Alitretinoin for the Treatment of Lichen Amyloidosis: A Retrospective, Observational Study and an In Vitro Study
Abstract
Background: Lichen amyloidosis (LA) is the most common form of localized cutaneous amyloidosis, often accompanied by severe pruritus. Treatment options are limited, and alitretinoin, a systemic retinoid, has shown promise in managing refractory cases.
Objective: This study aimed to evaluate the efficacy of alitretinoin in treating LA and its effects on keratinocytes.
Methods: We conducted a retrospective analysis of 13 patients diagnosed with LA treated with alitretinoin. In addition, an in vitro study assessed the impact of alitretinoin on keratinocyte inflammation markers.
Results: Of the 13 patients, 4 (30.77%) achieved complete clearance of lesions, demonstrating excellent improvement. The remaining 9 (69.23%) showed substantial improvement, with almost clear or mild residual lesions. In vitro, alitretinoin significantly decreased levels of IL-33, periostin, CCL5, and PTGES.
Conclusion: Alitretinoin demonstrated effectiveness in treating LA, with favorable outcomes in both clinical and in vitro settings. These findings support alitretinoin as a viable treatment option for LA.
1. Introduction
Lichen amyloidosis (LA) is the most common form of primary localized cutaneous amyloidosis and involves the deposition of heterogeneous amyloid proteins on the skin without systemic involvement [1, 2]. With cutaneous amyloidosis, amyloid deposits are derived from keratin intermediate filament proteins, and basal keratinocyte disruption is thought to have an important role. LA primarily occurs between the fifth and sixth decades of life and is more common among men than among women. Clinically, LA appears as a pigmented hyperkeratotic papule. Although the pretibial area is commonly involved, LA may also appear in the extensor area. The most prominent symptom is intense pruritus, which is suggested to contribute to its pathogenesis by causing itchiness and friction [3]. Histologic findings include amphophilic material collection and basal cell vacuolar changes on the papillary dermis. Case reports and case series of patients treated with etretinate, corticosteroids, cyclosporine, amitriptyline, colchicine, tacrolimus, dimethyl sulfoxide, laser treatment, and phototherapy have been published; however, large-scale research of primary localized cutaneous amyloidosis treatment has been limited. In addition, standard treatments and guidelines for cutaneous amyloidosis are lacking [2]. Only a limited number of cases of LA treatment with alitretinoin have been reported [4–6].
Alitretinoin is an endogenous retinoid with high affinity for the retinoid acid and retinoic X receptors [7]. Although alitretinoin is currently approved for the treatment of chronic hand eczema, it is effective for a variety of diseases that are refractory to conventional treatment [8]. Common side effects include headache, erythema, nausea, and flushing. In addition, increased cholesterol and triglyceride levels and decreased thyroid-stimulating hormone and free T4 levels may occur [7].
Therefore, during this study, we aimed to evaluate the efficacy and safety of alitretinoin for the treatment of LA. Furthermore, to elucidate the effects of alitretinoin on keratinocytes, we examined the changes in mediators by treating a keratinocyte culture model with 0.1 μM 9-cis-retinoic acid.
2. Materials and Methods
2.1. Patients
The retrospective study was approved by the Ethics Committee of Hallym University Medical Center (approval number: HALLYM 2022-11-009) and included patients diagnosed with lichen amyloidosis based on clinical and histopathological findings in the dermatology outpatient department of the Hallym University Sacred Heart Hospital, Anyang, Korea, between January 2014 and June 2022. Thirteen patients were retrospectively enrolled in this study, and their clinical and/or histopathological data were evaluated. Clinical information, including sex, age, duration of illness, previous treatment history, distribution of lesions, medical history, and histopathological findings, provided in the electronic medical records and clinical photographs were assessed.
2.2. Cell Culture
The in vitro study was approved by the institutional review board of Hallym University Sacred Heart Hospital (institutional review board no. HALLYM 2019-07-029; Republic of Korea). Human epidermal keratinocytes (HEKs) were isolated from foreskins obtained after circumcision was performed [9]. The epidermal layer was separated from the dermis by enzymatic digestion. The tissue was incubated with dispase (25 U/mL in Hanks’ balanced salt solution) for 20 h at 4°C. On the next day, the epidermis was removed, and keratinocytes were dissociated from the epidermal layer by digestion (15 min) with 0.05% trypsin–ethylenediamine tetraacetic acid (EDTA) solution. The cells were cultured in medium 154CF (0.07 mM Ca2+; Gibco) containing human keratinocyte growth supplement. HEKs from passages 2–8 were enzymatically dissociated using trypsin–EDTA for each passage and used for the experiments. HEKs were cultured without serum for 16 h, followed by the addition of 0.1 μM 9-cis-retinoic acid (alitretinoin) to the culture medium for 24 h.
2.3. Quantitative Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated with TRIzol® reagent (Molecular Research Center, Inc.) according to the manufacturer’s protocol. Complementary DNA was synthesized using the Maxime RT PreMix kit (Intron Biotechnology, Inc.) according to the manufacturer’s instructions [8]. The mRNA expression levels were detected using a quantitative reverse-transcription polymerase chain reaction. The quantitative polymerase chain reaction was performed using SYBR green dye and the KAPA SYBR® FAST qPCR Master Mix (2X) kit (Roche). The comparative Cq method (2−ΔΔCq) was used to quantify relative gene expression, and β-actin was used as an endogenous reference control for all transcripts. Primers were designed based on previously published complementary DNA or genomic sequences. The sequences of the primers were as follows: interleukin (IL)-31 (forward, GAA CTC TGC CGT GAT TCC TT; reverse, AAG CCT GCA GAA GAA AAG CA); IL-33 (forward, AAT CAG GTG ACG GTG TTG; reverse, ACA CTC CAG GAT CAG TCT TG); periostin (forward, GAG ACA AAG TGG CTT CCG; reverse, CTG TCA CCG TCA CAT CCT); prostaglandin E synthase (PTGES) (forward, GGA AGA AGG CCT TTG CCA ACC C; reverse, GTT CCA CGT CGG GGT CGC T); and CCL5 (forward, CTG AAC AAG GGC AAG CTT TGT C; reverse, TAA GTT CAG GTT CAA GGA CTC TC).
2.4. Statistical Analysis
All experiments were performed at least three times. Data were expressed as the mean ± standard error of the mean. Statistical significance between groups was determined using the two-tailed Student’s t-test, and p < 0.05 indicated statistical significance.
3. Results
3.1. Retrospective Review of 13 Cases
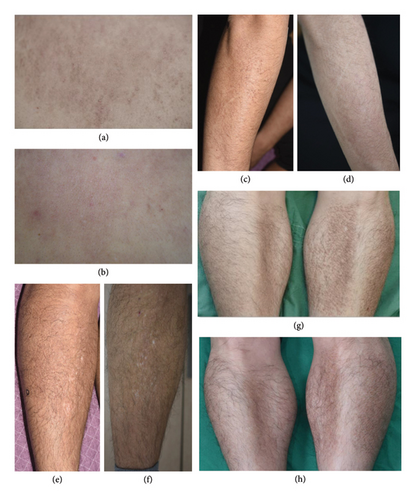
Thirteen patients diagnosed with LA based on clinical and histological findings were enrolled in the retrospective study. Electronic medical records, clinical photographs, and histological findings were reviewed, retrospectively. The patients’ baseline characteristics and responses to alitretinoin are summarized in Table 1. Of the 13 patients, 10 were men and 3 were women; their median age was 40 years (±11.21 years). Five patients had been previously diagnosed with atopic dermatitis and reported severe itchiness; therefore, oral antihistamines, topical corticosteroids, and topical calcineurin inhibitors were administered. To determine the diagnosis, a punch biopsy was performed for 10 patients; the results showed a collection of amphophilic material on the papillary dermis with epidermal hyperplasia. In addition, Congo red staining indicated that eight patients exhibited apple-green birefringence. These patients experienced itchiness and lesion improvement (Figure 1) at 4–8 weeks after treatment with alitretinoin (30 mg). The duration of alitretinoin use varied from 8 to 54 weeks. Of the 13 patients, 4 experienced improvements in lesions and itchiness, and 9 experienced partial improvement. Dyslipidemia was observed in one patient after alitretinoin administration; however, this symptom improved after treatment discontinuation. Four patients reported headaches after using alitretinoin; however, this symptom improved with continuous treatment administration. No additional drug-related adverse events were observed.
| Case | Sex | Age (yr) | Underlying disease | Duration of disease (yr) | Previous therapy | Clinical course | Duration of treatment | Physician global assessment | Adverse event |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | M | 58 | None | 5 | Antihistamine, topical corticosteroid, topical calcineurin inhibitor | Improvement in itching and skin lesions after 4 weeks | 16 week | 0 (clear) | Dyslipidemia |
| Case 2 | M | 53 | Hypertension | 1 | Antihistamine, topical corticosteroid, isotretinoin | Clinical improvement at week 4 | 8 week | 1 (almost clear) | None |
| Case 3 | M | 32 | None | 3 | Antihistamine, methylprednisolone, doxycycline, isotretinoin | Improvement in itching and skin lesions after 4 weeks | 54 week | 1 (almost clear) | None |
| Case 4 | M | 43 | Atopic dermatitis, dyslipidemia | 4 | Antihistamine, topical corticosteroid | Improvement in itching at 6 weeks, partial improvement of skin lesions at 12 weeks | 16 week | 2 (mild) | None |
| Case 5 | M | 31 | Atopic dermatitis | Unknown | Topical calcineurin inhibitor | Clinical improvement at week 6 | 25 week | 0 (clear) | None |
| Case 6 | F | 47 | None | 15 | None | Clinical improvement at week 8 | 21 week | 1 (almost clear) | None |
| Case 7 | M | 25 | Atopic dermatitis | 1 | Antihistamine, prednisolone | Clinical improvement at week 4 | 43 week | 2 (mild) | Headache |
| Case 8 | F | 30 | None | 0.5 | Antihistamine | Clinical improvement at week 5 | 26 week | 0 (clear) | Headache |
| Case 9 | M | 39 | Atopic dermatitis | 1 | Antihistamine, topical corticosteroid, topical calcineurin inhibitor | Improvement in itching at 2 weeks, partial improvement of skin lesions at 6 weeks | 45 week | 1 (almost clear) | Headache |
| Case 10 | M | 52 | None | 5 | Topical corticosteroid | Improvement in itching at 6 weeks | 14 week | 2 (mild) | None |
| Case 11 | M | 49 | None | 10 | Antihistamine, topical calcineurin inhibitor | Improvement in itching at 6 weeks, partial improvement of skin lesions at 14 weeks | 45 week | 0 (clear) | Headache |
| Case 12 | F | 25 | Atopic dermatitis | 0.25 | Antihistamine, cyclosporine, methylprednisolone, topical corticosteroid | Clinical improvement at week 8 | 38 week | 2 (mild) | None |
| Case 13 | M | 34 | None | 3 | Antihistamine | Improvement in itching at 2 weeks, partial improvement of skin lesions at 4 weeks | 27 week | 1 (almost clear) | None |
3.2. In Vitro Analysis of the Effects of 9-Cis-Retinoic Acid on Keratinocytes
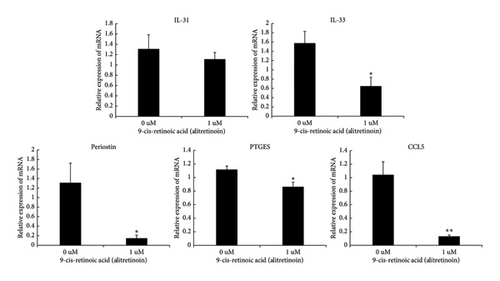
After the HEKs were treated with 0.1 μM of 9-cis-retinoic acid, the mRNA expression levels of the mediators decreased (Figure 2). The relative expression of IL-31 decreased from 1.31 to 1.11 after treatment, but this difference was not statistically significant (p = 0.55). Conversely, the IL-33 level decreased from 1.57 before treatment to 0.64 after treatment (p = 0.03). The periostin level decreased from 1.31 to 0.14, the PTGES level decreased from 1.11 to 0.86, and the CCL5 level decreased from 1.04 to 0.13 (p = 0.017, p = 0.017, and p = 0.006, respectively).
4. Discussion
Among the 13 patients included in the retrospective study, complete resolution was observed in 4 patients and partial resolution was observed in 9 patients after alitretinoin treatment. Most patients experienced symptom improvement during the fourth week of treatment, and alleviation of skin manifestations subsequently occurred. As this was a retrospective study, follow-up data beyond the treatment period were not uniformly available, and some patients were lost to follow-up. Among those who remained under observation, some experienced mild recurrence upon dose reduction. A few patients who discontinued the drug for approximately 3 months also reported recurrence but showed improvement again when treatment was resumed. Despite these fluctuations, no patient experienced a full relapse to the initial disease state, suggesting that alitretinoin may effectively control disease activity over time.
Alitretinoin exerts anti-inflammatory, immunomodulatory, antiproliferative, and antiapoptotic effects [7, 8]. Alitretinoin is a retinoid that may inhibit apolipoprotein E, which has an important role in amyloid formation [10]. Although the effects of alitretinoin on keratinocyte proliferation and differentiation have been established, the detailed mechanism has not yet been elucidated [11].
To analyze the mechanisms underlying the effectiveness of alitretinoin for LA, we investigated the changes in the mRNA levels of mediators involved with itchiness associated with keratinocytes. This was performed to determine whether alitretinoin could regulate itchiness associated with the pathogenesis of LA. This aligns with a previous case report, where alitretinoin was shown to improve pruritus in patients with lichen amyloidosis [6]. These combined results suggest that alitretinoin may effectively target both the inflammatory and pruritic aspects of LA, further supporting its therapeutic potential for this condition. The confirmation of keratinocyte-derived amyloid through CK 5/6 positivity in amyloid deposits, as demonstrated in earlier studies [6], provides a mechanistic explanation for the observed clinical improvements.
IL-31 belongs to the IL-6 family and acts as a pruritogen. Serum IL-31 and IL-33 levels of patients with atopic dermatitis were elevated compared with those of patients in the control group. IL-31 also causes epidermal thickening, impaired skin barrier function, and disrupted mechanical integrity [12]. In addition, IL-33 levels are elevated with atopic dermatitis and chronic pruritus of unknown origin, and it stimulates the secretion of IL-31 [12, 13]. Similarly, keratinocytes from patients with atopic dermatitis exhibited high CCL5 expression, and the injection of CCL5 increased the duration and intensity of itchiness in an itching model [14, 15]. Increased periostin levels have been observed with various pruritic and allergic diseases and are implicated in itchiness through nerve fiber activation and involvement with integrin receptors [16]. PTGES converts prostaglandin H2 to prostaglandin E2, and prostaglandin E2 induces mild pruritus [17, 18].
During this study, although the IL-31 level was not significantly altered, it decreased after treatment with 9-cis-retinoic acid. In contrast, the IL-33, periostin, CCL5, and PTGES levels were significantly decreased. These findings suggest that alitretinoin may effectively treat LA by regulating the various mediators associated with itchiness.
We aimed to identify the characteristics of patients who respond better to alitretinoin but found no significant differences based on itching, medical history, previous therapy, age, or disease duration. Given the small sample size and retrospective nature of this study, larger-scale prospective research is needed to better understand patient responses.
In addition, while 10 out of 13 patients underwent biopsy, and Congo red staining confirmed amyloid deposition in 8 cases, not all patients received histological confirmation. Despite these limitations, the positive clinical response in most patients supports the reliability of the therapeutic outcomes. Future studies incorporating immunohistochemistry could enhance diagnostic accuracy and help identify factors predicting a better response to treatment. Furthermore, prospective research should adopt objective grading methods to improve the assessment of pigmentation and skin roughness, which were not systematically evaluated in this retrospective study.
5. Conclusions
Alitretinoin can effectively control the signs and symptoms of cases unresponsive to antihistamines and topical agents. Furthermore, it can be used for up to 54 weeks without major complications. Alitretinoin may exert its effects on keratinocytes by regulating various mediators involved in itchiness.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Eun Joo Baek and Sun Mee Shin contributed equally to this work.
Funding
This study was supported by Hallym University Research Fund.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in reference number.




