◦
This study addresses patient concerns about potential hair whitening during oral tranexamic acid (TXA) treatment for melasma. By demonstrating no significant effects of TXA on hair melanin content and color, the findings provide evidence-based reassurance for clinicians and patients. Hair whitening is often perceived negatively, impacting emotional well-being. The results highlight the safety of TXA regarding hair pigmentation, emphasizing its selective action on ultraviolet (UV)-induced skin pigmentation without interfering with hair follicle melanogenesis. This study contributes to a deeper understanding of TXA’s effects, guiding informed clinical decisions and enhancing patient confidence in its use for pigmentation disorders.
- •
Research highlights
- ◦
Oral TXA (500 mg/day for 3 months) had no significant effect on hair melanin content or color in middle-aged East Asian women, addressing concerns about hair whitening during treatment and providing reassurance for patients and clinicians.
1. Introduction
Gray hair is one of the earliest and most prominent features of human aging, with profound effects on social cognition, emotional well-being, and mental health [1, 2]. The formation of white hair is mainly caused by the decrease of melanin. Melanin is the main pigment responsible for skin, eye, and hair color. Eumelanin, in particular, determines the dark color of hair, with higher levels indicating darker hair color [3].
Melanin is produced by melanocytes, specialized cells that synthesize and deliver the pigment to the hair stem. Hair follicle melanocytes play a key role in maintaining hair pigment, and their activity is regulated by complex biochemical pathways, such as the Raper–Mason pathway and the rate-limiting enzyme tyrosinase [4]. Either aging or dysfunction of melanocytes caused by external factors can trigger the gradual formation of gray hair.
A variety of drugs have been reported to affect melanin levels, leading to decolorization or deep pigmentation of hair. For example, medications such as chloroquine and hydroxychloroquine are associated with lighter hair color, whereas tamoxifen and cyclophosphamide are associated with darker hair color [5]. However, the effects of many commonly used drugs on hair pigmentation have not been fully studied.
One such agent of interest is oral or topical TXA, which is gaining recognition for its effectiveness in managing melasma [6, 7]. Oral administration of TXA modulates melanin synthesis and has shown superior efficacy in improving skin pigmentation. However, its potential effect on hair pigmentation has not been investigated. If oral administration of TXA results in a reduction in hair melanin content, it may cause significant hair color changes that may affect the patient’s appearance and emotional response.
The aim of this study was to investigate the effect of oral TXA on hair melanin content in middle-aged Asian women. By analyzing the hair pigment changes and their potential effects, this study will provide guidance for clinicians to consult patients and help patients understand the possible effects of TXA use in advance.
2. Materials and Methods
2.1. Study Design
This was an observational prospective study. Eight healthy Asian women aged 28–35 years were recruited. From May to August 2024, these recruited participants received oral TXA (Daiichi Sankyo Healthcare Co., Ltd., Tokyo, Japan), taking 500 mg once daily after breakfast. The treatment was administered continuously for 3 months, with interruptions during menstrual periods. Ultimately, seven participants completed the 3-month study.
After 3 months of oral TXA treatment, hair samples were collected from areas close to the scalp. Sampling sites included the left and right sides of the forehead, occiput, temples, and crown, as well as the left and right posterior hairline, totaling 10 regions. More than 15 hairs were collected from each region. Because distal and nascent proximal ends of long hair remain equally melanized [8], the distal end retains the melanin content generated earlier. The processing of hair samples was based on the human hair growth rate, commonly estimated to be 1 cm per month [9]. In the study, to obtain the pretreatment sample, a 1-cm segment was cut from the section of the hair shaft located 4-5 cm away from the root. For the post-treatment sample, another 1-cm segment was cut from the portion of the hair shaft located immediately above the root. This method leverages the typical growth rate of human hair, allowing segmental analysis to capture time-related biological changes.
2.2. Study Sample
Participants were excluded if they had a known hypersensitivity to TXA, were elderly, smoked, or had familial canities. Individuals with pre-existing medical conditions, including hypertension, hyperlipidemia, diabetes mellitus, impaired renal function, or a history of thromboembolism, such as venous thrombosis, were also excluded. Pregnant and lactating women were not eligible to participate. Additionally, those who had used medications known to affect hair color within the past 3 months, including chemotherapy drugs, tetracycline antibiotics, immunosuppressants, antidepressants, hormonal drugs, or high-dose oral vitamin C, were excluded from the study.
During the observation period (from 2 months before the study began to the end of medication use), participants refrained from using products that might affect hair color, such as selenium sulfide shampoos, medicated shampoos, scalp treatment products containing sulfur, salicylic acid, or certain herbal ingredients, as well as hair dyes or bleaching agents. In addition, participants did not experience major illness during this period, did not deliberately change their weight, and did not engage in intense physical activity. Their home and work environments remain stable.
2.3. Measurements
The primary measurement was the level of melanin in hair before and after taking 500-mg TXA daily for 3 months. The content of melanin in hair was determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS) using SCIEX ExionLC system (AB SCIEX, MA, USA) and API 4000 mass spectrometry system (AB SCIEX, MA, USA). The secondary measurement was the hair color. The colorimeter 3NH-ST70 was used to detect hair color.
2.3.1. Mass Spectrometry Analysis
Melanin content in hair was determined using LC-MS/MS using a SCIEX ExionLC system and API 4000 mass spectrometry system (AB SCIEX, USA). Pyrrolidine-2,3,5-tricarboxylic acid (PTCA) was chosen as the main marker for quantitative analysis of eumelanin because PTCA originates from the DHICA unit in eumelanin and has been validated as a reliable indicator of melanin content [10].
Hair samples were treated with alkaline H2O2 oxidation for 16 h before PTCA levels were measured by LC-MS/MS. Previous studies have demonstrated a significant correlation (p < 0.0001) between PTCA levels and other hair degradation products, such as pyrrole-2,3-dicarboxylic acid (PDCA) and thiazole-2,4,5-tricarboxylic acid (TTCA) [10]. Thus, PTCA is an appropriate marker to assess changes in melanin content. The PDCA/PTCA ratio has consistently maintained high reproducibility in melanin hairs ranging from black to blond [10]. The PDCA/PTCA ratio was used to estimate the amount of 5,6-dihydroxyindole (DHI) in eumelanin.
All participants in this study were middle-aged Asian women with dark hair. Given that melanin in black hair is mainly composed of eumelanin (> 99%) [11], PTCA was chosen as the main target for melanin quantitative analysis.
2.3.2. Colorimeter
Tristimulus colorimetry is a method developed to objectively reproduce color, similar to how the human eye perceives it [12]. This method determines the color by measuring the reflectance of light at specific wavelengths, using a photodiode array light source. The most commonly used color parameters are from the CIELAB (International Commission on Illumination Lab) system established in 1976. This system uses a specific combination of three wavelengths—green, blue, and red light—for in vivo quantitative assessment of pigmentation.
The CIELAB system includes three primary parameters: L∗, which represents lightness (ranging from black with L∗ = 0 to white with L∗ = 100); a∗, which indicates the change from red to green; and b∗, which reflects the change from yellow to blue. These three variables allow any color to be represented in a three-dimensional space.
In this study, a colorimeter (3NH-ST70, Sanenchi Technology Co., Ltd., Guangzhou, China) was used to measure hair grayscale. The instrument uses D/8 illumination, with a measurement wavelength range from 360 to 780 nm. Previous studies have shown that the measured lightness is closely related to melanin concentration [13, 14]. For example, Shriver and Parra [15] used a colorimeter to measure the L∗ values of hair from European Americans, African Americans, South Asians, and East Asians and found a strong correlation (R2 = 0.827, p < 0.001) between L∗ values and the melanin (M) index measured by a spectrometer, allowing for accurate estimation of hair pigmentation levels.
Since there is no interference from hemoglobin in hair, the L∗ value can more accurately reflect the true color of the hair, unlike skin melanin measurements, which are influenced by hemoglobin peak absorption [15] because L∗ is highly dependent on the reflectance of green light. Therefore, L∗ provides a reliable indicator for measuring hair color.
To minimize measurement error, all hair color values were measured by the same operator using this instrument. The measurement site for each bundle of hair was the same as the sampling site for mass spectrometry detection. Hair samples completely covered the measuring window of the instrument, and three measurements were performed at each position. The average of three readings was taken for analysis [16].
2.4. Ethical Consideration
This study was approved by the Institutional Review Board of Zibo Municipal Hospital (20240509). All patients were informed and consented to participate in this study.
2.5. Analysis
Univariate regression analyses were used to examine the differences in the melanin level and hair color between pre- (4-cm section from the hair root) and post-treatment (hair root), with patient cluster controlled. The significance level is 0.05. All analyses were conducted in STATA MP 17.0.
3. Results
3.1. Level of Melanin
The mean PTCA concentration in hair root was 234.2 ng/mg (SD = 65.8) following TAX intervention, compared to 228.5 ng/mg (SD = 57.2) at 4 cm from the hair root prior to the intervention (Figure 1). These differences were not statistically significant, controlled for the within-observation variations (p = 0.29).
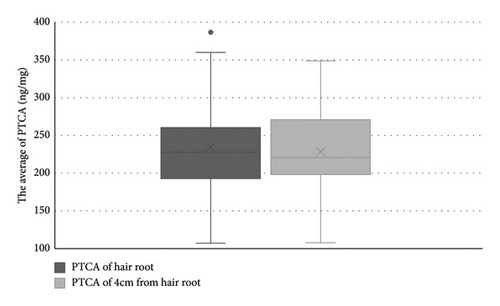
3.2. Hair Color
The average L∗ value of hair root color following TAX intervention was 16.8 (SD = 2.0), compared to 17.0 (SD = 1.9) for hair color measured 4 cm from the root prior to the intervention (Figure 2). These differences were not statistically significant, controlled for the within-observation variations (p = 0.59).
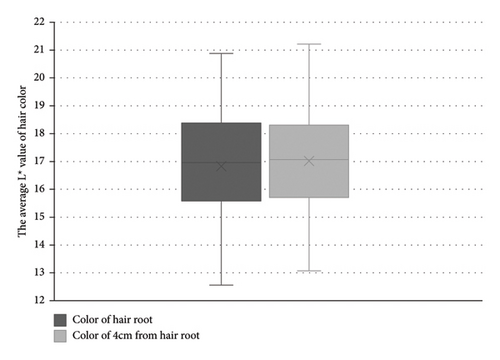
4. Discussion
In the present study, oral TXA treatment was found to have no significant effect on hair melanin content and hair color. The participants maintained stable health, behavior, and living environments throughout the study, with no major life events occurring. This inclusion criterion helped reduce the potential impact of illness, life stress, and other confounding factors on the experimental outcomes. Since oral TAX administration may lead to reduced menstrual flow, participants were instructed to discontinue TAX during menstruation to avoid additional psychological stress [17]. These measures were taken to minimize the influence of psychological stress and behavioral factors on hair whitening [18, 19]. These controlled conditions validate the reliability of the findings and suggest that TXA does not affect hair melanogenesis under normal conditions.
A possible explanation for this result is the protective effect of hair itself. Thick, long, dark hair significantly reduces UV exposure to the scalp. Previous studies have shown that hair blocks up to 81% of UVB radiation, especially in people with thick and darker hair [20]. This shading effect may limit the effect of UV light on hair follicle melanocytes, thereby reducing the potential effect of TXA on hair pigmentation.
In addition to the shading effect, the characteristic of limited UV penetration depth further explains why UV has little effect on hair melanin production. Studies have shown that UV light can only penetrate the superficial skin layer of 15–60 μm depending on the wavelength [21, 22], while the melanocytes of hair follicles are located in the deep skin layer (4–5 mm) [23, 24], far beyond the influence range of UV light. These factors together suggest that the mechanism of UV-induced pigmentation targeted by TXA has little to do with hair melanogenesis.
Some researchers [25, 26] have previously speculated that TXA may inhibit melanogenesis by competitively inhibiting tyrosinase, the rate-limiting enzyme of melanogenesis, but there is no definitive evidence. However, Maeda and Naganuma [27] found that TXA was effective in preventing UV-induced skin pigmentation but had no effect on epidermal tyrosinase activity, dopa-positive melanocyte count, and melanin production in unexposed skin. In another trial in which 5% TXA emulsion was administered to normal skin for 24 weeks, no depigmentation or hyperpigmentation was also observed [28]. Our test results showed no statistically significant difference in hair melanin levels before and after oral administration of TXA. Combined with the conclusions of the above two experiments, after excluding the effect of UV light on hair follicle melanocytes, it is speculated that TXA does not reduce the effect of melanin production by competitive inhibition of tyrosinase directly.
To summarize the discussion above, first, due to the shielding effect of normal-density hair, approximately 81% of UV radiation cannot reach the scalp. The remaining UV rays are also unable to penetrate to the depth of 4-5 mm where follicular melanocyte units reside and thus cannot activate melanin production in these deep structures. This suggests that TXA, which inhibits melanin production by blocking UV-induced activation of epidermal melanocytes, does not exert the same effect on follicular melanocytes. Second, two experiments using topical TXA on non–UV-exposed skin confirmed that TXA does not directly inhibit tyrosinase activity through competitive binding. This further indicates that TXA’s effect on melanogenesis is not due to direct suppression of the enzymatic pathway responsible for melanin synthesis. Taken together, these findings suggest that TXA has limited influence on follicular melanocytes and is unlikely to affect hair pigmentation through the conventional mechanisms known to reduce epidermal pigmentation.
In this study, we detected melanin content by measuring PTCA—which is a specific metabolite of eumelanin [29]—in hair using LC-MS/MS. This technique is highly accurate in the analysis of melanin degradation products [30]. Our results are consistent with previous studies on human hair, validating the reliability of the method. Specifically, the results of our determination of PTCA content in black hair are consistent with previous reports [10], further supporting the reliability of the analytical method.
Nonetheless, there are some limitations to this study. The sample size was small, and the nonrandomized open-label design may have introduced selection bias. In addition, the treatment period was relatively short, and the study population was limited to Asian women with long black hair. Future studies should include participants with different hairstyles and hair colors and extend the treatment period. In addition, the regulatory enzyme activities of hair follicle melanocytes and other possible influencing factors should be observed.
In conclusion, the present study demonstrates that oral administration of TXA does not significantly affect hair melanin content or color. These results suggest that the mechanism of action of TXA is specific to UV-induced pigmentation in the skin and does not interfere with the melanogenic function of hair follicle melanocytes. Future studies should further extend these findings to confirm the underlying mechanisms and assess the broad applicability of TXA in different populations and conditions.
Ethics Statement
This study was approved by the Institutional Review Board of Zibo Municipal Hospital (20240509).
Consent
All patients were informed and consented to participate in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jinglai Li should be considered as the joint first author.
Funding
No funding was received for this manuscript.
Acknowledgments
We express our sincere gratitude to the cryo-EM platform at the School of life Sciences, Peking University, and cryo-EM facility, Changping laboratory.
Supporting Information
The supporting information for this manuscript includes a detailed description of the procedure for detecting the melanin metabolite PTCA in hair using liquid chromatography-tandem mass spectrometry (LC-MS/MS), a supporting Figure 1 showing the representative MS chromatogram of PTCA and IS, and a supporting Figure 2 presenting the PTCA calibration curve. These materials provide further clarification and support the results presented in the main text.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




