Noncoding RNAs in Atopic Dermatitis: Insight Into Inflammation and Immune Regulation
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting approximately 20% of children and 10% of adults. While previous studies have linked AD to allergen exposure, disruption of the skin barrier, and Type 2 immune responses, the precise pathophysiology of AD remains elusive, significantly limiting the effectiveness of current treatments. Noncoding RNAs (ncRNAs), a diverse group of transcripts that do not encode proteins and account for at least 98% of the human genome, are implicated in numerous physiological and pathological processes. A growing body of evidence underscores the pivotal role of ncRNAs in the pathogenesis and progression of AD. This review offers a detailed synthesis of the latest insights into the involvement of ncRNAs in AD, as well as their potential as diagnostic biomarkers and therapeutic targets.
1. Introduction
Atopic dermatitis (AD) is a prevalent inflammatory skin disorder, often emerging in childhood and persisting into adulthood. It commonly manifests as recurring, itchy eczema, with symptoms that may fluctuate seasonally. A significant proportion of patients with AD also suffer from comorbid allergic conditions such as allergic asthma, rhinoconjunctivitis, and food allergies [1]. Recent years have seen a rise in both the prevalence and incidence of AD, which now affects approximately 20% of children and 10% of adults, making it one of the most prevalent nonfatal diseases and the skin condition with the highest disability-adjusted life year burden [2]. The economic impact is substantial, with higher healthcare utilization and treatment costs reported among patients with AD compared to non-AD individuals. Despite ongoing research efforts, the understanding of AD’s pathogenesis remains incomplete, limiting advances in treatment strategies [3].
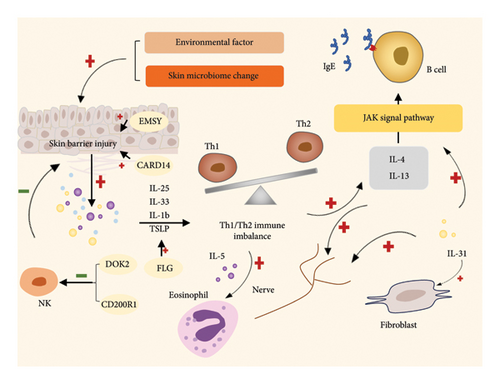
The etiology of AD is complex and not fully elucidated. Individuals with AD are prone to developing various allergies. There are two ways of pathogenesis of AD: outside-in and inside-out theory. External causes include skin barrier, microbiome and ecological imbalance, immunogen, nutrition and environmental pollution, etc. These external factors disrupt the homeostasis of the skin microenvironment and cause disease. Internal etiology includes keratinocytes, microvascular system, humoral factors, cellular factors, skin-enteric-lung epithelial permeability, neurosensory mechanisms, and epigenetics. Internal homeostasis changes can also be manifested through the skin as various symptoms of AD [4]. But the reality is that both mechanisms are often present at the same time, and immune activation seems to play an important role in both internal and external factors. The skin barrier dysfunction allows allergens to penetrate the skin, triggering immune responses primarily mediated by CD4+ helper T2 (Th2) cells, which promote B-cell antibody production. Experimental studies in mice have shown that exposure to specific antigens can induce AD-like conditions, mirroring the clinical and immunological features observed in humans [5]. The FLG gene, which encodes filaggrin—a key structural protein in the epidermis—plays a vital role in maintaining skin integrity. Research has linked FLG downregulation or inactivation to a heightened risk of developing AD [6]. Beyond FLG, over 30 genetic loci have been associated with AD susceptibility, involving disruptions in epidermal barrier function, immune regulation, and skin microbiome composition [7]. Key genes implicated include EMSY, docking protein 2 (DOK2), CD200 receptor 1 (CD200R1), and caspase recruitment domain–containing protein 14 (CARD14) [8–10]. AD is believed to result from a combination of barrier dysfunction, immune activation, and microbiome alterations, all underpinned by genetic and environmental factors. In the early stages of AD, the breakdown of the epidermal barrier and activation of inflammatory dendritic cells and innate lymphoid cells trigger inflammation. Inactivation of FLG can induce thymic stromal lymphopoietin (TSLP) expression, promoting Th2 activation and leading to a Th1/Th2 imbalance [11]. Activated T cells secrete cytokines such as IL-4, IL-13, and IL-31, which activate the JAK pathway, inducing inflammation and promoting the synthesis of antigen-specific IgE. Eosinophils and fibroblasts can also be regulated to influence AD progression [12–14]. Even IL-4, 13, 31 can act on the skin neural network to affect neural regulation [15]. Overexpression of EMSY is linked to reduced levels of long-chain ceramides and compromised cell–cell adhesion [8]. Mutations in DOK2 and CD200R1 may influence ERK signaling downstream of toll-like receptor 4 and affect natural killer (NK) cell maturation, which can protect the skin barrier [9]. Loss-of-function mutations in CARD14 are associated with a severe AD variant characterized by impaired epidermal secretion of antimicrobial peptides and hCCL20 [10] (Figure 1). These immunological mechanisms exacerbate skin barrier dysfunction, contributing to the chronicity of AD lesions.
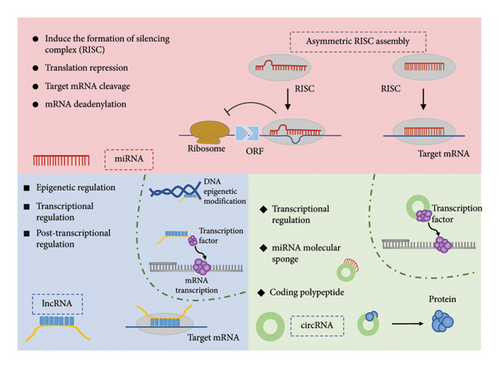
Noncoding RNAs (ncRNAs), a diverse group of transcripts that do not encode proteins, make up a substantial portion of the genome, accounting for at least 98% of its content. Previously dismissed as by-products of extensive transcription—referred to as “junk” or “dark matter” on the genome—ncRNAs were once believed to hold little biological significance [16]. However, recent research has illuminated their critical roles as regulators of heredity, epigenetic inheritance, and translation (Figure 2). To better understand these functions, ncRNAs are classified into three main categories: long noncoding RNAs (lncRNAs), which are over 200 nucleotides in length; small ncRNAs (sncRNAs), which are under 200 nucleotides; and circular RNAs (circRNAs). sncRNAs are further divided into microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), and transfer RNA–derived small RNAs (tsRNAs), based on their structural and functional attributes. NcRNAs perform a wide range of functions by interacting with proteins, DNA, and RNA, thereby playing a pivotal role in gene regulation, RNA processing, and posttranscriptional modifications. Significantly, ncRNAs are not isolated in their functions; they form complex regulatory networks [17]. Kleaveland et al. highlighted one such network involving four types of ncRNAs—comprising one lncRNA, one circRNA, and two miRNAs—shedding light on the intricate interconnections within the ncRNA landscape [18]. Additionally, recent findings suggest that RNA modifications are involved in immune infiltration and regulation [19–21]. NcRNAs have emerged as pivotal molecules in modulating skin inflammation and immune signaling [22]. In particular, specific ncRNAs are heavily implicated in skin diseases like psoriasis and AD, where they govern the inflammatory response and immune activity. Understanding the mechanisms by which ncRNAs influence these processes will be instrumental in slowing the progression of AD and paving the way for novel therapeutic approaches.
2. Functional Significance of Specific ncRNAs in AD
NcRNA networks are essential regulators of various physiological processes, including gene transcription, mRNA translation, and protein modification. These discoveries have unveiled new research avenues in the complex and fascinating realm of ncRNAs, with their potential implications for human health and disease just starting to be uncovered [23, 24]. Some ncRNAs are linked to specific diseases, while others exert broader effects by interacting with multiple ncRNAs [25]. Notably, ncRNAs have been identified as key molecules governing inflammatory signaling in the skin [26]. Unraveling the specific mechanisms by which ncRNAs contribute to conditions such as AD could significantly advance the development of diagnostic tools and therapeutic strategies.
2.1. miRNAs in AD
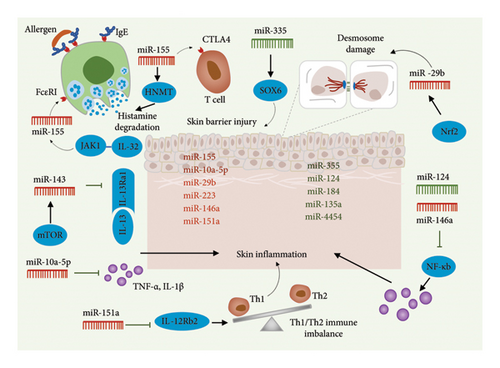
In 1993, scientists identified miRNAs, short RNA molecules consisting of 19–25 nucleotides. Although miRNAs are not translated into proteins, they play a pivotal role in regulating gene expression posttranscription [27, 28]. By binding to specific mRNA sequences, miRNAs guide RNA-induced silencing complexes to their target sites, leading to mRNA degradation or translation inhibition. miR-203 initially showed a highly skin-specific expression profile, and upregulation of miR-203 can regulate inflammation and keratinocyte function and participate in the pathogenesis of psoriasis [29]. Altered miRNA expression has been observed in various diseases, including inflammatory and immune-related skin disorders [30]. This finding presents novel opportunities for comprehending the development of these diseases, identifying biomarkers, and devising therapeutic strategies [31]. Recent studies have highlighted the significant role of miRNAs in the pathogenesis of AD. These studies show that miRNAs influence AD progression by inhibiting specific proinflammatory transcripts and suppressing signal transducers of the nuclear factor kappa-B (NF-κB) pathway. Table 1 summarizes the functions of upregulated and downregulated miRNAs in AD (Figure 3).
| ncRNA | Function in AD | Expression | Target gene |
|---|---|---|---|
| miRNA | |||
| miR-155 | Affect T-cell activation | Upregulated | CTLA-4 |
| miR-10a-5p | Anti-inflammatory effect | Upregulated | HAS3 |
| miR-29b | Affect desmosome function | Upregulated | Desmosome |
| miR-223 | Regulate the function of Treg cell | Upregulated | HNMT |
| miR-146a | Anti-inflammatory effect | Upregulated | NF-κB |
| MiR-143 | Maintain epidermal barrier function | Upregulated | Il-13 |
| miR-355 | Maintain epidermal barrier function | Downregulated | SOX6 |
| miR-124 | Anti-inflammatory effect | Downregulated | NF-κB |
| lncRNA | |||
| MALAT1 | Anti-inflammatory effect; affect keratinocyte differentiation | Upregulated | SIRT1 |
| GAS5 | Maintain epidermal barrier function | Upregulated | c-Myc |
| NEAT1 | Stimulate the inflammasome | Upregulated | NLRP3 |
| H19 | Maintain epidermal barrier function | Downregulated | CYP1AY |
| circRNA | |||
| circ004287 | Stimulate the inflammasome | Upregulated | NLRP3 |
2.1.1. miR-10a-5p
One such miRNA, miR-10a-5p, is associated with inflammation and is regulated by IL-6 [32]. miR-10a-5p has been shown to play a critical role in immune invasion across various cancers, regulating cancer cell proliferation, invasiveness, and the inflammatory response in endothelial cells. Its upregulation significantly enhances the proliferative and migratory capabilities of endothelial cells [33]. Vaher et al. reported a notable upregulation of miR-10a-5p in both cutaneous and noncutaneous lesions of patients with AD compared to controls [34]. miR-10a-5p exerts a beneficial regulatory effect on inflammation by suppressing TNF-α and IL-1 mRNA levels while simultaneously promoting the upregulation of transforming growth factor-b1 (TGFb1) mRNA and protein, as well as IL-3 protein [35]. Similarly, miR-10a-5p, which is overexpressed in AD, positively influences the regulation of inflammation. Transfection with miR-10a-5p results in a significant reduction in the proportion of human primary keratinocytes in the S-phase and diminishes the effects of IL-1 on genes involved in cell cycle regulation and cytokine signaling. Additionally, miR-10a-5p directly targets hyaluronate synthetase 3 (HAS3), a protein linked to injury that regulates keratinocyte proliferation and migration [34]. Therefore, miR-10a-5p may have a favorable anti-inflammatory effect in AD and could also inhibit keratinocyte proliferation, which is essential for maintaining the integrity of the skin barrier (Figure 3).
2.1.2. miR-29b
miR-29b is a key miRNA that significantly influences keratinocyte function. Gu et al. demonstrated that miR-29b levels were elevated in the epidermis and serum of patients with AD compared to healthy individuals, with serum miR-29b levels correlating with SCORAD, a clinical index used to assess AD severity [36]. Nrf2, a novel regulator of desmosomes in the epidermis, targets miR-29b, thereby affecting desmosome function. Overexpression of miR-29b, driven by the activation of the Nrf2-miR-29b pathway, impairs the formation of hyper-adherent desmosomes in keratinocytes, consequently compromising the skin’s defense and barrier systems [37]. Interestingly, miR-29b exerts a contrasting effect on tight junctions as opposed to desmosomes. Yuan et al. observed that introducing miR-29b-3p mimics into keratinocytes activated MMP-2 and MMP-9, which had been induced by nano toxins. Additionally, the introduction of these mimics reversed the downregulation of tight junction-related proteins [38]. miR-29b expression has also been linked to various cellular processes in epidermal cells, including proliferation, differentiation, apoptosis, and senescence. Notably, the expression of hsa-miR-29b-3p was significantly lower in human epidermal stem cells (ESCs) compared to differentiated keratinocytes [39]. This suggests that the abnormal upregulation of miR-29b may contribute to epithelial barrier dysfunction in AD (Figure 3).
2.1.3. miR-124
Existing studies have also implicated miR-124 in skin inflammation [40], a condition associated with the apoptosis of epidermal cells [41]. Yang et al. reported downregulation of miR-124 in chronic AD lesions, whereas the mRNA expressions of p65, IL8, CCL5, and CCL8 were upregulated in these lesions, showing a negative correlation with miR-124 levels [42]. miR-124 is known to directly target NF-κB in lymphomas, inhibiting the expression of p65, a key component of NF-κB involved in regulating inflammatory processes and immune responses [43]. P65 significantly upregulates IL8, CCL5, and CCL8, but miR-124 partially counteracts this effect. These findings suggest that miR-124 plays a critical role in controlling the NF-κB-dependent inflammatory response in keratinocytes and chronic skin inflammation. Consequently, restoring miR-124 expression presents a promising therapeutic strategy for treating AD (Figure 3).
2.1.4. miR-143
miR-143 is an miRNA highly specific to epithelial tissues, playing a critical role in regulating the phenotype of vascular cells, thus contributing to their differentiation and the progression of related diseases [44]. In keratinocytes at the site of skin lesions, the upregulation of miR-143 has been shown to inhibit local skin inflammation and promote wound healing [45]. IL-13, a cytokine associated with T helper cell 2 responses, impairs normal epidermal barrier function [46]. Jia and Zeng demonstrated that the upregulation of miR-143 counteracted IL-13-induced downregulation of epidermal barrier-related proteins in keratinocytes [47]. Moreover, the administration of rapamycin prevented the IL-13-induced downregulation of miR-143 in epidermal keratinocytes, thereby inhibiting IL-13R1 expression. This indicates the significant role of the mTOR-miR-143 signaling pathway in the pathogenesis of AD. Additionally, the JAK2 inhibitor CYT387 has been found to protect the epidermal barrier. Zu et al. revealed that miR-143 directly binds to the 3′-UTR and STAT3 of IL-13R1, exhibiting a synergistic protective effect with CYT387 [48]. By specifically targeting IL-13Ra1 in keratinocytes, miR-143 has the potential to reduce IL-13 activity and alleviate the associated inflammatory response. Thus, miR-143 emerges as a promising therapeutic target for AD treatment (Figure 3).
2.1.5. miR-146a
miR-146a is an miRNA known for its anti-inflammatory properties, particularly through the inhibition of multiple components of the NF-κB pathway in various cell types, including keratinocytes [49]. Previous research has highlighted the vital role of miR-146a in several inflammatory skin conditions, such as acne and psoriasis [50]. Deletion of the miR-146a gene leads to early onset of skin inflammation, increased expression of IL-17-induced inflammatory mediators from keratinocytes, epidermal hyperplasia, and enhanced neutrophil infiltration. Conversely, the introduction of miR-146a mimics has been shown to alleviate these symptoms [51]. In patients with AD, miR-146a is upregulated in both keratinocytes and affected skin. Transfection with miR-146a has been found to reduce several proinflammatory factors, including CCL5 and CCL8, which are associated with AD and induced by interferon, in both keratinocytes and AD mouse models. Conversely, suppression of miR-146a leads to an upregulation of proinflammatory molecules in keratinocytes [52]. Similarly, miR-146a-deficient mice display exacerbated inflammatory responses, characterized by increased cell infiltration in the dermis and elevated expression of inflammatory molecules in the skin (Figure 3).
2.1.6. miR-155
The upregulation of miR-155 has been documented in various malignancies and inflammatory diseases, including B-cell lymphoma and AD, where it influences both innate and adaptive immune responses [53]. miR-155 plays a pivotal role in autoimmune diseases, particularly in the differentiation of Th17 cells. Sonkoly et al. demonstrated that miR-155 is significantly upregulated in patients with AD, with its expression primarily observed in immune cells infiltrating the affected areas, especially during T-cell activation. Additionally, miR-155 expression is induced in peripheral blood mononuclear cells and epidermal allergen cells by T cell activators. A direct target of miR-155 is CTLA-4, a critical negative regulator of T-cell activation. Overexpression of miR-155 results in decreased CTLA-4 levels in T helper cells, leading to increased T helper cell proliferation, suggesting that the abnormal upregulation of miR-155 may contribute to chronic cutaneous inflammation by targeting CTLA-4 [54]. Chang et al. confirmed the overexpression of miR-155 in patients with AD and established a positive correlation between miR-155 levels and AD severity. Their research also revealed that JAK1 promotes miR-155 expression, further driving the development of AD [55]. Similarly, Ma et al. found that miR-155 exacerbates AD severity and increases the proportion of Th17 cells. Conversely, they observed a negative correlation between miR-155 expression and the expression and plasma concentration of cytokine signaling inhibitor 1 mRNA in both plasma and skin lesions of patients with AD [56]. These findings suggest that miR-155 overexpression may contribute to AD pathogenesis by influencing Th17 cell differentiation and function. However, a study involving pediatric patients with AD and healthy controls found no significant correlation between miR-155 and the Th17/Treg ratio, though increased responses to miR-155, FOXP3, and ROR were noted, indicating a potential link to immune dysregulation in AD [57]. Research by Mohammed highlighted miR-155’s role in regulating mast cell function. miR-155 targets prostaglandin and cytokine pathways, enhancing mast cell degranulation in FcεRI-induced mast cells [58]. miR-155-5p directly targets protein kinase inhibitor α (PKIα), showing a negative correlation with PKIα levels. Fluorescence in situ hybridization revealed that most cells expressing miR-155-5p are located in the epidermis. In these epithelial cells, PKIα expression was significantly suppressed, leading to reduced epidermal hypertrophy, inflammatory cell infiltration, and Th2 cytokine secretion in AD models [59]. These findings suggest that miR-155 plays a key role in regulating cytokine responses and maintaining cutaneous barrier function, thereby contributing to AD development. Traditional treatments, such as Pseudaspartate B, inhibit IL-17-induced inflammation by preventing miR-155 phosphorylation, improving AD-like skin lesions via the PPARγ pathway (Figure 3).
2.1.7. miR-223
miR-223 is an miRNA implicated in the regulation of inflammation, particularly in adipose tissue, and has been linked to conditions such as nonalcoholic fatty liver disease [60] and atherosclerosis [61]. A study by Jia et al. revealed a significant increase in miR-223 levels in the whole blood of individuals diagnosed with AD. The study also observed a concurrent rise in histamine-N-methyltransferase (HNMT), an enzyme essential for histamine degradation, in both patients with AD and AD model mice. This suggests that miR-223 may contribute to the degradation of excess histamine in AD by upregulating HNMT [62]. Moreover, miR-223 has been associated with cutaneous diseases related to smoking. Herberth et al. explored the relationship between perinatal exposure to tobacco smoke, miRNA expression, and regulatory T cell (Treg) counts. They identified a positive correlation between miR-223 expression in maternal and umbilical cord blood and cotinine levels in maternal urine [63]. Additionally, maternal miR-223 expression was linked to ambient benzene concentrations. Elevated miR-223 levels in both maternal and umbilical cord blood were associated with decreased Treg cell counts. This decrease in Treg cells during infancy has been linked to an increased risk of developing AD within the first 3 years of life. Therefore, perinatal maternal smoking may elevate miR-223 levels, which subsequently influences Treg cell counts in cord blood, potentially increasing the risk of AD (Figure 3).
2.1.8. Others
With advancements in transcription detection technology, the ability to identify various miRNAs has significantly improved, revealing their potential impact on differential expression in AD. miR-151a, a member of the miR-28 family located on chromosome 8q within the host gene adhesion kinase, has been identified as significantly elevated in the plasma of patients with AD compared to healthy controls [64]. miR-151a plays a role in immune regulation, particularly in the modulation of Th1/Th2 phenotypes [65]. Overexpression of miR-151a in human T helper cells results in a marked decrease in the expression of IL12RB2, a gene associated with AD. Another miRNA of interest, miR-335, is recognized for its role in promoting keratinocyte differentiation. This miRNA is consistently downregulated in patients with AD, which directly affects the inhibition of SOX6—a protein that impedes epidermal differentiation by recruiting various components of the SMARCA complex and epigenetically suppressing key genes involved in keratinocyte differentiation. In AD-affected epidermis, miR-335 is notably absent, while SOX6 is aberrantly expressed, contributing to the compromised skin barrier. Research has demonstrated that miR-335 expression is regulated by histone deacetylase through epigenetic mechanisms, and belinostat has emerged as a promising therapeutic candidate for restoring miR-335 expression and improving the impaired skin barrier in patients with AD [66]. The involvement of the miR-221/PI3K/Akt/Ca2+ pathway in mast cell degranulation has also been identified, suggesting its potential role in AD pathogenesis [67]. In immune-related diseases, it has been observed that IgG in adult patients with AD influences the maturation of nonatopic neonatal thymus IL-22-producing T cells and CLA+ T cells through miRNA regulation. Specifically, 10 mature miRNAs, including miR-181b-5p, hsa-miR-130b-3p, and hsa-miR-26a-5p, are modulated by AD-associated IgG [68]. Additionally, Gu et al. reported numerous abnormally regulated miRNAs in AD skin biopsies, such as upregulated miR-4270, miR-211, miR-4529-3p, and miR-29b, alongside downregulated miR-184, miR-135a, and miR-4454 [36]. Notably, the upregulation of a specific miRNA in AD tissue does not necessarily imply a pathogenic role in the disease’s development.
Recent advances in map analysis and functional studies have elucidated the role of miRNAs in AD [69], linking their dysregulation to skin barrier dysfunction, cytokine signaling abnormalities, NF-κB-dependent inflammatory responses, and the activity of Th17 and Treg cells. As statistics and bioinformatics continue to evolve, new prediction models for miRNA-disease associations have emerged [70], offering more accurate predictions than classic algorithms. For example, Peng et al. developed a novel prediction model that has proven more precise than existing methods [71]. It is anticipated that the role of miRNAs in various diseases, including AD, will become increasingly clear. However, the specific functions of many ncRNAs related to AD remain largely unexplored. Further research is needed to fully understand the contributions of these miRNAs to AD pathogenesis.
2.2. lncRNA
lncRNAs, a subclass of ncRNAs, typically range from 200 nucleotides to several hundred bases in length. Unlike miRNAs or piRNAs, lncRNAs are highly heterogeneous in size, interaction partners, and mechanisms of action. Although predominantly localized in the nucleus, lncRNAs are also present in the cytoplasm. Previous studies have shown that nuclear lncRNAs play key roles in gene expression and epigenetic regulation [72]. In the cytoplasm, lncRNAs are involved in essential functions such as modulating mRNA and protein stability, regulating translation, and influencing miRNA activity [17]. Through their roles in endogenous RNA regulatory networks, lncRNAs are integral to physiological processes like alternative splicing, chromatin remodeling, gene expression, and RNA metabolism. Additionally, lncRNAs exhibit distinct spatial and temporal expression patterns during tissue differentiation and development. Recent research has emphasized the significant role of lncRNAs in the pathogenesis of AD, particularly in their ability to inhibit proinflammatory transcripts. For example, silencing the lncRNA c7orf30-2 has been shown to suppress IL-6 expression and reduce local skin inflammation in AD [73]. Functional lncRNAs in AD are summarized in Table 1.
2.2.1. lncRNA H19
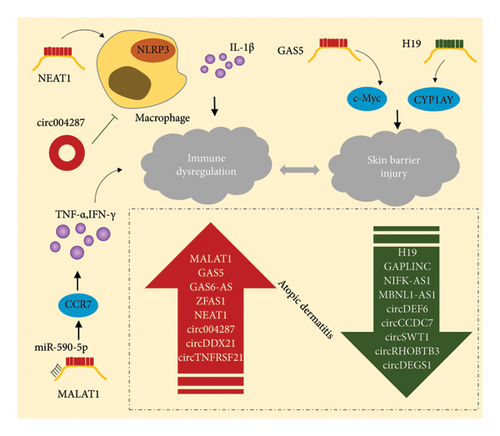
The lncRNA H19 gene, which is paternally imprinted, produces a 2.3-kb splice variant that is blocked and polyadenylated. This lncRNA is mainly localized in the cytoplasm and is coregulated by a maternally imprinted gene, insulin-like growth factor 2, located at the same genomic locus. H19 has been implicated in fetal tumorigenesis and various tumors, contributing to their occurrence and malignant progression [74]. Furthermore, H19 has been shown to influence endothelial–mesenchymal transition (EndMT) in endothelial cells through the regulation of TGF-β [75]. In AD, H19 expression was significantly reduced in keratinocytes from affected individuals compared to healthy controls. This downregulation was negatively correlated with CYP1A1 expression, a gene involved in drug metabolism and the maintenance of skin barrier homeostasis in keratinocytes. Additionally, SPRR2C, a potential regulator of IL-22 stimulation and downstream signaling of IL-17A and IL-17 + TNF in keratinocytes, seems to contribute to H19 downregulation in AD [76]. RNA-Seq analysis has provided insights into the cell-type specificity of H19, including its basal expression characteristics in the epidermis (Figure 4).
2.2.2. lncRNA MALAT1
MALAT1, an lncRNA extensively studied in cancer research [77], has recently been shown to significantly influence various other diseases through its involvement in inflammatory responses [78], osteoarthritis [79], diabetes, pyohemia [80], tympanitis, and inflammatory skin conditions. Cremer et al. observed a marked increase in endothelial cell adhesion and elevated levels of proinflammatory mediators in MALAT1-knockout mice [81]. Overexpression of MALAT1 in epidermal cells leads to enhanced SIRT1 expression and promotes keratinocyte differentiation [82]. RT-qPCR analysis revealed that MALAT1 expression in the plasma of AD mice was significantly higher compared to healthy controls [83]. Furthermore, MALAT1 functions as a sponge for miR-590-5p, regulating CCR7 through a competitive endogenous RNA (ceRNA) mechanism, thereby promoting inflammation. Targeting MALAT1 has shown therapeutic potential in inflammatory skin diseases such as AD. For instance, Morinda officinalis extract can inhibit MALAT1 in AD, significantly reducing mast cell infiltration and inflammatory cytokine secretion, thereby alleviating the symptoms of AD (Figure 4). Kuang found that exosomes derived from human keratinocytes containing MALAT1 can significantly enhance the biological functions of abnormal macrophages in a high-glucose environment [84].
2.2.3. lncRNA NEAT1
NEAT1, another lncRNA, is known for its elevated expression in various human cancers [85]. Acting as a ceRNA, NEAT1 modulates the expression of target genes during disease progression by sponging miRNAs. NEAT1 is also a key mediator of inflammasome activation in innate immunity (Figure 4). It is associated with the NLRP3, NLRC4, and AIM2 inflammasomes in mouse macrophages, stabilizing mature caspase-1 and promoting the production and pro-death of IL-1β [86]. AD is driven by both immune dysregulation and inflammatory activation. Peng et al. identified differences in immune infiltration profiles between normal controls and patients with AD, with key genes such as S100A7, S100A8, and LCE3D being strongly associated with the primary infiltrating cells in AD. They also discovered that the lncRNA-miRNA-mRNA network, including NEAT1, plays a pivotal role in AD development. RT-qPCR further confirmed that NEAT1, a core component of the ceRNA network, is significantly overexpressed in patients with AD [87].
2.2.4. lncRNA GAS5
GAS5 is an lncRNA localized in mitochondria, playing a key role in maintaining cellular energy homeostasis [88] and skin homeostasis. The expression of growth arrest-specific genes, including GAS1, GAS5, and GAS6, significantly influences the regulation of skin tissue proliferation, with these genes being notably expressed in keratinocytes [89]. In cutaneous squamous cell carcinoma, GAS5 expression is relatively low and primarily localized in the cytoplasm. However, when GAS5 is overexpressed, it impedes cell survival, growth, migration, and invasion while promoting cell death [90]. Additionally, plasma levels of GAS5 are significantly elevated in patients with AD compared to healthy individuals [76] (Figure 4). GAS5 also directly targets the transcription factor c-Myc, a biomarker for nonhealing wounds, thereby enhancing its expression and influencing the healing process of skin lesions [91]. This suggests that GAS5 may contribute to the progression of AD by upregulating c-Myc, which in turn inhibits the healing of skin wounds. Consequently, reducing GAS5 levels or activity may offer a novel therapeutic approach to treating AD.
Microarray detection has revealed that several lncRNAs, including GAS5, GAS6-AS, MALAT1, and ZFAS1, are significantly upregulated in AD, while others such as GAPLINC, NIFK-AS1, and MBNL1-AS1 are downregulated in patients with AD. These differentially expressed lncRNAs are considered potential targets for regulating AD recurrence in mouse models [83]. Increasing empirical evidence suggests that lncRNAs exert regulatory effects on miRNA targets through competitive binding, acting as miRNA sponges, or functioning as ceRNAs [92]. Substantial evidence supports the widespread involvement of the lncRNA-miRNA-mRNA ceRNA network in the development of various human diseases [93, 94]. In AD, MALAT1 has been identified as a ceRNA, interacting with miR-590-5p. lncRNAs may thus represent a key to developing new treatment strategies for AD.
2.3. circRNA
circRNAs are a unique class of ncRNAs generated through a distinct splicing process known as back-splicing in eukaryotes. Unlike linear RNAs, circRNAs form a covalently closed-loop structure, which makes them more resistant to degradation by exonucleases. The advent of high-throughput sequencing and advanced bioinformatics algorithms has led to the identification of a large number of circRNAs in eukaryotes, many of which exhibit specific expression patterns in certain tissues or cells. circRNAs play vital regulatory roles in various physiological and pathological processes, including the innate immune response, neuronal function, and cell differentiation and proliferation [95]. In the context of skin diseases, circRNAs are implicated in pathogenesis by modulating gene expression in different tissues, including the skin, and are essential for maintaining physiological homeostasis and function [96]. Extensive research has focused on the regulatory roles of circRNAs in skin wound healing. For example, circ_0000250 enhances SIRT1 expression and promotes wound healing by sequestering miR-128-3p [97], whereas circ_0084443, which is downregulated during regular wound healing, negatively impacts keratinocyte migration [98]. The regulatory effects of circRNAs are often amplified within the circRNA–miRNA–mRNA network. For instance, circ_0084443 positively influences FOXO4 expression by acting as an miR-17-3p sponge, thereby modulating the TGF-β signaling pathway through the miR-17-3p/FOXO4 axis. Xiang et al. also identified the circRNA-Krt13/miR-665-3p/Itga3 and circRNA-Krt14/miR-706/Mylk4 pathways as potential contributors to diabetic wound healing.
In AD, the circRNA transcriptome exhibits distinct characteristics. Compared to nondiseased and healthy skin, the expression of circRNAs is significantly altered in AD lesions. CircRHOBTB3, circDEF6, circCCDC7, circSWT1, and circDEGS1 are downregulated, while circDDX21 and circTNFRSF21 are upregulated. Overall, circRNAs are generally downregulated in diseased skin relative to healthy skin, with expression levels inversely correlated with the AD score index [99] (Figure 4). One circRNA, hsa_circ_0004287, has been shown to have increased expression in the PBMCs of patients with AD, primarily in macrophages under inflammatory conditions [100]. In vitro studies have demonstrated that hsa_circ_0004287 inhibits M1 macrophage activation, and overexpression of this circRNA in macrophages reduces cutaneous inflammation in AD rodent models, suggesting its potential as a therapeutic candidate for AD. The study of circRNAs has gained significant attention in medical research, with implications for understanding, diagnosing, and treating various diseases. circRNAs hold promise as biomarkers for disease diagnosis and severity assessment, and as potential therapeutic targets for inflammatory skin conditions [101]. However, there is still a lack of detailed understanding of the specific mechanisms by which circRNAs contribute to AD, including the signaling pathways and molecular mechanisms involved. It remains unclear whether changes in circRNA expression are a cause or consequence of the disease. Therefore, further investigation is needed to elucidate the role of circRNAs in AD, which could pave the way for circRNA-based therapeutic interventions. Notably, large-scale studies are required to validate the significance of circRNA expression in diagnosing and assessing AD severity and to explore their potential as therapeutic targets.
3. ncRNAs’ Roles in AD
3.1. Diagnostic Biomarkers
AD is a chronic disease. Early diagnosis and intervention are important to alleviate symptoms and improve quality of life, which requires highly sensitive biomarkers. And, given the highly heterogeneous nature of AD, it is unlikely that each patient will respond the same to a particular treatment. In recent years, the introduction of novel targeted therapies for AD has driven the need for biomarker-based patient stratification. The application of biomarkers will lead to better characterization and stratification of patients, and allow for better comparison of current and new treatments, enabling more personalized “patient-specific” management [102]. Many diseases lack specific markers or are often misdiagnosed, leading to delayed detection and diagnosis, which significantly affects treatment outcomes and prognosis. Single nucleotide polymorphisms (SNPs), due to their stable presence within individuals, are considered valuable disease markers. The advent of gene sequencing has enabled the identification of numerous disease-specific SNPs, which have been crucial in the timely detection of various genetically based diseases [103–105]. Similarly, advancements in omics technologies have been extensively utilized in the search for disease markers [106, 107]. ncRNAs, which predominantly exist in a stable state in circulation, have disease-specific types and contents, making them promising candidates for new biomarkers. The role of various ncRNAs has been demonstrated in cardiovascular diseases [108] and numerous cancers [109, 110]. Leveraging the characteristics of ncRNAs to develop noninvasive liquid biopsy-based assays offers significant potential for advancing the identification of disease biomarkers.
The study of miRNAs in AD has progressed considerably, and the development of biosensor-based methods for miRNA detection has greatly improved the accuracy and sensitivity of identifying these molecules [111]. Several miRNAs have been confirmed as promising biomarkers. For instance, Lv et al. conducted a comprehensive analysis of miRNAs in serum and urine samples from patients with AD, revealing a significant elevation in the levels of miR-203 and miR-483-5p in the serum of children with AD compared to healthy controls. Conversely, miR-203 levels in urine were significantly reduced in the same group [112]. Elevated serum miR-203 levels were strongly correlated with increased levels of soluble tumor necrosis factor receptor I (sTNFRI) and sTNFRII, both recognized markers of inflammation. On the other hand, reduced miR-203 levels in urine were significantly associated with abnormal serum IgE levels in patients with AD. Moreover, the dysregulation of three specific miRNAs—miR-451a, miR-143-3p, and miR-223-3p—was confirmed in a large number of samples, with miR-451a emerging as a potential biomarker for early detection of AD. The experimentally validated targets of miR-451a, including IL6R and proteasome subunit β-8 type (PSMB8), were found to be elevated in patients with AD, exhibiting a negative correlation with miR-451a levels, and were upregulated following miR-451a inhibition in PBMCs [113]. Additionally, Yan et al. identified a significant inverse relationship between miR-146a levels and the Th1/Th2 ratio in AD, suggesting its potential as a marker for assessing immune dysfunction in AD [114].
Microarray technology has confirmed the specificity of circRNA and lncRNA expression profiles in AD. circRNAs, with their covalently bonded closed-loop structures, exhibit remarkable resistance to exonuclease degradation, ensuring exceptional stability in circulation. Likewise, lipid bilayers in various biological substances effectively protect lncRNAs from RNA enzymes within exosomes. These characteristics underscore their potential as diagnostic biomarkers and therapeutic targets for cutaneous diseases. However, our understanding of lncRNAs and circRNAs in skin disorders remains in its nascent stages, leaving several critical questions unanswered regarding their viability as clinical biomarkers. One key challenge is the limited understanding of the mechanisms by which most lncRNAs and circRNAs contribute to the onset and progression of specific skin diseases. While certain lncRNAs and circRNAs display differential expression in skin tissues or cells, further research is essential to determine their prevalence in plasma or other body fluids and to assess their potential as noninvasive biomarkers for diagnosing or predicting skin diseases. Additionally, rigorous validation is required to establish the safety and reliability of lncRNAs and circRNAs as biomarkers or therapeutic targets for cutaneous diseases. Moreover, the standardization of bioinformatics tools and the creation of a comprehensive RNA database specific to the epidermis are essential for advancing the field. Such efforts would ensure that ncRNAs can be effectively utilized in clinical settings.
Given their unique structural and functional attributes—such as stability, specificity, and sensitivity—ncRNAs hold significant promise as innovative biomarkers in dermatology. As biotechnology and bioinformatics continue to advance, an expanding array of ncRNAs is expected to be identified and recognized as promising biomarkers with potential clinical applications in dermatology.
3.2. ncRNA-Targeted Drugs
AD is a multifactorial condition that results from a complex interplay of immune dysregulation, genetic mutations affecting the epidermis, and environmental triggers. Despite recent advancements in treatment, managing moderate-to-severe cases of AD remains challenging [115]. Identifying safe and effective treatments for AD is therefore of paramount importance. NcRNAs have emerged as key players in various inflammatory skin diseases, offering potential therapeutic targets, particularly in conditions where the molecular mechanisms are not yet fully understood. The ability of ncRNAs to regulate gene expression makes them promising candidates for drug development, leading to the exploration of ncRNA-based therapies for complex diseases such as cardiomyopathies, diabetes, and cancer [116–118]. As a result, numerous innovative approaches have been developed to manipulate ncRNA activity.
MiRNA mimics are artificially synthesized double-stranded RNA molecules designed to replicate the biological function of naturally occurring miRNAs. These mimics, crafted based on complementary sequences of related miRNAs, are used to disrupt the interaction between endogenous miRNAs and their target molecules. They can act as inhibitors, miRNA sponges, or small antisense oligonucleotides (ASOs). Compared to traditional small molecules that inhibit protein function, designing an oligonucleotide to bind to mRNA and inhibit protein production is generally more straightforward [119]. This ease of design and rapid identification of lead compounds makes miRNA mimics particularly advantageous in drug development, often taking weeks rather than months or years. In both cell cultures and mice, the cell-penetrating peptide PepFect6 (PF6) has been shown to effectively deliver small interfering RNA (siRNA). When used for transfection of miR-146a mimics, PF6 demonstrated superior efficacy in keratinized cell cultures compared to lipid-based reagents, with minimal impact on cell proliferation and similar effects on cell viability. In a mouse model of irritant contact dermatitis, subcutaneous administration of PF6-miR-146a nanocomplexes significantly reduced ear edema and decreased the expression of proinflammatory cytokines and chemokines, including IL-6, CCL11, CCL24, and CXCL1 [120]. These findings suggest that PF6-miR-146a nanoparticles hold promise as therapeutic interventions for inflammatory skin conditions.
Current therapeutic strategies targeting lncRNAs involve the use of oligonucleotide compounds, such as sulfur-modified main chains and various sugar modifications. Additionally, lncRNAs can be targeted by inhibiting or silencing their expression using ASOs or siRNAs [121]. These approaches have shown promising results in clinical trials. The CRISPR-Cas9 system has also emerged as a powerful tool for gene mapping and modification, with the Cas9 endonuclease being employed for DNA editing or gene knockout. An innovative study by Wan et al. demonstrated the use of Cas9 ribonucleoprotein (RNP) to target NLRP3 for treating inflammatory skin diseases, highlighting the potential of transdermal drug delivery systems [122].
The therapeutic application of circRNAs has advanced significantly, particularly in treating tumors, infectious diseases, and various inflammatory conditions [123]. A novel circRNA vaccine has been developed that induces the production of neutralizing antibodies and T cell responses by expressing the trimer RBD of the spike protein. This vaccine has demonstrated promising protective effects against COVID-19, offering a valuable approach to preventing and treating challenging infectious diseases [124]. Additionally, circ-ITCH, found in bone marrow mesenchymal stem cell exosomes, has been shown to promote angiogenesis and accelerate the healing process of diabetic foot wounds [125].
The biological processes regulated by ncRNAs not only serve as direct drug targets but also hold significant therapeutic potential for treating conditions like AD. For example, targeting the activity of NF-κB, which has been effective in treating AD [126], has revealed that IL-32 isotypes reduce AD severity by inhibiting miR-205 expression through the suppression of NF-κB activity [127]. These emerging research areas point toward the future of novel AD therapies. Empirical validation of ncRNAs linked to AD, along with their downstream effectors, as viable pharmaceutical targets, will undoubtedly contribute to the development of mechanistically driven treatments for this complex dermatological condition. The investigation of ncRNA networks is essential for understanding the intricate mechanisms underlying complex diseases. Advances in bioinformatics, network-based algorithms, and machine learning methods for predicting drug targets offer promising avenues for exploring ncRNA networks with complex mechanisms [128, 129]. However, caution is warranted in ncRNA-based drug discovery. While numerous studies highlight the significant biological activity of RNA, necessitating bold hypotheses and innovative findings, the field has also seen a trend toward nonreplicable investigations. Thus, balancing the ambition to push scientific boundaries with healthy skepticism about the relationship between molecular interactions and biological outcomes is essential for drug discovery and development [130]. Moreover, research should focus on optimizing the delivery of ncRNA mimics or inhibitors in a cell-type-specific manner, as ncRNAs may serve different functions in various tissues. The emergence of advanced skin treatment methods, such as exosomes, hydrogels, and wound dressings, underscores the need for comprehensive investigations into the mechanisms underlying ncRNAs in conjunction with these innovative therapeutic technologies. Such research will provide precise insights into the treatment of skin diseases and further enhance the therapeutic potential of ncRNAs [131].
4. Conclusion and Discussion
AD is a debilitating skin disease, particularly affecting children, with complex underlying causes that have impeded the development of targeted treatments. Research has increasingly focused on exploring diagnostic and therapeutic strategies, particularly emphasizing ncRNAs due to their remarkable functional stability, diversity, and adaptability. The involvement of ncRNAs in AD primarily encompasses the regulation of skin barrier function, cytokine signaling, and inflammatory responses. Bioinformatics tools have been employed to identify numerous ncRNAs with abnormal expression patterns in cutaneous diseases, indicating their potential as reliable diagnostic biomarkers or therapeutic targets in AD. Despite these insights, the understanding of ncRNAs in the context of AD remains in its early stages. Advancing this field will require a deeper elucidation of the precise mechanisms through which ncRNAs operate, the creation of AD-specific ncRNA databases, and continued research efforts. These steps are crucial for unlocking the full potential of ncRNAs in developing effective treatments for this complex dermatological condition.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.D. and P.B.: conceptualization, reviewing and editing; L.Z. and Y.L.: writing–original draft preparation; Z.G. and B.Z.: visualization and investigation; C.Z., H.Z. and W.L.: writing and reviewing; S.H.: figures and polishing. All the authors contributed to and approved the final version of the manuscript. All authors read and approved the final manuscript. L.Z. and Y.L. authors shared co-first authorship.
Funding
This work was supported by the Hunan Provincial Education Commission Foundation (20A056, 22C0565, 23A0664, 24A0683); The Hunan Provincial Health Commission Foundation (No. 202112041226, D202302088596); The Hunan Provincial Natural Science Foundation of China (No. 2022JJ40220); The Innovation and Entrepreneurship Education Base of Public Health and Preventive Medicine (Hunan Education Bureau Notice 2019 No. 333-93); Funding by Young Backbone Teachers of Hunan Province Training Program Foundation of Changsha Medical University (Hunan Education Bureau Notice 2021 No. 29-26); and the National College Students’ Innovative Entrepreneurial Training Plan Program (No. 202210823010, S202310823003).
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.