The Role of Negativity Bias in Emotional and Cognitive Dysregulation: A Neuroimaging Study in Anxiety Disorders
Abstract
Negativity bias, the cognitive tendency to prioritize negative stimuli, is commonly observed in anxiety disorders and contributes to emotional and cognitive dysregulation. This study investigated the associations between conscious negativity bias, brain function, reported symptoms, and cognitive performance in 1990 patients with anxiety disorders. We hypothesized that greater negativity bias would be linked to altered brain function in regions involved in cognitive control and attention, deficits in memory, stress and anxiety control, resilience, and symptoms of negative affect and emotional instability. Voxel-based analysis of single photon emission computed tomography (SPECT) scans revealed significant hypoperfusion in the frontal, temporal, and parietal lobes, regions critical for cognitive control and emotional regulation. Increased perfusion in cerebellar lobules IV–VI, associated with somatomotor and emotional processing, was also observed. These neural patterns were strongly aligned with patient-reported symptoms, including elevated anxiety, depression, intrusive thoughts, decreased motivation, and suicidal ideation. In addition, cognitive assessments from the total brain platform showed that higher negativity bias was linked to deficits in emotional regulation, memory, stress and anxiety control, and resilience. These findings suggest that negativity bias contributes to widespread brain dysfunction, exacerbating emotional instability, and cognitive control deficits in patients with anxiety disorders. This study highlights the importance of targeting negativity bias in therapeutic interventions to improve emotional and cognitive outcomes. Future research should investigate the neural mechanisms linking negativity bias to mental health outcomes.
1. Introduction
Negativity bias refers to the greater subjective impact of negative events compared to positive events of the same objective magnitude, as well as the higher likelihood of interpreting ambiguous stimuli as negative [1–3]. In individuals with heightened conscious negativity bias, negative aspects or threats in a situation are magnified, significantly influencing decisions and behaviors, rather than emphasizing the positive aspects. This affects everyday regulation of thoughts and emotions. Conversely, nonconscious negativity bias involves an automatic, uncontrolled focus on negative emotional states without subjective awareness. The phenomenon of negativity bias influences various psychological processes, including physiological arousal, perception, attention, decision-making, emotion, learning, memory, and social interactions [3, 4]. It is also central to prospect theory, particularly the concept of loss aversion, which highlights the greater emotional weight of negative events compared to positive ones [2]. The prospect function illustrates that losses are felt more intensely than equivalent gains, reinforcing our tendency to prioritize negative information [5]. This connection between negativity bias and loss aversion highlights how these biases shape psychological responses, decision-making processes, and judgments. At a higher cognitive level, negative events are thought to hold greater significance than positive ones, requiring more attention, cognitive processing, and complex mental representations [6]. Supporting this, studies suggest that people spend more time focusing on negative stimuli than positive ones [7–9].
Negativity bias encompasses several key aspects that shape our emotional and cognitive processes. Negative potency refers to the idea that negative events have a stronger subjective impact compared to their positive counterpart, making them more memorable, vivid, and of greater emotional impact. This heightened negative potency is paired with the greater steepness of negative gradients, which suggests that negative events tend to escalate in emotional intensity more rapidly than positive experiences as they draw near. Another key aspect is negativity dominance, where our perception of an experience tends to skew more negative, often leading to a more pessimistic overall assessment. Finally, greater negative differentiation suggests that negative stimuli or events are typically perceived as more complex and distinct than their positive counterparts, making them stand out more in our minds [3].
Negativity bias has been associated with various psychological and physiological effects. A study investigating brain region activation during conscious fear found that brain regions such as the midbrain, bilateral amygdala, right dorsal and ventral medial prefrontal cortices (mPFC), and bilateral anterior cingulate cortex were significantly more active in individuals with negativity bias compared to those with positivity bias [10]. The negativity bias group also exhibited a heightened startle response and elevated heart rate during fear perception. These findings suggest that negativity bias is linked to an increased sensitivity to fear cues of potential danger, even at the earliest stages of automatic emotional processing [10]. Additionally, negativity bias has been linked to improved learning of negative contingencies, meaning individuals with this bias are more adept at recognizing and learning about situations or stimuli associated with negative outcomes or threats [11]. Similarly, negativity bias has been linked to depression, with depressed adults exhibiting higher negativity bias and lower positivity offset compared to healthy individuals [12]. Furthermore, high-risk individuals for depression were found to be more accurate in consciously identifying disgust, more attuned to nonconsciously recognizing sad faces, and more likely to interpret neutral faces as angry [13]. Anxiety disorders have also been associated with a bias toward negative stimuli. Individuals exhibiting higher levels of anxiety tend to direct their attention to emotionally negative or threatening stimuli, as observed in tasks like the dot-probe and emotional Stroop [14, 15]. Such cognitive biases are notably linked to social anxiety disorders [16]. At the neural level, dorsal regions of the prefrontal cortex are involved in behavioral responses to threats. These regions are typically activated during conscious threat appraisal in healthy individuals but show overactivity during threat appraisal in those with pathological anxiety [17].
Functional magnetic resonance imaging (fMRI) studies in both anxiety and depression reveal neural patterns associated with emotional processing and negativity bias. In anxiety disorders, fMRI studies have shown reduced activation of the central striatum [18] and deactivation in the default mode network, particularly in regions like the MPFC, posterior cingulate cortex (PCC), and inferior parietal cortex [19]. Similarly, in depression, biased attention—such as negativity bias—has been linked to decreased activation in brain regions such as the superior parietal lobe, ventrolateral prefrontal cortex (VLPFC), and dorsolateral prefrontal cortex (DLPFC) [20–22]. Patients with depression also exhibit reduced positive affect and a diminished response to rewards, which are associated with decreased activity in the nucleus accumbens and prefrontal regions [21]. Negativity bias itself appears to be encoded at the neural level, where the right inferior frontal/insular cortex showed greater activation in response to negative stimuli compared to positive stimuli [23]. Research on the neural processing of emotional intensity and valence has also highlighted the role of the amygdala, which is more heavily involved in processing emotional intensity than in distinguishing between positive and negative valence [24, 25]. Notably, negative stimuli tend to be rated as more emotionally intense than positive stimuli [26] and several studies have shown that the amygdala exhibits a greater response to negative stimuli, even when their intensity is matched with positive stimuli [27, 28]. Together, these findings suggest that negativity bias is encoded at the neural level.
Overall, negativity bias has been linked to a range of mental health conditions and brain function deficits, underscoring its clinical relevance in treating mental health illnesses. In this paper, we contribute to the existing literature by exploring the relationships between conscious negativity bias, brain function, resilience, memory, and symptoms in patients diagnosed with anxiety disorders. We hypothesize that patients with anxiety disorders who exhibit greater conscious negativity bias will demonstrate altered brain function in regions involved in cognitive control and attention, experience deficits in memory, stress and anxiety control, resilience, and display symptoms related to negative affect and emotional dysregulation.
2. Methods
This study utilized a retrospective clinical dataset consisting of 1990 patients diagnosed with an anxiety disorder (Table 1), who were evaluated at Amen Clinics Inc. (ACI) mental health facilities over the past 2 years. The anonymized database was made available for research use with informed consent from all patients during their initial clinic visit (Integ Review Board #004-Amen Clinics Inc.). Patients were excluded if they had comorbid diagnoses of epilepsy, substance abuse or dependence-related disorders, toxic encephalopathy, brain lesions, tumors, brain resections, any dementias, mild cognitive impairment, or if they reported taking psychiatric medications at the time of intake. All patients had an anxiety disorder diagnosis, along with other comorbidities (see Supporting Information S1 for comorbid frequencies). Each patient underwent a single photon emission computed tomography (SPECT) brain scan and completed a 300-item self-reported DSM-based assessment battery, covering topics related to brain health, general symptoms, and learning disabilities (5-point Likert scale 0 = Never to 4 = Very Frequently), and a Total Brain assessment battery. Additionally, conscious negativity bias was measured within the Total Brain platform1, which assesses an individual’s ability to interpret and respond to primary emotions, particularly negative ones like fear, anger, and sadness. SPECT scans were acquired during rest at one of 11 ACI clinics as part of patients’ clinical intake evaluations. The scans and symptom checklists were collected prior to any treatments and/or diagnoses at ACI. Diagnosis data were obtained from the patients’ medical charts and assigned by the ACI treating physician based on DSM IV/V criteria.
| N | Age | Sex at birth | Comorbidities | Conscious negativity bias z-scores | Race and ethnicity |
|---|---|---|---|---|---|
| 1990 | 38.8 ± 14.8 |
|
4.9 ± 2.1 | −1.1 ± 1.1 |
|
SPECT scans were acquired using InterMedical MultiCam 3000eco triple-headed gamma cameras (Intermedical Medizintechnik GmbH, Lubbecke, D-32312, Germany). For each procedure, an age- and weight-appropriate dose of 99mTc-hexamethylpropyleneamine oxime (HMPAO) was administered intravenously. Participants received the injection while sitting in a dimly lit room with their eyes open and were scanned ~30 min post-injection. Data acquisition yielded 120 images per scan, each separated by three degrees, covering a full 360-degree rotation. A low-pass filter with a high cutoff was applied, followed by Chang attenuation correction [29, 30]. The resulting reconstructed image matrices had dimensions of 128 × 128 × 78 with voxel sizes of 2.5 mm3.
For the neuroimaging analyses, the images were aligned to Montreal Neurological Institute (MNI) space using a SPECT template and the Advanced Normalization Tools (ANTs version 2.2.0; [31]; RRID:SCR_004757), consistent with the methods from Keator et al. [32]. SPECT images were scaled to the within-scan maximum voxel, as per clinical practice, and noise outside the brain was removed using a 50% maximum threshold prior to registration. After alignment to MNI space, a brain mask derived from the MNI 152 template [33, 34] was applied to delineate the un-thresholded images for use in the statistical models. Registered SPECT scans had an image matrix of 79 × 96 × 68 with isotropic voxel sizes of 2.0 mm3 and were visually inspected to ensure the absence of severe anatomical abnormalities and proper alignment to MNI space. Voxel-based linear models were constructed using the SPM122 Statistical Parametric Mapping tool (RRID:SCR_007037) [35]. A voxel-based multiple regression model was used to assess cerebral perfusion in relation to lower conscious negativity bias z-scores (i.e., greater negative thinking) with ANCOVA normalization. Nuisance covariates included sex at birth, acquisition site, age, and the number of comorbid diagnoses (see Supporting Information S1 for diagnostic frequencies). Results were corrected for multiple comparisons using whole-brain False-Discovery Rate (FDR) correction as implemented in SPM12, with a threshold of p < 0.05. Anatomic localization of significant differences was assessed using the AAL3 toolbox [36] in SPM12. Additionally, to further explore differences observed in cerebellar sub-regions and their association with cognitive processes and networks, we used a cerebellar atlas derived from resting-state functional connectivity data across 1000 subjects. This was overlaid with the seven-network atlas developed by Buckner and Yeo et al. [37, 38].
To explore symptoms associated with conscious negativity bias, ordinal regression models were developed for each assessment question using the R statistical analysis software (RStudio version 2023.06.0+421; RRID:SCR_001905). The models included age, sex at birth, acquisition site, and the number of comorbid diagnoses as nuisance covariates. Additionally, to understand the associations with memory, stress and anxiety control, and resilience from the Total Brain platform, multiple regression models were constructed in R using the Total Brain reported z-score measurements while also covarying for age, sex at birth, acquisition site, and number or comorbid diagnoses. Mean differences for each regression model were evaluated and corrected for multiple comparisons using the Holm [39] method.
3. Results
3.1. Voxel-Based Neuroimaging Results
Results from the voxel-based whole-brain neuroimaging analysis at rest (t [1, 1984] = 3.56; p < 0.05 FDR) yielded predominantly decreased brain function in association with higher conscious negativity bias, with the exception of the cerebellum (see Figure 1, Table 2). Specifically, decreased function was observed in the bilateral frontal, temporal, and parietal lobes, as well as the insula. In contrast, increased function was observed in lobules IV–VI of the cerebellum and an area anterior to the brainstem near the ventral aspect of the hypothalamus. When evaluating the functional role of these cerebellar lobules using a resting-state functional connectivity-based atlas [37, 38], we found these subregions to be associated with the somatomotor network.
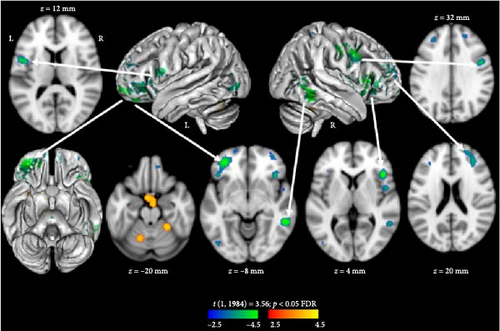
| Coordinate (x,y,z) | Region | Direction | T (1, 1984) | p-Value FDR |
|---|---|---|---|---|
| Frontal lobe | ||||
| 44.0, 24.0, 2.0 | Frontal_Inf_Tri_B |  |
4.76 | 8.76e−03 |
| 48.0, 2.0, 38.0 | Precentral_R |  |
4.61 | 8.76e−03 |
| 52.0, 8.0, 26.0 | Frontal_Inf_Oper_R |  |
3.72 | 2.43e−02 |
| −34.0, 44.0, −10.0 | Frontal_Mid_2_L |  |
4.58 | 8.76e−03 |
| −44.0, 26.0, −6.0 | Frontal_Inf_Orb_2_L |  |
3.99 | 1.57e−02 |
| −52.0, 6.0, 10.0 | Frontal_Inf_Oper_L |  |
4.13 | 1.29e−02 |
| −52.0, 6.0, 10.0 | Rolandic_Oper_L |  |
4.13 | 1.29e−02 |
| −44.0, 2.0,12.0 | Rolandic_Oper_L |  |
4.12 | 1.29e−02 |
| 50.0, 4.0, 2.0 | Rolandic_Oper_R |  |
3.99 | 1.59e−02 |
| 34.0, 38.0, 20.0 | Frontal_Mid_2_R |  |
3.83 | 2.02e−02 |
| 28.0, 56.0, 18.0 | Frontal_Sup_2_R |  |
3.73 | 2.41e−02 |
| −10.0, 50.0, −14.0 | Frontal_Med_Orb_L |  |
3.76 | 2.28e−02 |
| 12.0, 48.0, −18.0 | OFCmed_R |  |
3.72 | 2.41e−02 |
| 12.0, 48.0, −18.0 | Rectus_R |  |
3.72 | 2.41e−02 |
| 8.0, 54.0, −14.0 | Frontal_Med_Orb_R |  |
3.40 | 3.77e−02 |
| 8.0, 54.0, −14.0 | Rectus_R |  |
3.40 | 3.77e−02 |
| −28.0, 38.0, 30.0 | Frontal_Mid_2_L |  |
3.56 | 3.04e−02 |
| −28.0, 38.0, 30.0 | Frontal_Sup_2_L |  |
3.56 | 3.04e−02 |
| 38.0, 54.0, −8.0 | Frontal_Mid_2_R |  |
3.55 | 3.10e−02 |
| 18.0, 44.0, 36.0 | Frontal_Sup_2_R |  |
3.50 | 3.31e−02 |
| −32.0, 10.0, 48.0 | Frontal_Mid_2_L |  |
3.34 | 4.04e−02 |
| −18.0, 64.0, −6.0 | Frontal_Sup_2_L |  |
3.33 | 4.08e−02 |
| Temporal lobe | ||||
| 54.0, −42.0, −10.0 | Temporal_Mid_R |  |
4.91 | 8.76e−03 |
| −58.0, −46.0, −16.0 | Temporal_Inf_L |  |
3.41 | 3.73e−02 |
| Sub-cortical | ||||
| 50.0, 4.0, 2.0 | Insula_R |  |
3.99 | 1.59e−02 |
| Occipital | ||||
| −46.0, −76.0, −2.0 | Occipital_Mid_L |  |
3.90 | 1.74e−02 |
| 26.0, −36.0, 52.0 | Postcentral |  |
3.57 | 3.02e−02 |
| Cerebellum | ||||
| −16.0, −60.0, −16.0 | Cerebelum_6_L |  |
4.34 | 2.39e−02 |
| 26.0, −44.0, −22.0 | Cerebelum_4_5_R |  |
4.23 | 2.39e−02 |
- Note: The table includes the MNI-Talairach coordinates in millimeters from the origin (coordinate), brain region (region), direction of perfusion (direction) (
 = decreased perfusion or
= decreased perfusion or  = increased perfusion), t-score (t), and false discovery rate corrected p-values (p-value FDR).
= increased perfusion), t-score (t), and false discovery rate corrected p-values (p-value FDR).
3.2. Symptom and Total Brain Associations
To understand the patient-reported symptoms associated with higher conscious negativity bias, we evaluated associations with participant-reported symptoms from the 300 question assessments administered at intake for all patients in the cohort who responded to each question. Table 3 highlights a subset of significant symptom-related differences that align with the neuroimaging findings (see Supporting Information section S2 for the complete list of significant differences). Notably, the symptoms most consistent with the neuroimaging results fall into several categories, including depressive and anxiety-related symptoms, repetitive and intrusive thoughts, decreased interest and motivation, suicidal ideation, and emotional instability. These findings suggest a pattern of emotional dysregulation, difficulties in cognitive control, and challenges in managing anxiety.
| Symptoms | R | Lower | Upper | df | p unc. | p FDR |
|---|---|---|---|---|---|---|
| Depressive symptoms | ||||||
| Experiencing frequent feelings of sadness | −0.55 | −0.58 | −0.51 | 1617 | 3.53E−127 | 1.57E−124 |
| Having feelings of helplessness or powerlessness | −0.53 | −0.57 | −0.50 | 1616 | 2.54E−120 | 5.65E−118 |
| Having feelings of negativity | −0.52 | −0.55 | −0.48 | 1623 | 5.71E−111 | 6.34E−109 |
| Feeling excessive guilt | −0.44 | −0.48 | −0.40 | 1608 | 1.88E−78 | 8.33E−77 |
| Having crying spells | −0.42 | −0.46 | −0.38 | 1604 | 3.17E−70 | 9.39E−69 |
| Anxiety symptoms | ||||||
| Tending to predict the worst | −0.41 | −0.45 | −0.37 | 1598 | 3.62E−67 | 9.45E−66 |
| Experiencing excessive or senseless worrying | −0.41 | −0.45 | −0.37 | 1538 | 1.91E−64 | 4.47E−63 |
| Frequently feeling nervous or anxious | −0.40 | −0.44 | −0.36 | 1616 | 1.78E−63 | 3.95E−62 |
| Having unrealistic or excessive worry in at least a couple areas of your life | −0.41 | −0.45 | −0.37 | 1533 | 2.11E−63 | 4.46E−62 |
| Repetitive and intrusive thoughts | ||||||
| Tending to have repetitive negative thoughts | −0.46 | −0.49 | −0.42 | 1623 | 3.40E−84 | 1.89E−82 |
| Fearing going crazy or doing something out of control | −0.40 | −0.44 | −0.35 | 1543 | 1.68E−59 | 2.77E−58 |
| Decreased interest and motivation | ||||||
| Having lowered interest in things that are usually fun | −0.46 | −0.50 | −0.43 | 1610 | 4.56E−87 | 2.89E−85 |
| Having a marked decreased interest in important activities | −0.43 | −0.47 | −0.39 | 1530 | 5.21E−71 | 1.93E−69 |
| Feeling overwhelmed by the tasks of everyday living | −0.42 | −0.46 | −0.38 | 1541 | 2.30E−66 | 5.66E−65 |
| Feeling apathetic or unmotivated | −0.38 | −0.42 | −0.34 | 1620 | 3.91E−57 | 5.98E−56 |
| Suicidal thoughts | ||||||
| Having suicidal feelings | −0.43 | −0.47 | −0.39 | 1589 | 3.67E−73 | 1.48E−71 |
| Having recurrent thoughts of death or suicide | −0.43 | −0.47 | −0.39 | 1538 | 1.44E−70 | 4.90E−69 |
| Emotional instability | ||||||
| Having feelings of moodiness | −0.42 | −0.46 | −0.38 | 1618 | 3.40E−70 | 9.42E−69 |
| Feeling socially isolated or withdrawn | −0.41 | −0.45 | −0.36 | 1514 | 3.43E−61 | 6.34E−60 |
| Feeling detached or distant from others | −0.40 | −0.44 | −0.35 | 1537 | 4.69E−59 | 7.44E−58 |
- Note: Table shows the selected symptoms (symptoms), coefficient (R), lower and upper bounds of the confidence interval (lower, upper), degrees of freedom (df), uncorrected (p unc.), and false discovery rate (p FDR) corrected p-values.
To further explore the associations between conscious negativity bias and cognitive and emotional functioning, we evaluated measures from the Total Brain [40] assessment battery. The significant results indicate that higher conscious negativity bias in patients with anxiety disorders is linked to reduced performance in measures of emotion regulation, mood control, cognition, memory, and resilience (Table 4). The “feeling domain score” represents the average across three types of control: stress, anxiety, and depressed mood. The questions comprising this variable focus on behavioral, cognitive, and emotional responses to stressful situations, autonomic nervous system overactivity, thoughts of worry, feelings of despair, low motivation, and the likelihood of achieving personal goals. Notably, each of these control variables remained significant when evaluated separately. The “overall score” is an average of markers related to self-control, emotion, feeling, and cognition. These markers emphasize the shaping and planning of cognition and emotion over time to maximize well-being, as well as automatic and nonconscious processes that help minimize danger and maximize reward. This score also reflects the conscious experience of negative emotions and selective awareness in information processing. The capacity score for “resilience” represents the ability to navigate stress and adversity, allowing them to cope and recover from challenging events. Furthermore, there was a significant association between conscious negativity bias and a “verbal memory recognition task,” which assessed both delayed and immediate recall. This task aimed to measure an individual’s capacity to retrieve past information and evaluate their encoding and storage capabilities. Finally, the “thinking domain score” is a summary score averaged across cognitive tasks, including digit span, verbal interference, continuous performance (1-back), and a maze task. These tasks are designed to assess memory, controlled attention, sustained attention, and planning.
| Total brain variables | coef | SE | t-score | df | p unc. | p FDR |
|---|---|---|---|---|---|---|
| Feeling domain score (averaging across stress, anxiety, and depressive mood control) | 0.53 | 0.01 | 57.79 | 1988 | 0.00E+00 | 0.00E+00 |
| Capacity scores; depressive mood control | 0.61 | 0.01 | 53.13 | 1988 | 0.00E+00 | 0.00E+00 |
| Overall score (Averaging across self-control, emotion, feeling and thinking) | 0.27 | 0.01 | 41.53 | 1988 | 5.75E−272 | 1.61E−270 |
| Capacity scores—stress control | 0.51 | 0.01 | 38.48 | 1988 | 1.34E−242 | 2.25E−241 |
| Capacity scores—anxiety control | 0.47 | 0.02 | 30.09 | 1988 | 2.92E−164 | 4.09E−163 |
| Capacity scores—resilience | 0.56 | 0.02 | 24.09 | 1988 | 9.31E−113 | 1.12E−111 |
| Verbal memory recognition task—delayed memory | 0.11 | 0.03 | 3.65 | 1941 | 2.67E−04 | 2.49E−03 |
| Thinking domain score; Summary score averaging across cognitive tasks (excluding Emotion Awareness and Emotion Bias tasks) | 0.04 | 0.01 | 3.30 | 1988 | 1.00E−03 | 8.41E−03 |
| Verbal memory recognition task—immediate memory | 0.10 | 0.03 | 3.21 | 1980 | 1.33E−03 | 1.01E−02 |
- Note: Table shows the significant variables (total Brain variables), coefficient (coef), standard error (SE), t-score, degrees of freedom (df), uncorrected (p unc.), and false discovery rate (p FDR) corrected p-values.
4. Discussion
This study aimed to investigate the brain- and symptom-based associations with negativity bias in patients diagnosed with anxiety disorders, providing valuable insights into both neural and psychological processes. Our findings revealed that increased negativity bias correlates with decreased activation in several key brain regions, particularly the bilateral frontal, temporal, and parietal lobes, as well as the insula. Conversely, we observed increased activation in specific cerebellar regions and brainstem. These results suggest that conscious negativity bias is linked to widespread dysfunction across brain networks critical for emotional regulation, cognitive control, and motor functions.
The decreased function observed in the frontal lobe, particularly in the DLPFC, aligns with prior research linking the DLPFC to mood regulation and cognitive control. Dysfunction in this region has been associated with anxiety and depressive disorders, where patients often exhibit impaired cognitive control and difficulty managing negative emotions [41, 42]. The reduced DLPFC activity observed in this study supports these findings, suggesting that patients with greater negativity bias experience more severe disruptions in cognitive and emotional regulation. This dysfunction may manifest in depressive and anxiety-related symptoms, including emotional instability, repetitive thoughts, and diminished motivation [43, 44].
Our findings of increased cerebellar activation are particularly intriguing. Traditionally viewed as a center for motor coordination, the cerebellum’s role in emotional regulation is increasingly recognized [45]. The hyperactivity observed in lobules IV–VI, which have been associated with the somatomotor network, suggests a relationship between negativity bias and heightened autonomic arousal, possibly linked to anxiety [46]. Previous studies have reported cerebellar hyperactivity in major depressive disorder [47], further supporting the notion that the cerebellum contributes to emotional dysregulation and may play a role in anxiety disorders. Additionally, the observed increased activation of an area anterior to the brainstem near the ventral aspect of the hypothalamus could potentially reflect hypothalamic–pituitary–adrenal (HPA) axis dysregulation. The HPA axis is involved in stress response and causes an increase of cortisol, the stress hormone which affects the sympathetic nervous system [48]. Increased and prolonged activity of the HPA axis has been linked to various psychiatric disorders, including depression, anxiety, panic disorders, and phobias. These findings of hyperactivity of the HPA axis have led some researchers to propose that a disrupted balance of postsynaptic 5-HT1A and 5-HT2A receptors may be contributing to the pathophysiology of depression and anxiety [49]. This cortisol elevation has also been linked to more negative ratings of ambiguous facial expressions and with more direct response trajectories toward negative ratings [1].
The results from the Total Brain assessments provided additional insights into how negativity bias may influence cognitive and emotional functioning. Patients exhibiting higher levels of conscious negativity bias demonstrated poorer performance in emotional and cognitive control, memory, and resilience—key components of mood and anxiety disorders. Notably, decreased resilience scores suggest a reduced ability to cope with stress and adversity, which is a hallmark of both anxiety and depression [50]. Furthermore, deficits observed in verbal memory recognition tasks underscore the cognitive impairments linked to negativity bias, particularly in the areas of information retrieval and encoding. These deficits contribute to more global cognitive dysfunction in individuals with heightened conscious negativity bias.
The strong associations between negativity bias and elevated symptoms of anxiety and depression, including suicidal ideation, reinforce previous findings that heightened negativity bias exacerbates emotional and cognitive difficulties. Research indicates that repetitive negative thinking (RNT)—a common feature in patients with anxiety and depression—is driven by dysregulation in the prefrontal cortex and insula, both of which are crucial for emotional processing and cognitive control [51]. Our results further substantiate the role of these brain regions in mediating the cognitive and emotional effects of negativity bias, contributing to psychiatric symptoms such as intrusive thoughts and suicidal ideation [52]. Furthermore, the associations between negativity bias, apathy, and reduced motivation highlight how negative cognitive patterns can diminish goal-directed behavior and interest in daily activities. The medial prefrontal cortex, particularly the orbitofrontal cortex, is critical for motivational processes, and dysfunction in this region has been linked to both apathy and depression [53, 54]. Our study’s results suggest that negativity bias may directly influence motivational deficits, further exacerbating symptoms of depression and emotional instability.
The findings from this study underscore critical areas for clinical application in treating patients with anxiety disorders, emphasizing the importance of addressing the neural correlates of negativity bias. Reduced activity in the DLPFC and hyperactivation of cerebellar regions and the hypothalamus are key contributors to cognitive control deficits and emotional instability. Therapies like transcranial magnetic stimulation (TMS) or neurofeedback could be employed to stimulate underactive regions like the DLPFC, enhancing cognitive control and emotional stability. Stress-reduction strategies, such as mindfulness-based therapy or diaphragmatic breathing, could modulate HPA axis dysregulation and reduce autonomic arousal. Additionally, tailored behavioral interventions, including cognitive–behavioral therapy (CBT), can focus on reframing repetitive negative thoughts and building resilience. These insights could further guide personalized treatment plans that incorporate cognitive training, resilience-building exercises, and potentially pharmacological approaches to address hyperactivity in the cerebellum and regulate the stress response. This neuroscience-informed approach offers a pathway to reduce anxiety symptoms while improving emotional and cognitive health in patients.
This study has several limitations. First, the study group consists of a clinical sample of patients diagnosed with anxiety disorders amongst other comorbidities. It is unclear how these comorbidities may have affected the results presented here; although, we suspect these will only increase variability and reduce our power to detect significant associations. We tried to reduce these confounds by adding regressors to our models, but we acknowledge linear models will not entirely mitigate the confounding effects on our results. Next, the treating physicians gave each patient the DSM-based diagnoses used in this study. The physicians had access to all available data including patient-reported symptoms, Total Brain summary reports, and neuroimaging readings from trained scan readers who rated broad regions in the SPECT scans as either over or underactive compared to healthy participants. Although this is a confound, none of the physicians had access to the quantitative results shown here, and diagnoses were based on DSM IV/V criteria. In interpreting the neuroimaging findings, we identified brain regions using a brain atlas and MNI-space coordinates of the largest (i.e., peak-level) associations after whole-brain thresholding. Due to signal smoothness and SPECT scan resolution constraints (~6.5 mm), additional studies should be done to validate these findings. In addition, we attempt to interpret the neuroimaging findings with respect to the anatomical regions implicated by the maximum statistical strength and associate these with prior literature, but we acknowledge that the brain is highly interconnected, and we expect there are more complex interactions occurring between brain regions that would require other modalities to investigate.
5. Conclusion
In conclusion, our findings indicate that negativity bias is associated with widespread dysfunction across multiple brain networks, resulting in symptoms of emotional instability, deficits in cognitive control, and mood dysregulation. These results underscore the importance of targeting negativity bias in therapeutic interventions aimed at improving emotional and cognitive outcomes for individuals with anxiety and depression. Future research should explore the mechanistic pathways connecting these neural and psychological factors, with an emphasis on developing strategies to mitigate the negative impact of this cognitive bias on mental health and emotional well-being.
Ethics Statement
This study was approved by the Integ Review Board (004-Amen Clinics Inc.) on 09/19/2014 and determined to be except category 4.
Conflicts of Interest
Stephanie Norris is an unpaid student intern working on this research study and has no conflicts of interest. Frank Salgado and Sydnyy Murray are employed at the Amen Clinics Inc. and support research done at the clinics and have no conflicts of interest. Daniel Amen is the founder and CEO of Amen Clinics and the Change Your Brain, Change Your Life Foundation. He receives a salary from Amen Clinics. David B. Keator has an appointment at the University of California, Irvine, studying neuroimaging biomarkers in psychiatric disorders using PET and MRI modalities in the department of Psychiatry and Human Behavior. David B. Keator is further employed at the Amen Clinics Inc. and Change Your Brain, Change Your Life Foundation and receives a salary for research studies and technological innovations for SPECT neuroimaging in psychiatry.
Funding
The authors received no specific funding for this work.
Acknowledgments
We would like to thank the patients for their participation in this exploratory study and giving their consent for the use of their anonymized data as part of their self-paid clinical evaluations at the Amen Clinics Inc. Further, we would like to thank the Amen Clinics Inc. for providing these data for this research contribution.
Endnotes
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




