Real-World Impact of Calcipotriol/Betamethasone Dipropionate Aerosol Foam on Quality of Life in Patients With Plaque Psoriasis: A Prospective Observational Study
Abstract
Background and Aim: Quality of life (QoL) of psoriasis patients treated with calcipotriol/betamethasone dipropionate (Cal/BD) foam has not been thoroughly evaluated in real-world settings. This study evaluated the change in plaque psoriasis patients’ QoL after 4 weeks of first treatment with Cal/BD foam and after 6 months under daily practice conditions.
Methods: A prospective, noninterventional study evaluated QoL, treatment adherence, satisfaction, and efficacy through the dermatology life quality index (DLQI), the Morisky-Green scale, the treatment satisfaction questionnaire for medication (TSQM-9), and the change in the body surface area (BSA) with plaque psoriasis, among others.
Results: A total of 172 adult patients with plaque psoriasis were included. After 4 weeks of treatment, 53.5% of patients had a DLQI score ≤ 1. Mean absolute change in the DLQI score from baseline was −4.2 after 4 weeks of treatment and −4.0 after the 6-month follow-up (p < 0.0001). Improvement in the BSA was statistically significant after the first treatment period and after the 6-month follow-up with a mean reduction of 2.4% and 2.6%, respectively (p < 0.0001). Mean absolute change in global satisfaction between the end of the 4-week treatment period and the 6-month follow-up was −4.3 (p = 0.0049). In total, 41% of patients were compliant after the first treatment period, and 55.3% were moderately compliant. Higher patient treatment satisfaction was moderately correlated with lower DLQI scores after 4 weeks (r = −0.527; p < 0.0001). Statistically significant differences between DLQI groups were found in the BSA: patients with DLQI ≤ 1 after 4 weeks of treatment had a lower BSA than patients with DLQI > 1 (1.3 ± 1.8 vs. 2.8 ± 2.7, respectively; p < 0.0001).
Conclusion: After 4 weeks of treatment, daily use of Cal/BD foam in plaque psoriasis patients resulted in an improvement in QoL that was related to an improvement in both satisfaction and efficacy.
1. Introduction
Psoriasis is a chronic and immune-mediated skin disease affecting around 125 million people worldwide [1], with a prevalence of 1.92% in Western Europe [2]. Plaque psoriasis is the most common type (80%–90%) [1, 3]. Despite not being life-threatening, psoriasis has a negative association with quality of life (QoL), and plaque psoriasis may have a disproportionate effect on it [1]. Besides the skin, psoriasis can also have systemic effects and may be associated with comorbidities that substantially diminishes patients’ QOL [1]. Topical therapies are fundamental in the treatment of mild psoriasis. Its success rate reaches approximately 70% in the cases of mild or moderate disease [4]. Topical treatments are also effective as the coadjunctive therapy to systemic treatment in patients with moderate-severe psoriasis [5].
Topical options for adults with psoriasis are as follows: steroids, calcineurin inhibitors, vitamin D analogs, tazarotene, emollients, salicylic acid, anthralin, and coal tar [6]. The efficacy of the combination of calcipotriol, a vitamin D analog, and betamethasone dipropionate (Cal/BD) has been shown in different clinical trials in mild-to-moderate plaque psoriasis [7–9]. It accelerates the onset of action and improves the convenience when compared to other topical treatments [10, 11]. Furthermore, the aerosol foam combination has been proven to have a superior efficacy to both of its active components used separately [6, 12] and to other formulations of Cal/BD [9, 13, 14] for the treatment of plaque psoriasis. Additionally, real-world effectiveness of the two-compound aerosol foam formulation is being assessed in some European countries through observational studies [15]. In fact, the aerosol foam formulation showed high satisfaction with effectiveness and convenience compared with other previous topical treatments in a routine practice setting [16]. However, its real-world impact on plaque psoriasis patients’ QoL has not been evaluated thoroughly, despite the importance of subjective QoL as a standard health outcome measure for chronic diseases [17]. This prospective, noninterventional study aimed to assess the change in plaque psoriasis patients’ QoL after 4 weeks of first treatment with Cal/BD foam and after 6 months under daily practice conditions.
2. Methods
2.1. Study Design
This was a prospective, observational, noninterventional, open-label, multicenter (25 centers in Spain and 9 in Portugal), and single-arm study, conducted from October 2019 to May 2022. The study was carried out in adult patients (≥ 18 years old) with plaque psoriasis who had been prescribed Cal/BD foam (Enstilar, LEO Pharma A/S, Denmark) in monotherapy for the first time and were about to start treatment, who agreed to participate and had read, understood, and signed the informed consent form. The exclusion criteria were as follows: (1) uncontrolled psoriasis vulgaris on the body affecting more than 30% of the body surface area (BSA), (2) Cal/BD foam contraindication use according to current approved conditions, (3) pregnancy or breastfeeding, (4) inability to understand the informed consent form, scales, or study questionnaires, (5) systemic or biologic therapy within 4–16 weeks prior to treatment with Cal/BD, and (6) current or past enrollment in any clinical trial or other observational studies in the last 3 months prior to signing the informed consent form. Data were collected at baseline after the first treatment period (4 weeks) and after the observational period of 6 months.
2.2. Sample Description
The description of the patients at baseline included demographic and anthropometric data, comorbidities, psoriasis diagnosis, previous treatments, global assessments (BSA with plaque psoriasis and severity of psoriasis [PGA and PaGA]), and dermatology QoL, assessed by means of the dermatology life quality index (DLQI).
2.3. Study Endpoints
The primary endpoint was the proportion of patients with DLQI scores ≤ 1 after the 4-week treatment period. Other QoL variables were also evaluated, such as the mean change in the DLQI score between baseline and follow-up visits and the proportion of patients with the DLQI score ≥ 5 at baseline that reached an improvement ≥ 4.5 points and an improvement of ≥ 4 points after the first treatment period with Cal/BD. Additionally, the percentages of patients with an improvement ≥ 4.5 points and ≥ 4 points during the first period and who have maintained it after 6 months were also analyzed.
Secondary endpoints were treatment adherence during the first treatment period (assessed by means of the Morisky-Green scale), patient treatment satisfaction [treatment satisfaction questionnaire for medication (TSQM-9)] after the 4-week treatment period and at the end of the 6-month observational period, and efficacy (mean change in the BSA between baseline and after the first treatment period and after the observational period). Global assessment by means of both physicians and patients scales (PGA and PaGA, respectively) and pruritus assessment through a visual analog scale (VAS) were also carried out and evaluated during follow-up visits, together with overall physician satisfaction. Safety was also evaluated throughout the study.
The TSQM-9 questionnaire, which measures treatment satisfaction, has three domains with three items each (global satisfaction, effectiveness, and convenience), yielding a value ranging from 0 to 100 for each item [18]. For the Morisky-Green scale, an adequate response is the combination of no/yes/no/no for items 1, 2, 3, and 4, classifying the patient as “compliant”; one or two inadequate responses as “moderately compliant”; three or four inadequate responses as “noncompliant” [19]. The PGA and PaGA values ranged from 0 to 4, where 0 is clear and 4 is severe [20, 21], and VAS pruritus comprised values between 0 and 10, where 0 is no itching and 10 is maximum itching [22]. Overall physician satisfaction was assessed with a VAS ranging from 0 to 10, where 0 is very disappointed and 10 is very satisfied.
Moreover, additional analyses were performed to study the relation between QoL and adherence, efficacy, and satisfaction and between adherence and efficacy.
2.4. Statistical Analysis
Analyses were carried out based on the available data, with the only exception of those who attended the visit after the first 4 weeks of treatment with the invalid or incomplete DLQI questionnaire; in this case, last observation carried forward (LOCF) was conducted using baseline values for each patient. Categorical variables were presented by absolute and relative frequencies. Continuous variables were described using mean ± standard deviation (SD). Parametric (paired Student’s t) or nonparametric (Wilcoxon) tests were used for paired comparisons for continuous variables, and McNemar’s test or Bowker’s test of symmetry were used for categorical variables. Comparisons between groups were performed in a continuous variable by means of parametric (Student’s t) or nonparametric (U Mann–Whitney or Kruskal–Wallis) tests, and Chi-square or Fisher exact tests were also used in categorical variables. Statistical significance was considered when p < 0.05. All statistical analyses were performed using the SAS 9.4 software (SAS Institute Inc., Cary NC, USA).
3. Results
3.1. Study Population
A total of 176 adult patients with plaque psoriasis were initially included, of which 172 were evaluable for all analyses by giving the informed consent form, meeting all the inclusion criteria, and completing the DLQI questionnaire at baseline. Of those, 171 and 151 attended visits after the four-week treatment period and at the end of the observational period, respectively, and were evaluated. Patients had a mean age of 51.0 ± 16.1, including 55.8% of male subjects. The mean percentage of the BSA with plaque psoriasis was 4.5 ± 2.8%, the mean PGA score was 2.1 ± 0.7, the mean PaGA score was 2.4 ± 0.8, and the mean VAS pruritus score was 5.6 ± 2.9 (Table 1). Most patients (82.6%) presented mild or moderate PGA scores, and 78.5% had mild or moderate PaGA scores. Other baseline demographic and disease characteristics of the patients are displayed in Table 1. The mean DLQI score at baseline was 7.2 ± 5.5, with most patients reporting psoriasis had a small (33.1%) or moderate (30.8%) effect on their life, and 11.6% of patients had a DLQI score ≤ 1 (Table 2).
| Characteristics | N = 172 |
|---|---|
| Demographic data | |
| Age (years), mean (SD) | 51.0 (16.1) |
| Male, n (%) | 96 (55.8) |
| Female, n (%) | 76 (44.2) |
| Anthropometric data | |
| BMI (kg/m2), mean (SD) | 26.9 (5.0) |
| Overweight (BMI ≥ 25), n (%) | 98 (57.3) |
| Psoriasis diagnosis | |
| Time elapsed since psoriasis diagnosis1 (years), mean (SD) | 11.0 (13.2) |
| Location of psoriasis2, n (%) | |
| Elbows | 96 (55.8) |
| Lower extremities (without knees) | 92 (53.5) |
| Scalp | 63 (36.6) |
| Trunk | 59 (34.3) |
| Upper extremities (without elbows) | 57 (33.1) |
| Knees | 47 (27.3) |
| Back | 29 (16.9) |
| Palms of the hands and soles of the feet | 9 (5.2) |
| Nails | 9 (5.2) |
| BSA with plaque psoriasis (%), mean (SD) | 4.5 (2.8) |
| PGA, mean (SD) | 2.1 (0.7) |
| PaGA, mean (SD) | 2.4 (0.8) |
| Pruritus VAS, mean (SD) | 5.6 (2.9) |
| Concomitant comorbidities associated to psoriasis3, n (%) | 70 (100.0) |
| Hypertension | 42 (60.0) |
| Dyslipidaemia | 22 (31.4) |
| Diabetes | 17 (24.3) |
| Depression | 10 (14.3) |
| Psoriatic arthritis | 7 (10.0) |
| Cardiovascular disease4 | 6 (8.6) |
| Previous treatments for psoriasis5, n (%) | 135 (100.0) |
| Topical | 125 (92.6) |
| Phototherapy | 7 (5.2) |
| Conventional systemic6 | 6 (4.4) |
- Note: PGA, physician global assessment scale.
- Abbreviations: BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; PaGA, patient global assessment scale; QoL, quality of life; SD, standard deviation; VAS, visual analog scale.
- 1Time elapsed between psoriasis diagnosis and baseline visit date.
- 2A single patient might report more than one location of psoriasis.
- 3A single patient might simultaneously have reported more than one comorbidity (n = 70 patients with concomitant comorbidities reported).
- 4n = 1 acute myocardial infarction; n = 1 stroke; n = 2 heart failure; n = 1 transient ischemic attack; n = 1 vascular coronary disease.
- 5A single patient might report more than one previous treatment (n = 135 patients who reported previous treatment for psoriasis).
- 6Per protocol, a patient could not be treated with conventional systemic or biologic treatment within 4–16 weeks prior to treatment with Enstilar.
| Baseline n = 172 | After the first 4 weeks of treatment n = 1721 | After the observational period of 6 months n = 148 | |
|---|---|---|---|
| DLQI score2 | |||
| Mean (SD) | 7.2 (5.5) | 3.0 (4.6) | 3.0 (4.2) |
| 95% CI | (6.4; 8.0) | (2.3; 3.7) | (2.3; 3.7) |
| Median [P25; P75] | 6.0 (3.0; 10.0) | 1.0 (0.0; 4.0) | 2.0 (0.0; 4.0) |
| DLQI cut-off point, n (%) | |||
| No effect at all on patient’s life (0-1) | 20 (11.6) | 92 (53.5) | 72 (48.6) |
| Small effect on patient’s life (2–5) | 57 (33.1) | 48 (27.9) | 51 (34.5) |
| Moderate effect on patient’s life (6–10) | 53 (30.8) | 22 (12.8) | 15 (10.1) |
| Very large effect on patient’s life (11–20) | 36 (20.9) | 6 (3.5) | 9 (6.1) |
| Extremely large effect on patient’s life (21–30) | 6 (3.5) | 4 (2.3) | 1 (0.7) |
| DLQI ≤ 1, n (%) | |||
| Score ≤ 1 | 20 (11.6) | 92 (53.5) | 72 (48.6) |
| Score > 1 | 152 (88.4) | 80 (46.5) | 76 (51.4) |
- Abbreviations: DLQI, dermatology life quality index; LOCF, last observation carried forward; SD, standard deviation.
- 1LOCF procedure was applied in n = 6 patients without data in the DLQI after the first 4-week treatment period.
- 2Range between 0 and 30, where the higher the score, the more quality of life is impaired.
For the first treatment period of 4 weeks, the patients used the foam once daily, following the authorized prescribing information [23]. A total of 97.1% of the subjects completed the initial 4 weeks of treatment with Cal/BD, with a mean duration of 30.2 ± 3.2 days. Afterward, 77 patients kept using it as maintenance: the majority used the treatment twice weekly in not consecutive days (58.4%), 28.6% kept using it once a day, and 13.0% used the treatment in different schedules. The mean duration of treatment was 115.1 ± 67.3 days for those who reported the end of the maintenance therapy.
3.2. Primary Endpoint
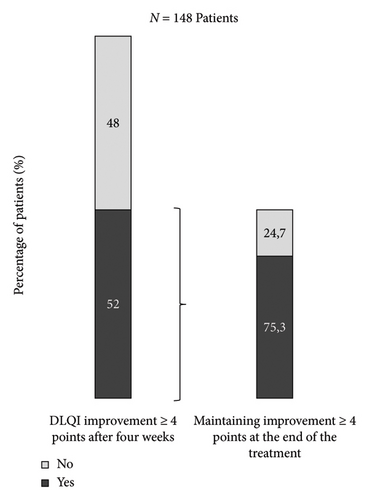
After the first 4 weeks of treatment with Enstilar, 53.5% of patients had reached a DLQI score ≤ 1 (Table 2). Of all patients who had a DLQI score ≤ 1 at baseline, 19 (95.0%) maintained a score ≤ 1 after the first four weeks of treatment, and 73 patients (48.0%) with a score > 1 at baseline had a score ≤ 1 after this period (p < 0.0001). Mean absolute change in the DLQI score was −4.2 between baseline and the end of the first 4-week treatment period and −4.0 between baseline and the end of the 6-month observational period (p < 0.0001 in both cases). Moreover, 72 patients (65.5%) who had a DLQI score ≥ 5 at baseline had an improvement of ≥ 4.5 points at the end of the first four-week treatment period. That figure was 74.5% for subjects whose improvement was of ≥ 4 points after 4 weeks. Finally, of all patients with a DLQI improvement ≥ 4 points at the end of the first 4 week, 75.3% maintained the same result at the end of the study (Figure 1).
3.3. Secondary Endpoints
The mean BSA with plaque psoriasis was 4.5 ± 2.8% (median [P25; P75]; 4.0 [2.0; 6.0]), 2.0 ± 2.4% (median [P25; P75]; 1.0 [0.0; 3.0]), and 2.1 ± 2.8% (median [P25; P75]; 1.0 [0.0; 3.0]) at baseline, after the first four weeks of treatment and at the end of the six-month observational period, respectively. The improvement of the BSA compared to baseline was statistically significant after the first treatment period with Cal/BD foam and after 6 months of the observational period with a mean reduction of 2.4 and 2.6, respectively (p < 0.0001).
The mean PGA score at baseline was 2.4 (0.8) and decreased to 1.1 ± 0.9 and 1.0 ± 0.9 points after the first 4-week treatment and at the end of the observational period, respectively. These values were 2.4 (0.8) at baseline and 1.4 ± 1.0 and 1.3 ± 1.0 at follow-up visits for the mean PaGA score. A total of 68.3% and 73.2% of subjects reported PGA scores ≤ 1, respectively. Those proportions are 55.7% and 61.4% for PaGA scores. As for the pruritus VAS, the score at baseline was 5.6 ± 2.9 (median [P25; P75]; 6.0 [3.5; 8.0]) and decreased to 2.5 ± 3.0 (median [P25; P75]; 1.0 [0.0; 4.0]) and 2.6 ± 2.7 (median [P25; P75]; 2.0 [0.0; 4.0]) after the first four-week treatment and at the end of the observational period, respectively, yielding a mean reduction of 3.1 ± 3.2 and 3.0 ± 3.2 compared to baseline (p < 0.0001 in both cases).
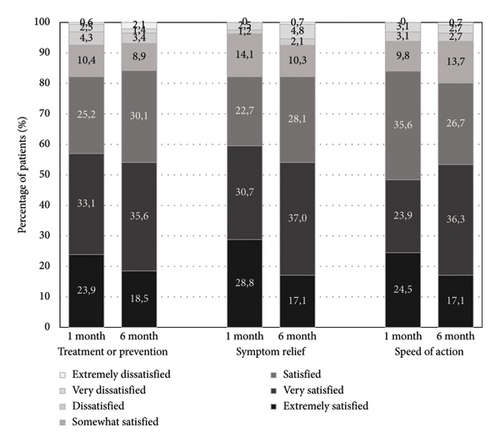
The TQSM-9 global satisfaction score was 78.3 ± 17.7 after 4 weeks of treatment and 74.0 ± 19.5 at the end of the observational period, with a mean absolute change of −4.3 (p = 0.0049). At 4 weeks of treatment, 85.3% of the patients were satisfied, and this satisfaction rate remained at 6 months (86.3%). The detailed data of effectiveness, convenience, and global satisfaction are depicted in Figure 2. As for overall physician satisfaction, VAS mean scores in follow-up visits were 8.1 ± 2.1 and 7.8 ± 2.3, respectively.
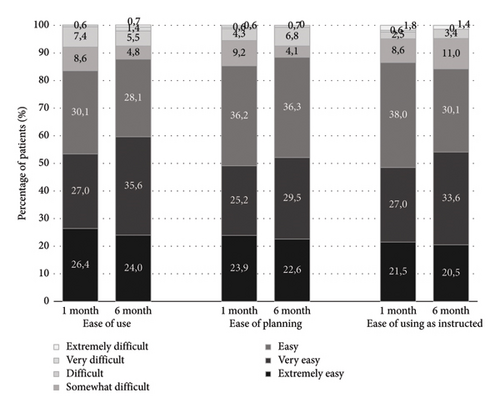
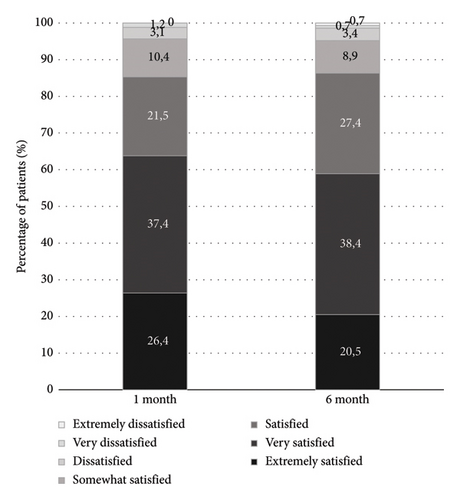
Out of 161 patients who completed the compliance Morisky-Green questionnaire, 155 (96.3%) were at least moderately compliant (66 patients were compliant and 89 patients were moderately compliant), while only 6 (3.7%) were noncompliant.
3.4. Factors Related With the Achievement of the DLQI and Adherence
Statistically significant differences between DLQI groups were found in % BSA after the initial 4 weeks of treatment: patients with DLQI ≤ 1 had lower % BSA in plaque psoriasis than patients with DLQI > 1 (1.3 ± 1.8 vs. 2.8 ± 2.7, respectively; p < 0.0001). The BSA with plaque psoriasis at baseline was significantly related with the achievement of DLQI ≤ 1 after the first 4 weeks of treatment (p = 0.0362); the mean value was 3.9 ± 2.0% and 5.1 ± 3.4% for subjects who achieved and did not achieve a DLQI score ≤ 1, respectively. A negative relation was found between the TSQM-9 global satisfaction score and the DLQI score at the first visit (r = −0.527; p < 0.0001) in a way that higher patient treatment satisfaction was moderately correlated with lower DLQI scores after 4 weeks. Moreover, statistically significant differences were found in the TQSM-9 global satisfaction score between DLQI groups (85.6 ± 12.1 for DLQI ≤ 1 and 64.9 ± 20.7 for DLQI > 1; p < 0.0001).
After the first 4-week treatment period, there were statistically significant differences referred to the moderately compliant group in the DLQI score (1.8 ± 3.0, 3.5 ± 5.1, and 1.5 ± 1.9 for compliant, moderately compliant, and noncompliant patients, respectively; p = 0.0201) and DLQI groups (score ≤ 1 or > 1) between treatment adherence groups (66.7%, 47.2%, and 66.7% for compliant, moderately compliant, and noncompliant patients with the DLQI score ≤ 1, respectively; p = 0.0468). However, no statistical differences were found between adherence groups in absolute DLQI score change (p = 0.6569), the smallest absolute change being observed for the noncompliant group. Statistically significant differences between treatment adherence groups have been observed in the BSA at baseline and after the observational period. Noncompliant patients had a higher BSA at baseline (4.5 ± 2.8%, 4.3 ± 2.6%, and 8.0 ± 4.0% for compliant, moderately compliant, and noncompliant patients, respectively; p = 0.0364) and also after 6 months of the observational period (1.8 ± 1.9%, 2.2 ± 3.3%, and 4.3 ± 3.0%, respectively; p = 0.0356); the same tendency has been observed after the 4-week treatment period (2.1 ± 2.7%, 1.9 ± 2.2%, and 3.7 ± 2.4%, respectively) but not statistically significant (p = 0.1048).
3.5. Safety
A total of three patients reported three adverse events (AEs) (rib fracture, spinal osteoarthritis, and psoriasis worsening), none of which were serious. All AEs reported were CTC grade 2 of moderate severity and nonrelated with the study treatment. No action was required for any of them.
4. Discussion
Despite the non-negligible impact of plaque psoriasis on QoL [1], the effects of Cal/BD on patients’ QoL have not been thoroughly studied in routine clinical practice. Our results analyzed the impact of Cal/BD foam on QoL in a real-life context in detail for the first time. Health-related QoL was assessed in the clinical study setting of the randomized, double-blind, phase III PSO-FAST study in patients with psoriasis vulgaris. After 4 weeks of treatment, there was a greater improvement from baseline in the DLQI score for Cal/BD foam versus vehicle (−7.0 vs. −4.4; p < 0.001). A total of 48.1% of patients using the foam reported no effect of psoriasis on their lives after 4 weeks of treatment, and 81.2% of subjects who used Cal/BD with a baseline score ≥ 5 achieved a ≥ 5-point improvement [8]. In our study, at 4 weeks, approximately half of the patients had reached a DLQI score ≤ 1, which is in accordance with the aforementioned phase III study. Moreover, we found that 65.5% of patients with an initial DLQI score ≥ 5 had an improvement of ≥ 4.5 points at 4 weeks, a smaller figure than the one obtained in the PSO-FAST study under strict conditions. [8]. The literature available at the time of the elaboration of the study protocol and therefore the ≥ 4.5-point improvement limit is currently outdated. When the prevailing minimal clinically important difference (MCID) of ≥ 4-point improvement in the DLQI score was considered [24], the figure was higher (74.5%). In a daily use evaluation by Navarro-Triviño, Lozano-Lozano, and Ruiz-Villaverde, the mean DLQI score was 10.67 ± 4.96, 2.41 ± 3.87, and 2.24 ± 5.25 at baseline, week 4, and week 12, respectively. However, no changes in the DLQI were evaluated [25]. In a real-life study in Austria, the DLQI significantly decreased by 5.38 points (p < 0.001) from an average of 7.81 ± 6.14 to 2.43 ± 2.91 points after 4 weeks of therapy with Cal/BD aerosol foam, with 50.56% of the patients presenting a DLQI score ≤ 1 [21]. Similarly, in our study, 53.5% of patients had a DLQI score ≤ 1 after the first 4 weeks of treatment.
Other parameters such as satisfaction and adherence have been previously assessed in real-world settings. Velasco et al. studied both patient (TQSM-9) and physician (5-point Likert scale) satisfaction. As for patients, 85% were satisfied, very satisfied, or extremely satisfied with Cal/BD treatment [16]. In another study, 100% and 73.8% of patients showed high adherence (Morisky-Green-Levine scale) at week 4 and 12, respectively. A significant relationship between adherence and efficacy and significant intergroup differences in favor of high adherence were also found [25]. Conversely, in our hands, no differences between treatment adherence groups and efficacy (% BSA) were found after the first 4-week treatment period. The sample size of the noncompliant group (n = 6) was very low compared to the other groups, which could explain statistical significance not being reached. Importantly, our results showed a statistically significant relation between QoL and global treatment satisfaction and QoL and efficacy (% BSA): after four weeks of treatment with Cal/BD, patients who reached lower DLQI scores were more satisfied with the therapy, and patients with the DLQI ≤ 1 presented a smaller % BSA with plaque psoriasis than patients with the DLQI > 1. As for satisfaction and QoL, our results are in disagreement with a previous study carried out in 41 patients that failed to demonstrate an association between QoL and treatment satisfaction (p = 0.051), despite 87.7% of the patients declaring to be satisfied with their psoriasis treatment and 80.5% referring a good QoL [26]. Regarding efficacy and QoL, our results seem in accordance with the ones of a cross-sectional questionnaire survey of 200 patients with moderate to severe psoriasis. That study found a strong correlation between the DLQI and the psoriasis area and severity index (PASI) (r = 0.81, p < 0.05) [27]. Furthermore, in our study, there was an improvement in severity of psoriasis according to both physicians’ and patients’ global assessment. While at baseline only 15.1% and 10.5% of PGA and PaGA scores were ≤ 1, those figures were of 68.3% and 55.7% after the initial 4 weeks of treatment and 73.2% and 61.4% after the 6-month observational period. This improvement was accompanied by a decrease in pruritus (p < 0.0001). Therefore, treatment with Cal/BD showed consistent efficacy in improving severity of psoriasis by means of four different variables: % BSA, PGA, PaGA, and pruritus VAS. Finally, physician treatment satisfaction was around 8/10 points in both follow-up visits, which is in accordance with a previous study where physicians were very satisfied with the Cal/BD aerosol foam therapy in patients with psoriasis [16].
The observational nature of the study comprises some limitations: the usual clinical practice possible biases and that no causal interpretations can be made from the study due to its design. Moreover, the DLQI endpoints were based on pre- and post-treatment assessments, which allow temporal bias to take place, for example, seasonal variations. However, the Mediterranean weather is not associated with strong seasonal variations. Thus, they were not expected throughout the study. Restrictions on visits due to the COVID-19 pandemic, which took place during the course of the study, have hindered both the selection of patients and the follow-up period. Finally, although the initial sample size planned was 330 subjects and despite the extension of the recruitment period, a total of 176 patients were initially included in the study. Consequently, some patient profiles, particularly those with the BSA between 15% and 30%, were underrepresented. The study sample size was limited, especially for the analyses related to compliance groups, since the noncompliant group included only six patients, which was very low compared to the other groups. Thus, further studies with a higher number of participants are needed.
5. Conclusion
This study evaluated in daily practice the impact of Cal/BD foam on plaque psoriasis patients’ QoL, resulting in an improvement in QoL that was related with an improvement in both treatment satisfaction and efficacy.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki. Procedures were approved by the local Ethics Committee of each center, and all patients signed an appropriate informed consent.
Conflicts of Interest
Antonio Martorell has received honoraria and/or travel grants and/or has acted as an advisory board member for Novartis, AbbVie, Janssen Cilag, UCB, Lilly, LEO Pharma, L’Oréal, Sanofi, Boehringer Ingelheim, Almirall, Bristol Myers Squib, and Amgen. He has also worked as a principal investigator in clinical trials supported by AbbVie, UCB, Jansen, Bristol Myers Squibb, Lilly, Galderma, Sanofi, and Novartis.
Miquel Ribera has been a speaker, consultant, and investigator for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gebro Pharma, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Pierre-Fabre, Sandoz, and UCB, all of which make psoriasis drugs.
Tiago Torres, Paulo Leal Filipe, Caridad Soria, Fernando Mota, José Pardo, and Ricardo Ruiz-Villaverde have received honoraria from LEO Pharma.
Funding
This study was funded by LEO Pharma SA.
Open Research
Data Availability Statement
The data presented in this study are available on request from the corresponding author.




