Circulating Cytokine Levels and the Risk of Hypertrophic Scar: A Two-Sample Mendelian Randomization Study
Abstract
Background: Chronic inflammation has been implicated in hypertrophic scar (HS) formation based on experimental evidence and clinical observations. However, the existence of a causal relationship between circulating cytokines and the risk of HS remains uncertain. This study aimed to elucidate whether genetically predicted circulating cytokine levels are associated with HS risk using a two-sample Mendelian randomization (MR) analysis.
Methods: We used genetic variants associated with circulating levels of cytokines in a genome-wide association study (GWAS) meta-analysis involving 8293 European populations as instrumental variables. HS data were obtained from an open GWAS dataset comprising 208,248 individuals of European descent, including 766 diagnosed HS cases and 207,482 controls. Analysis was performed using MR methods including inverse-variance weighted (IVW), MR-Egger, weighted median, simple median, and weighted mode. The MR-Egger intercept and Cochran’s Q test were applied to assess pleiotropy and heterogeneity, and MR Steiger test was employed to examine the causative direction.
Results: Our findings revealed a suggestive causal relationship between elevated circulating levels of interleukin-2 (IL-2) and an increased risk of HS (OR: 1.48, 95% CI: 1.04–2.10, p = 0.028); platelet-derived growth factor-BB (PDGFbb) was also causally associated with the risk of HS (OR: 1.38, 95% CI: 1.07–1.77, p = 0.012).
Conclusion: Our MR analysis provides suggestive evidence supporting a potential causal relationship between circulating IL-2, PDGFbb, and HS risk. Further research is warranted to explore how these cytokines influence the development of HS.
1. Introduction
Hypertrophic scar (HS) is a fibroproliferative disorder developed from aberrant wound healing. It usually occurs within 1-2 months after injury (such as trauma, burn, or surgery) and stays within the edge of the original wound [1, 2]. Under the influence of various factors including genetics [3], stretch/contraction [4], and infection [5], damage to the deep reticular dermis leads to the accumulation of a large number of inflammatory cells, fibroblast proliferation, neovascularization, and collagen accumulation, resulting in forming HS [5]. This disorder can not only affect the appearance but also induce itch and pain, which may have a negative impact on the physical and mental health of patients [6].
Previous studies had indicated that inflammation plays a dominant role in the process of forming HS [5, 7]. For example, local inflammation can be involved in the regulation of collagen synthesis, and the intensity of inflammation exhibits a positive correlation with scar dimensions [8]. In the early stage of wound healing, most inflammatory factors play a proinflammatory role, transitioning to an anti-inflammatory role in the late stage. The imbalance of these factors is one of the causes of abnormal scar hyperplasia [7]. Cytokines and growth factors may affect wound healing by modulating cellular activities and the extracellular matrix at the wound site [9]. Notably, in a patient with Castleman disease, characterized by unregulated overproduction of interleukin-6 (IL-6), her auricular keloids were aggravated when accompanied with increasing levels of circulating inflammatory factors. Additionally, it was shown that systemic inflammation may increase the risk of keloids and HS in patients undergoing burn repair [5]. Identifying systemic cytokines associated with scar formation can enable early selection of appropriate treatment strategies for patients with high-risk factors upon wound occurrence. Furthermore, it may facilitate the development of targeted therapeutics aimed at specific cytokine-related pathways to prevent HS formation and mitigate its severity. Currently, most studies focus on the mechanisms of local inflammatory factors in scar formation, and there is insufficient evidence to determine which circulating inflammatory factors influence scar development. Whether through clinical laboratory tests or basic experiments, detecting multiple circulating inflammatory factors is costly. With the rise of Mendelian randomization (MR) analysis, this research method offers the possibility of exploring the relationship between circulating cytokines and HS formation from a genetic perspective.
MR analysis adheres to the Mendelian principle wherein parental alleles are randomly assigned to offspring. This method employs genetic variants as instrumental variables (IVs) to infer causal associations between phenotypes and diseases [10]. MR analysis can minimize confounding effects and reverse causality bias, addressing the scarcity of case-control data and the difficulty of measuring exposure levels. A genome-wide association study (GWAS) in 2017 summarized genetic variants associated with circulating levels of 41 cytokines [11], including common inflammatory and growth factors that may be closely related to the scar microenvironment. In 2021, the FinnGen study released GWAS data on HS. These data allows us to explore the relationship between circulating cytokines and HS from a genetic susceptibility perspective.
In this study, we used genetic variants associated with these cytokines as IVs and genetic variants related to HS as outcome variables to investigate the correlation between circulating cytokines and the risk of HS.
2. Materials and Methods
2.1. Study Design and Data Sources
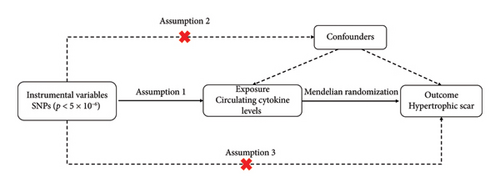
A two-sample MR analysis model was employed to assess the potential causal effects of circulating cytokines on HS risk. To ensure the credibility of the findings, MR studies need to meet the following three foundational assumptions: (1) genetic variants are significantly associated with circulating cytokine levels (association hypothesis); (2) genetic variants remain independent of other confounders (independence assumption); and (3) genetic variants exert influence on outcomes solely through exposure factors (exclusivity hypothesis) (Figure 1) [12].
Summary data on genetic variants associated with circulating cytokines and growth factors were derived from a GWAS comprising 8,293 Finnish participants across three cohorts: the Young Finns Cardiovascular Risk Study, the FINRISK 1997 study, and the FINRISK 2002 study [11]. Summary statistics on HS were derived from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_HYPETROPHICSCAR/). This study included a sizable cohort comprising 766 cases (diagnosed based on ICD-10 L91.0 criteria) and 207,482 controls, constituting a total of 208,248 Finnish individuals from the FinnGen study. The GWAS rigorously evaluated the association of 16,380,443 genotypic single-nucleotide polymorphisms (SNPs) with HS. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines to ensure methodological rigor in the study, as shown in Additional file: STROBE-MR checklist.
2.2. Instrument Selection
We first set p < 5 × 10−8 as the genome-wide significance threshold to select strongly correlated IVs. Since only a few SNPs were picked out under this criterion for some cytokines, a more permissive significance threshold (p < 5 × 10−6) was employed. To minimize potential confounding factors and pleiotropy, we adopted the stringent default clumping criteria (r2 < 0.001 and distance = 10,000 kb) to remove IVs with linkage disequilibrium (LD) [12]. F statistics were used to assess the strength of the association between clumped IVs and exposure. IVs with an F statistic > 10 are considered strong, while those with F < 10 may lead to severe bias in causal effect estimation. The F statistic is calculated by F = R2(N − 2)/(1 − R2) and R2 = 2 × EAF × (1 − EAF) × beta2, where R2 is the proportion of exposure variability explained by each instrument and N is the sample size of the cytokines [13]. Subsequently, a harmonization process was executed, resulting in the identification of 410 SNPs associated with 41 cytokines. Details of all SNPs are provided in Additional file: Table S1.
2.3. Statistical Analysis and Annotation
A total of five MR analysis methods were used in this study. These methods include inverse-variance weighted (IVW), MR Egger, weighted median, simple median, and weighted mode. The IVW method served as the primary analytical tool for deriving credible causal effect estimates [14]. Assessment of potential pleiotropy and heterogeneity was conducted through MR-Egger regression analysis and Cochran’s Q statistics. If the MR-Egger intercept did not deviate significantly from 0 (p > 0.05), there was no pleiotropy [15]. Heterogeneity, as assessed according to Cochran’s Q statistic, was considered nonexistent when p > 0.05, leading to the adoption of the fixed-effects IVW model as the primary outcome. Additionally, a leave-one-out analysis was performed to estimate the impact of individual aberrant SNPs on the overall MR analysis, ensuring result robustness. We also used PhenoScanner to detect potential phenotypes of individual SNPs to eliminate potential influence on the results. Bonferroni correction was applied for multiple comparisons due to the inclusion of multiple exposures, setting a significant evidence threshold at a p value of 1.22 × 10−3 (0.05/41). A p value less than 0.05 but more than 1.22 × 10−3 indicates suggestive evidence. A potential causal relationship can be considered if the results of MR analysis meet the following three conditions: (1) at least the p value of IVW method is less than 0.05; (2) consistency in the direction of the beta in IVW and other methods; and (3) no pleiotropy and heterogeneity were found [16, 17]. We further applied Steiger directionality test and filtering to determine the causal direction of the association [18]. All statistical analyses were performed using the “TwoSampleMR” software package in R Version 4.3.0, with a significance threshold set at a two-tailed p value of less than 0.05. We also performed annotations on the significant SNPs using the software ANNOVAR on human genome hg19, which is an efficient tool to assess the functional consequences of genetic variation. Annotations are mostly relied on precompiled databases, avoiding the need for new multiple sequence alignments or remote SQL queries [19].
3. Results
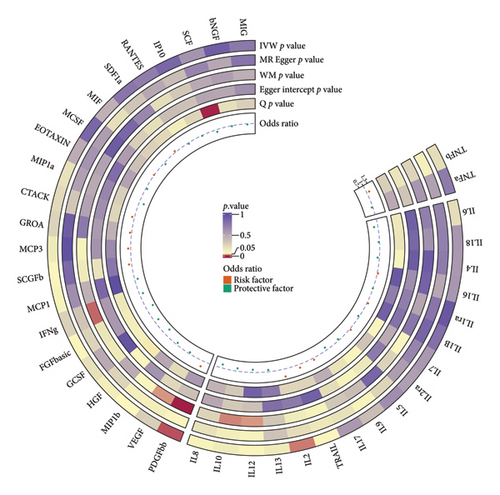
By the IVW method, three cytokines (FGFbasic, IL-2, and PDGFbb) were suggested to be related to the risk of HS, and other cytokines had no significant correlation with HS. The main results of the MR analyses for all 41 cytokines are presented in a circle plot (Figure 2), and detailed summary data for specific genetic variant associations are provided in Additional file: Table S2.
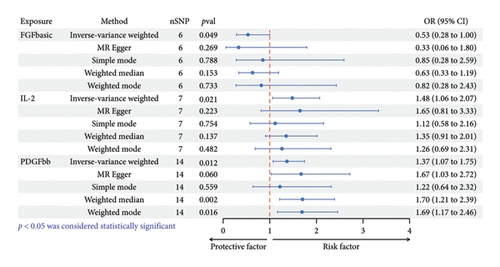
Elevated circulating FGFbasic levels, predicted by genetic factors, exhibited an association with a reduced risk of HS (OR: 0.53, 95% CI: 0.28–1.00, p = 0.049 < 0.05). Sensitivity analysis showed consistent trend (MR Egger-OR: 0.33, 95% CI: 0.06–1.80, p = 0.269; simple mode-OR: 0.85, 95% CI: 0.28–2.59, p = 0.788; weighted median-OR: 0.63, 95% CI: 0.33–1.99, p = 0.153; weighted mode-OR: 0.82, 95% CI: 0.28–2.43, p = 0.733), albeit without statistical significance (Figure 3; Additional file: Figure S1A). Cochrane’s Q analysis indicated no evidence of heterogeneity in the association between FGFbasic and HS (p = 0.123). In addition, MR-Egger regression analysis did not detect potential SNP directional pleiotropy (intercept p value = 0.581) (Table 1).
| Exposure | Methods | OR | 95% CI | p value | Q | Q-p value | Egger-intercept | p value |
|---|---|---|---|---|---|---|---|---|
| FGFbasic | IVW | 0.532 | 0.284–0.999 | 0.0496 | 8.666 | 0.123 | 0.068 | 0.581 |
| MR Egger | 0.331 | 0.061–1.795 | 0.269 | 7.951 | 0.093 | 0.068 | 0.581 | |
| Simple mode | 0.851 | 0.280–2.592 | 0.788 | NA | NA | 0.068 | 0.581 | |
| Weighted median | 0.63 | 0.334–1.187 | 0.153 | NA | NA | 0.068 | 0.581 | |
| Weighted mode | 0.819 | 0.276–2.428 | 0.733 | NA | NA | 0.068 | 0.581 | |
| IL-2 | IVW | 1.483 | 1.062–2.070 | 0.021 | 7.803 | 0.253 | −0.021 | 0.746 |
| MR Egger | 1.648 | 0.815–3.335 | 0.223 | 7.623 | 0.178 | −0.021 | 0.746 | |
| Simple mode | 1.117 | 0.578–2.158 | 0.754 | NA | NA | −0.021 | 0.746 | |
| Weighted median | 1.352 | 0.908–2.014 | 0.137 | NA | NA | −0.021 | 0.746 | |
| Weighted mode | 1.261 | 0.688–2.312 | 0.482 | NA | NA | −0.021 | 0.746 | |
| PDGFbb | IVW | 1.368 | 1.072–1.746 | 0.012 | 11.478 | 0.571 | −0.035 | 0.368 |
| MR Egger | 1.672 | 1.029–2.718 | 0.06 | 10.603 | 0.563 | −0.035 | 0.368 | |
| Simple mode | 1.218 | 0.640–2.320 | 0.559 | NA | NA | −0.035 | 0.368 | |
| Weighted median | 1.702 | 1.212–2.391 | 0.002 | NA | NA | −0.035 | 0.368 | |
| Weighted mode | 1.692 | 1.166–2.457 | 0.016 | NA | NA | −0.035 | 0.368 | |
Increased genetically predicted circulating IL-2 levels were associated with a 48% increased risk of HS (OR: 1.48, 95% CI: 1.06–2.07, p = 0.021). Other analysis methods displayed similar trends without achieving statistical significance (MR Egger-OR: 1.65, 95% CI: 0.81–3.33, p = 0.223; simple mode-OR: 1.12, 95% CI: 0.58–2.16, p = 0.754; weighted median-OR: 1.35, 95% CI: 0.91–2.01, p = 0.137; weighted mode-OR: 1.26, 95% CI: 0.69–2.31, p = 0.482) (Figure 3; Additional file: Figure S1B). The results of Cochrane’s Q indicated no heterogeneity in the correlation between IL-2 and HS (p = 0.253), and MR-Egger regression analysis did not reveal potential SNP directional pleiotropy (intercept p value = 0.746) (Table 1).
Increased levels of genetically predicted circulating PDGFbb were associated with a 37% elevated risk of HS (IVW-OR: 1.37, 95% CI: 1.07–1.75, p = 0.012). The results of weighted median method (OR: 1.70, 95% CI: 1.21–2.39, p = 0.049) and weighted mode method (OR: 1.11, 95% CI: 1.02–1.21, p = 0.020) were consistent with the IVW results, while the simple mode method displayed a similar trend without statistical significance (OR: 0.85, 95% CI: 0.28–2.59, p = 0.788) (Figure 3; Additional file: Figure S1C). Cochrane’s Q analysis indicated no heterogeneity in the correlation between PDGFbb and HS (p = 0.571). Potential SNP directional pleiotropy was not detected by MR-Egger regression analysis (intercept p value = 0.368) (Table 1).
Least-one-out sensitivity analysis results suggested that the association of FGF-basic and IL-2 with HS risk was not driven by any single SNP (Additional file: Figure S2A, B), but the association of PDGFbb with HS was influenced by an outlier SNP (rs13412535) (Additional file: Figure S2C), which was strongly associated with all three cytokines and several other traits, as revealed by PhenoScanner search (Additional file: Table S3). Exclusion of this SNP resulted in no significant evidence of the genetically predicted association between FGFbasic and HS (IVW-OR: 0.53, 95% CI: 0.28–1.02, p = 0.055), while genetic predictions of IL-2 (IVW-OR: 1.48, 95% CI: 1.04–2.10, p = 0.028) and PDGFbb (IVW-OR: 1.38, 95% CI: 1.07–1.77, p = 0.012) remained associated with HS risk (Additional file: Table S4). The Steiger test for IL-2 and PDGFbb on HS confirmed that the causative direction was correct (Additional file: Table S5). All selected SNPs showed the right direction of IVs on HS by Steiger filtering (Additional file: Table S1).
We identified six independent SNPs associated with circulating IL-2 level and 13 SNPs associated with circulating PDGFbb level. Then gene-based annotations were applied to identify the direct relationships between variants and known genes. Among the six genetically IL-2-associated SNPs, three SNPs were located in the intronic regions of the following genes: ITGB5, MEGF11, and LEKR1. The other three SNPs were located in the intergenic regions involving PDGFRA and KIT, LINC01372 and LOC102723427, and NT5C1B-RDH14 and MIR4757. Out of the 13 SNP variants genetically associated with PDGFbb, four were found within the intronic regions of the following genes, SLC37A2, PCSK6, RBFOX1, and TRIB3, while nine SNPs were located in the intergenic regions of PCSK6 and TM2D3, ISL1 and PELO, TPK1 and CNTNAP2, RNF24 and SMOX, RWDD2A and ME1, PITX2 and C4orf32, SERPINE2 and FAM124B, YWHAQ and TAF1B, and PLSCR5 and ZIC4 (Table 2).
| SNP | Chr:pos | Ref | Alt | Function | refGene | |
|---|---|---|---|---|---|---|
| IL-2 | rs16836080 | 3:124,548,684 | A | G | Intronic | ITGB5 |
| rs170117 | 4:55,390,380 | T | C | Intergenic | PDGFRA, KIT | |
| rs4634519 | 7:67,192,928 | A | G | Intergenic | LINC01372, LOC102723427 | |
| rs61335305 | 15:66,453,074 | A | C | Intronic | MEGF11 | |
| rs62124990 | 2:19,238,636 | T | G | Intergenic | NT5C1B-RDH14, MIR4757 | |
| rs7615304 | 3:156,675,703 | A | G | Intronic | LEKR1 | |
| PDGFbb | rs11247305 | 15:102,100,210 | C | G | Intergenic | PCSK6, TM2D3 |
| rs116445074 | 5:51,534,600 | T | G | Intergenic | ISL1, PELO | |
| rs11766649 | 7:144,839,247 | A | G | Intergenic | TPK1, CNTNAP2 | |
| rs12289510 | 11:124,947,051 | A | G | Intronic | SLC37A2 | |
| rs13037046 | 20:4,054,634 | A | T | Intergenic | RNF24, SMOX | |
| rs2324229 | 6:83,918,131 | T | C | Intergenic | RWDD2A, ME1 | |
| rs35859699 | 4:112,184,751 | A | G | Intergenic | PITX2, C4orf32 | |
| rs4965869 | 15:101,990,320 | T | C | Intronic | PCSK6 | |
| rs55680718 | 2:225,166,877 | T | C | Intergenic | SERPINE2, FAM124B | |
| rs72777070 | 2:9,798,877 | T | G | Intergenic | YWHAQ, TAF1B | |
| rs73162807 | 3:146,474,790 | A | C | Intergenic | PLSCR5, ZIC4 | |
| rs9936075 | 16:7,321,909 | A | G | Intronic | RBFOX1 | |
| rs9941733 | 20:374,061 | A | G | Intronic | TRIB3 | |
- Note: Intronic represents that the variant overlaps an intron of the refgene; intergenic means that the variant is in the intergenic region of the refgenes. Alt, alternative allele; Chr, chromosome; Pos, position; Ref, reference allele.
4. Discussion
In this study, we investigated the causal relationships between circulating cytokines and HS risk using a two-sample MR approach. Evidence suggested that genetically predicted circulating levels of IL-2 and PDGFbb were risk factors for HS formation.
IL-2 is a cytokine that initiates complex signaling cascades by binding to the IL-2 receptor (IL-2R) on the cell surfaces, primarily regulating the immune activity of leukocytes, especially lymphocytes [9]. This cytokine is actively secreted by various cells engaged in wound healing, including T-helper cells, cytotoxic T lymphocytes, macrophages, and keratinocytes [20, 21]. IL-2R subunits are also distributed on the surfaces of skin cells such as keratinocytes and fibroblasts [22–24]. IL-2 signaling may affect local immune response by promoting the recruitment and activation of immune or skin cells in the process of scar formation. In the HS tissues, more than one-third of infiltrated lymphocytes were activated IL-2R+ T cells, and some T-cell lymphokines act on keratinocytes, fibroblasts, and other cell types, inducing characteristic changes of scars [25]. After binding to its receptor, IL-2 primarily recruits Janus kinase (JAK) to phosphorylate IL-2R, which subsequently recruits and phosphorylates signal transducer and activator of transcription (STAT), activating downstream genes [26]. Alternatively, it can recruit Shc family signaling molecules, activating the phosphatidylinositol 3-kinase (PI3K) and extracellular regulated protein kinase (ERK) pathways, both of which may promote cell proliferation [27]. Some studies suggested that fibroblasts express IL-2Rβ and γ subunits which are involved in the signaling pathway. IL-2 stimulates fibroblasts to release proinflammatory cytokines [28, 29] and enhances monocyte chemokine production via the IL-2Rγ-JAK3 signaling pathway [29]. Alterations in circulating IL-2 levels can also affect the local wound environment. A study on burn patients revealed elevated circulating IL-2 levels on days 1 and 5 post-burn, positively correlating with burn area [30]. Interestingly, the IL-2 level at the burn wound site is lower than that in peripheral blood, suggesting nuanced roles for IL-2 at different stages of the healing process [31]. By examining the phosphorylation status of downstream mediators of the IL-2 pathway, such as STAT3, ERK1/2, and Akt, studies on fracture patients indicated that systemic IL-2 signaling might coordinate wound healing by stimulating cell migration to the wound or promoting growth factor production through the PI3K and ERK signaling pathways [32]. Our study revealed that genetically predicted elevated level of circulating IL-2 was associated with an increased risk of HS. Until recently, no experiments have provided direct evidence of circulating IL-2 involvement in scar formation. Through the annotations of IL-2-related SNPs, it was observed that all genetic variants located within the intronic and intergenic regions of genes do not directly alter the amino acid composition of the gene-encoded protein. This presents a challenge in deciphering the mechanism by which IL-2 affects scar formation. However, combining with the aforementioned studies, IL-2-associated signaling pathways emerge as potential research targets and further elucidation of the specific mechanism is warranted.
Platelet-derived growth factor (PDGF) is a potent mitogenic factor that can stimulate the division and proliferation of various cells, including vascular smooth muscle cells, fibroblasts, and glial cells. It has a regulatory effect on ontogenesis and cell differentiation and plays a prominent role in wound healing [33]. PDGFbb, a subtype of PDGF, particularly exhibits a strong mitogenic effect on fibroblasts [34], thereby contributing to a profibrotic effect and serving as a common component in fibrosis models [35]. Scar-derived fibroblasts have been reported to express higher levels of PDGF receptor (PDGFR) than fibroblasts from normal skin [36], with elevated PDGF expression observed in both epidermis and dermis of HS [37, 38]. Endothelial cells can enhance the proliferation and migration of fibroblasts by secreting cytokines such as PDGF, thus participating in the process of HS formation [39]. Besides, PDGFbb has also been shown to induce cyclooxygenase gene expression in keloid and HS fibroblasts [40]. The PDGF/PDGFR signaling pathway plays a key role in angiogenesis and fibrosis [41, 42] and is closely associated with the activation of transmembrane receptor tyrosine kinases [43]. Studies have shown that pleiotrophin (PTN) is upregulated in HS and promotes keratinocyte mitosis [44]. PTN expression is regulated by PDGF [45], and PTN can modulate lung epithelial cell proliferation through the β-catenin and Notch pathways [46], suggesting that PDGF may contribute to HS formation by regulating PTN levels and subsequently activating the β-catenin and Notch pathways. Additionally, PDGF can stimulate the formation of myofibroblasts and increase TGF-β receptor expression [47], implying that PDGF may be involved in scar fibrosis through TGF-β-related pathways. PDGFbb also induced the activation and proliferation of hepatic fibroblasts, while its associated PI3K and ERK pathways serve as effective targets for inhibiting fibrosis [48]. Recent research has indicated that the inhibitory effect of endostatin on HS by regulating the PDGFRβ/ERK pathway further emphasizes the promise of targeting the PDGF-related pathway in HS management [49]. Our study aligns with these findings, suggesting that increased circulating PDGFbb levels are associated with an elevated risk of HS. Further analysis of the PDGF-related genetic variants in the noncoding regions of genes will aid in understanding more mechanisms involved in regulating HS formation.
FGF-basic, a mesenchymal-derived growth factor, plays a crucial role in promoting cell growth and differentiation, with significant involvement in processes such as mitosis, angiogenesis, and wound healing [50]. In both animal models and clinical practice, FGF-basic had been shown to accelerate the healing of acute and chronic wounds [51–53]. Its expression increases in response to injury, which helps reduce fibrosis following tissue damage [54]. Direct application of FGF-basic to skin wounds also inhibits scar hypertrophy and expansion [50]. Studies involving FGF-basic gene knockout mice demonstrated delayed wound healing in excised skin, underscoring the importance of FGF-basic in wound healing and scar reduction [55]. Animal experiments indicated that FGF-basic can reduce scar weight and collagen content, potentially due to the activation of signaling pathways that upregulate MMP1 expression, a matrix metalloproteinase responsible for collagen degradation [56]. Moreover, FGF-2 significantly lowers IL-6 levels, thereby mitigating excessive collagen and extracellular matrix production during fibrosis [57]. FGF-basic can promote the downregulation of TGF-β1 activity by inhibiting the Smad signaling pathway, thereby counteracting the profibrotic effects of TGF-β [21]. It had also shown that triamcinolone can enhance FGF-basic expression while reducing TGF-β production in the skin [58]. In rabbit corneal fibroblasts, FGF-basic promotes cell migration, reduces myofibroblast numbers, and decreases scar formation. This process may be mediated through the ERK1/2 and p38 signaling pathways, which are part of the mitogen-activated protein kinase (MAPK) cascade [59]. FGF-basic also activates the Notch1/Jagged1 pathway to inhibit the differentiation of epidermal stem cells into myofibroblasts, thereby reducing scar formation [60]. Additionally, FGF-basic induces myofibroblast apoptosis through the PI3K-Akt and Rho-Rho signaling pathways, though it has no effect on fibroblasts [61]. Our findings did not prove a significant association between the genetically predicted circulating FGF-basic levels and HS, although previous studies suggested a close relationship between the two. Future research on the pathways involving FGF-basic in HS remains promising.
A variety of methods, including surgical and conservative approaches such as z-plasties, radiotherapy, and glucocorticoid injections, are currently employed for HS treatment [5]. Despite considerable advancements in the treatment strategies, a comprehensive cure for HS remains elusive [62]. As mentioned above, the intricate interplay of diverse inflammatory responses during wound healing hints the complexity of the pathogenesis. In the future, multiple experiments targeting cytokines may provide advanced treatment options for HS. This paper represents, to the best of our knowledge, the first study to explore the association between circulating cytokines and the risk of HS using MR approaches. Leveraging genetic information mitigates confounding factors and diminishes the likelihood of reverse causality, thereby offering robust causal associations. However, two-sample MR analysis provides genetically predicted associations, necessitating subsequent studies, including integrated multiomics analysis, in vitro cell experiments, animal experiments, and clinical research, to accelerate the exploration of cytokine-related mechanisms. We can collect HS or keloids tissues for RNA sequencing [63] or utilize publicly available sequencing data from the GEO database [64] to identify differentially expressed genes between scar samples and normal samples and then perform enrichment analysis to find relevant signaling pathways. For example, Jiayi Mao et al. have employed mRNA, miRNA, and single-cell RNA sequencing to elucidate a comprehensive transcriptomic landscape and intercellular communication network in keloid, providing insights for future research [65]. Next, we can verify the expression of target genes by PCR, western blotting, and immunohistochemistry, selecting appropriate pathways for regulation analysis. Overexpression, inhibition, or knockout of pathway genes in cell and animal experiments can be used to compare the levels of fibronectin, collagen, and other proteins in fibroblasts, the changes of cell proliferation and migration, and the growth of scar, thereby clarifying the regulatory mechanism of the pathway and providing a basis for selecting drug targets [63, 64, 66]. Given that circulating cytokine levels are associated with the risk of HS, measuring circulating levels of cytokines may also aid in the scar prevention and prognosis. For instance, early identification of HS risk in patients with wound through assessment of cytokine levels or predicting therapeutic effect in patients with HS based on cytokine levels represents potential clinical research directions for exploration in the future.
However, certain limitations in the study merit consideration. First, there are differences in genetic structure, mutation frequency, and other factors across populations. Effective population size may influence genetic structure by reducing selective strength and increasing the frequency of certain genetic variants through genetic drift. In small populations, genetic variants change more rapidly, leading to fluctuations that cause the disappearance of some alleles and the fixation of others, thus altering the genetic structure of the population. The population’s history also impacts its genetic structure, which may be related to diverse evolutionary responses [67]. The data in this study were derived from the Finnish population, which limits the generalizability of the results. Future research will need to validate these findings using data from other populations of different ethnicities. Second, there may be some sample overlap between the populations from which the exposure and outcome data were derived. Although the overlap rate (assume that the outcome population entirely encompasses the exposure population: 8293/208,248 × 100% = 3.98%) is below 10%, allowing for over 90% statistical power in the full sample subgroup analysis [68], this overlap may introduce potential weak instrumental bias, increasing the likelihood of Type I errors. Third, a relatively relaxed significance threshold of p < 5 × 10−6 was used to select the IVs. It raised the possibility of bias due to some weak IVs. However, the F statistics for the IVs are all greater than 10, reducing the likelihood of weak instrumental bias. Fourth, although efforts were made to address pleiotropy by searching for potential IV’s secondary phenotypes, complete elimination of this possibility is challenging. Fifth, genetic variation typically has a small effect on most risk factors, and MR analysis can only be applied to risk factors with suitable genetic variants. By utilizing multiple genetic variants as IVs, statistical power can be enhanced, often requiring larger sample sizes [69]. However, large sample data are constrained by resources and technology. Currently, there are limited GWASs on HS, and the relatively small sample size of HS in this study may increase the risk of statistical insignificance and potential bias. As sample sizes become more widely available in the future, this limitation can be addressed.
In conclusion, our study established a suggestive causal relationship between circulating IL-2/PDGFbb levels and the risk of HS. These findings provided an epidemiological foundation for drug therapies targeting cytokines to prevent and treat HS. The clinical significance of IL-2 and PDGFbb in relation to the HS risk warrants further exploration.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Xi Xu, Liwei Fang, and Xinyi Li made substantial contributions to conception and design. Liwei Fang and Jinpeng Hu analyzed the data. Xi Xu, Liwei Fang, and Wei Zhang drafted the manuscript. Xiaojing Li revised the manuscript. All authors have read and approved the final submitted version of manuscript. Xi Xu, Liwei Fang, and Jinpeng Hu have contributed equally to this work and share first authorship.
Funding
This study was supported by grants from Scientific Research Projects of Colleges and Universities in Anhui Province (No. 2022AH051191).
Acknowledgments
We thank all the GWASs for making the summary data accessible, and we thank all of the researchers and participants who contributed to those studies.
Supporting Information
Figure S1. Scatter plots of genetic associations between cytokines and hypertrophic scar using different MR methods. (A) FGFbasic; (B) IL-2; (C) PDGFbb.
Figure S2. Leave-one-out analysis of estimating the impact of individual SNPs on the overall MR analysis. (A) FGFbasic; (B) IL-2; (C) PDGFbb.
Table S1. Details of all instrumental variables (SNPs) associated with 41 circulating cytokine levels after clumping and harmonization.
Table S2. Effect estimates of the associations between circulating levels of 41 cytokines and risk of hypertrophic scar in MR analyses.
Table S3. Details of the genetic variants with potential pleiotropy among instrumental variables.
Table S4. Effect estimates of the associations of circulating levels of FGFbasic, IL-2, and PDGFbb with risk of hypertrophic scar after excluding potential pleiotropic SNPs.
Table S5. Steiger test for circulating levels of 41 cytokines on hypertrophic scar. STROBE-MR checklist. STROBE checklist of recommended items to address in the report of the Mendelian randomization study.
Open Research
Data Availability Statement
The majority of the data presented in the study are included in the article and supporting files; the original datasets and R codes used and/or analyzed are available from the corresponding author on reasonable request.