Exploring Paediatric Jaw Osteosarcoma: A Detailed Case Report of a 12-Year-Old Girl and Review of the Literature
Abstract
Osteosarcoma, the most common primary bone malignancy, rarely affects the jaw, representing only 6%–10% of cases. Jaw osteosarcoma typically occurs in individuals between the ages of 30 and 50 years and is uncommon in children. It often involves the mandible, especially in males, presenting with swelling, tooth mobility, and paraesthesia rather than pain. We present a rare case of jaw osteosarcoma in a 12-year-old girl who exhibited paraesthesia and a significant mandibular mass. Imaging demonstrated extensive mandibular involvement with Codman’s triangle formation, and a biopsy confirmed the diagnosis of conventional osteosarcoma. The treatment approach included a hemimandibulectomy, followed by temporary reconstruction using a titanium plate and silastic spacer. This case underscores the importance of comprehensive evaluation and multidisciplinary management in diagnosing and treating osteosarcoma of the jaws in children. A review of 12 similar cases highlights the variability in presentation and treatment outcomes, emphasizing the need for individualized treatment plans to optimize patient prognosis.
1. Introduction
Although osteosarcoma is the most common primary malignancy of bone, only 6%–10% [1] of these lesions are localized to the jaws. When osteosarcoma of the jaw (OSJ) does occur, the affected individuals are usually between the ages of 30 and 50 years [2]. In children, the jaw is an uncommon location for osteosarcoma development.
Osteosarcoma of the axial skeleton typically affects the metaphysis of long bones during periods of skeletal development in children and adolescents, often in the second decade of life [3, 4]. However, OSJ usually occurs in the mandible, peaking one or two decades after adolescence. Males are more commonly affected by OSJ than females, with most tumours located in the mandibular molar region [1, 5].
Unlike osteosarcomas of long bones, OSJ typically presents with swelling rather than pain [3]. Patients may also report a rapidly growing soft tissue mass, tooth mobility, ulceration, nasal obstruction, and paraesthesia [6–8]. The mandibular body is the most frequently affected area, followed by the angle, symphysis, and ascending ramus. In the maxilla, the posterior alveolar process and maxillary sinus are commonly involved [9]. Osteosarcoma in the maxilla typically peaks in the 5th decade of life, while mandibular osteosarcoma occurs with similar frequency in both middle-age and older individuals [10].
Several case reports have documented paediatric OSJ, emphasizing its rarity and diagnostic challenges. Among them, cases reported by Nirmala et al. [11] and Madiraju [12] involved female patients with conventional osteosarcoma of the mandible, contributing to the understanding of its clinical presentation and management. However, certain radiographic features remain infrequently described despite these reports.
To the best of our research, no previously reported paediatric cases have demonstrated the presence of Codman’s triangle, making our case unique. This report presents a rare instance of OSJ in a child with no known contributing factors. By contextualizing our findings within the existing literature, this review aims to enhance the understanding of its diagnosis and treatment.
2. Case Report
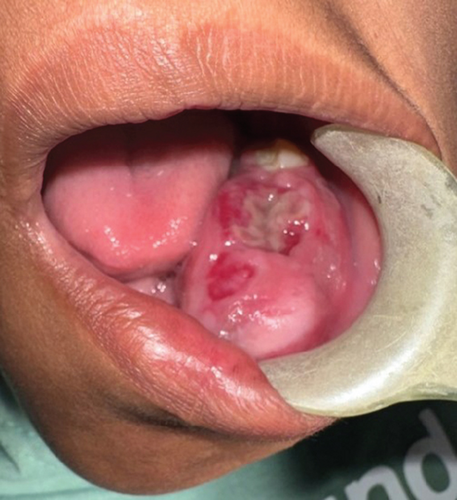
In January 2024, a 12-year-old mixed-race female presented at the University of The Western Cape Oral Health Centre with a 2-month history of an extensive mass in the left mandible, resulting in notable facial asymmetry. The patient’s medical history was unremarkable. No palpable lymph nodes were evident, but paraesthesia was present along the left inferior alveolar nerve. Intraorally, an irregular, nonpulsatile, expansive lesion with ulcerated areas was evident (Figure 1).
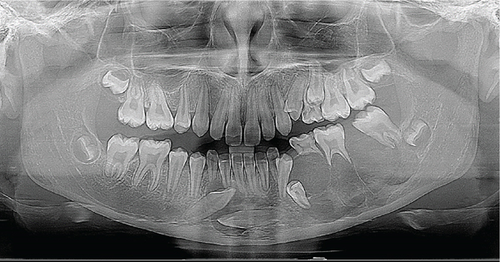
Panoramic imaging (Figure 2) revealed a partially defined, irregular, multilocular radiolucency with thin radiopaque septa in the third quadrant. The lesion extended medially, crossing the midline and encompassing the crowns of the impacted Teeth Nos. 33 and 43 (FDI classification). There was also root resorption of Tooth 36, displacement of both erupted and unerupted permanent teeth, and loss of lamina dura, giving the appearance of “floating teeth.”
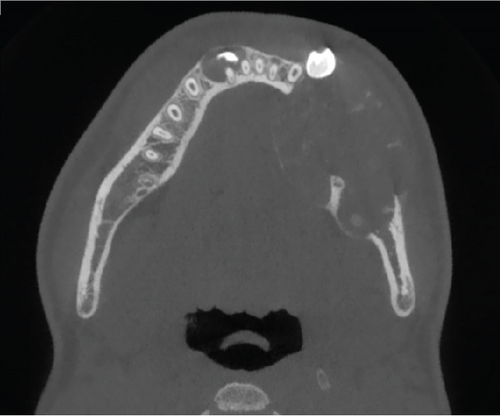
Cone-beam computed tomography (CBCT) imaging (Figures 3, 4, 5, and 6) showed a partially defined, expansile hypodensity containing hyperdense spicules in the body of the left mandible, extending from the angle to the right parasymphysis region, around the impacted canines. Codman’s triangle was observed on the lingual aspect of the mandible, along with involvement of the inferior alveolar nerve on the left side. The differential diagnosis included osteosarcoma, leukaemia, lymphoma, haemangioma, and ameloblastoma.

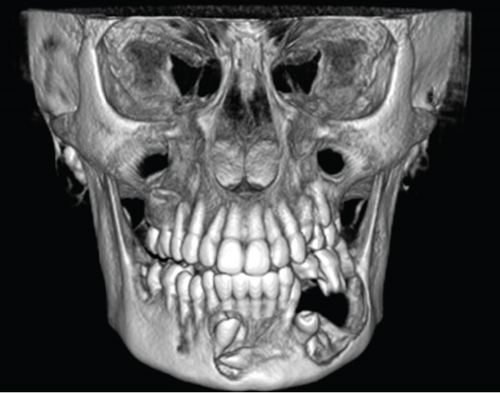
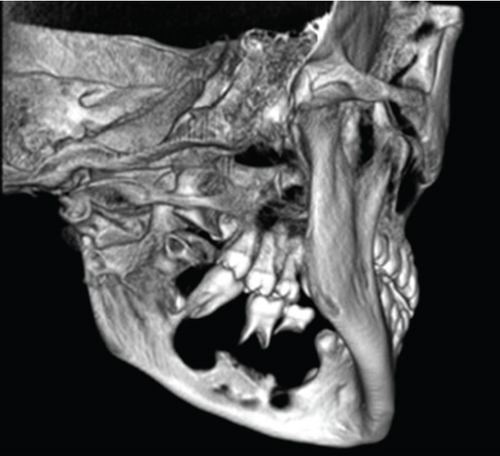
Following the initial consultation, the patient had an incisional biopsy done under general anaesthesia. Dental extractions were performed concurrently (Teeth 74 and 36) and the lesion biopsied via the extraction sockets.
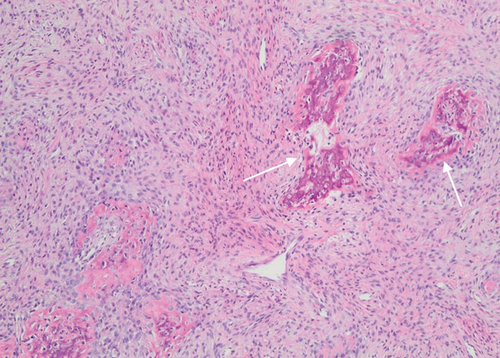
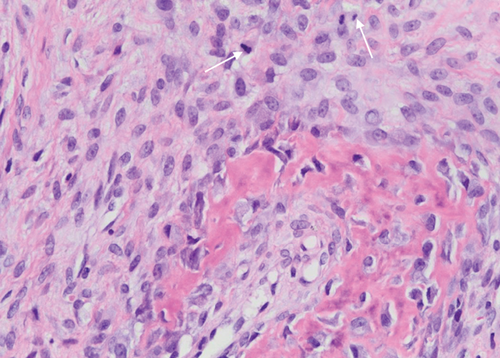
The biopsy revealed tumour tissue consisting of malignant osteoid with regions of mineralization (Figures 7 and 8). The tumour cells in between were spindled, exhibiting eosinophilic cytoplasm and irregular hyperchromatic nuclei. Scattered osteoclast-type giant cells were observed. Atypical mitotic figures were detected (arrows). This confirmed a diagnosis of conventional osteosarcoma.
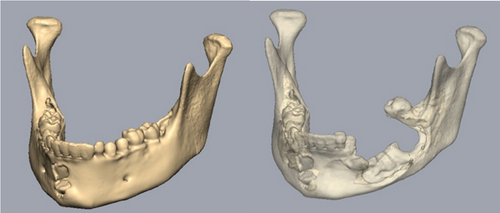
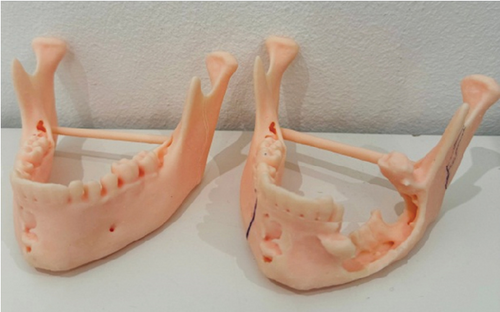
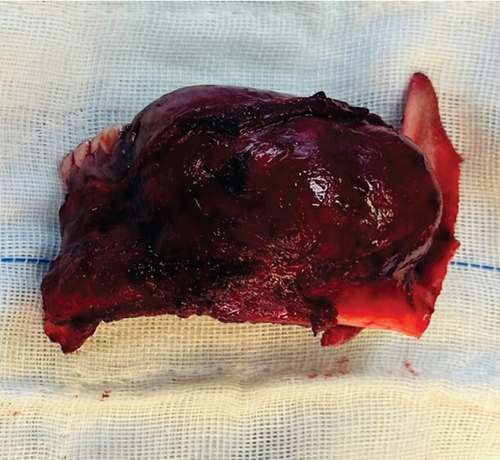
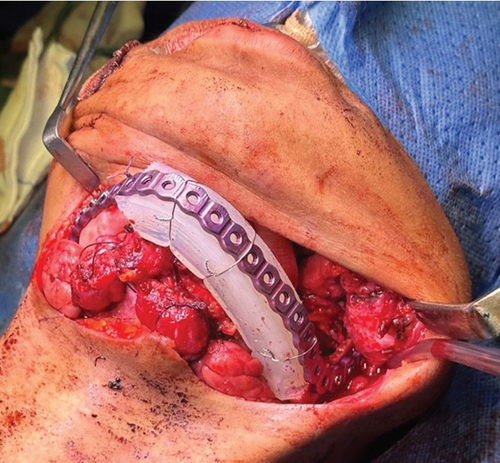
After establishing a histopathological diagnosis, surgical preparation commenced, and the patients’ CBCT scan was processed to generate a digital three-dimensional model. Tumour margins were then easily demarcated and excised digitally with the defect reconstructed by a mirror image of the unaffected contralateral side (Figure 9). A titanium reconstruction plate was then manually adapted to the three-dimensional model prior to surgery to re-establish the bony mandibular contour. A hemimandibulectomy with 10 mm clear surgical margins was performed (Figure 10) using extraoral approaches and the remaining mandibular segment fixated with the preadapted titanium reconstruction plate. A silastic spacer was secured to the titanium plate by means of wires (Figure 11) which would allow for the reconstruction of the defect using a particulate posterior iliac crest bone graft in the future. Bilateral Level I and Level II neck lymph nodes were also biopsied at the same time of surgery and demonstrated no features of malignancy. Three weeks after surgery, the patient was initiated on EURAMOS protocol of doxorubicin and cisplatin [13]. The patient has since completed the chemotherapy regimen and, as of February 2025, is no longer on chemotherapy. Currently, the patient is undergoing evaluation for a fibula free flap reconstruction to address the osseous defect.
3. Discussion
A search on PubMed and Google Scholar (“Osteosarcoma” [MeSH] AND “Jaw” [MeSH] AND “Case Reports” [Publication Type] AND “Child” [MeSH]) for case reports on OSJ in children aged 12 years and younger found 12 cases (in English) from accredited journals. These cases are summarized in Table 1.
| Authors, year of publication | Patient’s age, sex | Anatomic site | Clinical findings | Radiological features | Histological subtype | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| Kaveri et al., 2009 [14] | 4 years old, M | Maxilla | Swelling, medical/dental history not specified | CT: Hypodense lesion extending into nasal cavity, maxillary sinus, and orbital cavity. Destruction of the medial and lateral wall of the maxillary antrum, inferior orbital walls, and nasal septum | Epithelioid OS | Weber–Ferguson approach—excision of mass. Pt’s parents refused any further treatment | Not specified |
| Jot et al., 2023 [15] | 6 years old, M | Maxilla (anterior) | Swelling, history of trauma to the anterior maxilla by a wooden stick while playing |
|
Telangiectatic OS | ×3 cycles neoadjuvant chemotherapy followed by surgical resection and ×4 cycles of adjuvant chemotherapy | No evidence of recurrence 1 year, 3 months post-op |
| Miceli et al., 2020 [16] | 7 years old, M | Mandible (body) | Pain, swelling, medical/dental history not specified |
|
Osteosarcoma, NOS | Marginal resection | No evidence of recurrence 2 years post-op |
| Donaldson et al., 2004 [17] | 8 years old, M | Mandible (body) | Swelling. No significant medical/dental history |
|
Parosteal (juxtacortical) OS | Marginal resection of the mandible and surrounding soft tissue | No signs of recurrence 3.5 years post-op |
| Cozza et al., 2009 [18] | 8 years old, F | Mandible | Swelling, fever, pain | CT: Hypodense lesion on the left mandible with soft tissue oedema | Epithelioid OS | Pre-op chemotherapy (×2 cycles) followed by a hemi-mandibulectomy and fibular flap reconstruction. Post-op adjuvant chemotherapy was administered (×13 cycles) | No signs of recurrence 42 months post-op |
| Tomar et al., 2016 [19] | 9 years old, M | Mandible (body) | Swelling, pain, palpable submandibular and cervical lymph nodes. No significant medical/dental history |
|
Telangiectatic OS | Not specified | Not specified |
| Bilodeau et al., 2010 [20] | 9 years old, M | Mandible (body) | Pain, swelling, medical/dental history not specified |
|
Low-grade OS | Marginal resection with fibula graft | No signs of recurrence 2 years post-op |
| Kupeli et al., 2012 [21] | 10 years old, F | Maxilla | Swelling, pain, medical/dental history not specified | CT: Ill-defined, expansive mass at left maxillary alveolar process, extending to the palate and maxillary sinus. Bone destruction present | Low-grade OS | Maxillectomy followed by chemotherapy (×6 cycles) | No signs of recurrence 5 years post-op |
| Nirmala et al., 2014 [11] | 10 years old, F | Mandible (body) | Swelling. No significant medical/dental history |
|
Conventional OS (fibroblastic type) | Marginal mandibulectomy | Not specified |
| Yamamoto et al., 2011 [22] | 11 years old, M | Maxilla (anterior) | Swelling, history of afebrile convulsions |
|
Conventional OS (fibroblastic type) | Not specified | Not specified |
| Goncalves et al., 2024 [23] | 12 years old, F | Mandible | Pain, tooth mobility, history of parameningeal alveolar rhabdomyosarcoma at 2 years old—treated with chemo- and radiotherapy | Not specified | High-grade OS | Palliative | Pt succumbed to her illness 7 months after diagnosis |
| Madiraju, 2021 [12] | 12 years old, F | Mandible (body) | Swelling. No significant medical/dental history |
|
Conventional OS (fibroblastic type) | Surgical resection | No signs of recurrence 6 months post-op |
- Abbreviations: CT = computerized tomography, F=female, M = male, MRI = magnetic resonance imaging, NOS = not otherwise specified, OPG = orthopantomogram, OS = osteosarcoma, PDL = periodontal ligament.
While the precise cause of osteosarcoma is unknown, mutations in the RB and p53 genes significantly contribute to its onset. Individuals with RB germline mutations face a 1000-fold increased risk of developing osteosarcoma. Similarly, those with p53 germline mutations, known as Li–Fraumeni syndrome, also show a higher incidence of this tumour [24]. Notably, osteosarcoma is more likely to arise in areas of bone growth, probably due to the proliferation of osteoblasts, which are more prone to transformation [25].
OSJ can be classified into two types: primary and secondary. The aetiology of the primary type is unknown but may be associated with rapid bone growth during adolescence, environmental factors such as ultraviolet radiation, exposure to methylcholanthrene, chromium salts, beryllium oxide, asbestos, aniline dyes, and a genetic predisposition [8]. The secondary type occurs in older individuals with conditions like Paget’s disease, fibrous dysplasia, chronic osteomyelitis, or as a late complication of radiation therapy to the craniofacial region [9]. In the case described by Goncalves et al. [23], the patient had a history of malignancy in the orofacial region, which was subsequently treated with chemo- and radiotherapy. Although this patient was a 12-year-old, the radiotherapy could have been the initiating factor for the development of secondary OSJ.
A significant radiographic feature of OSJ on conventional imaging is Garrington’s sign, which is the symmetric widening of the periodontal ligament (PDL) due to tumour cell infiltration, often seen in the early stages [9]. This was a radiographic feature in three [11, 16, 19] of the cases we identified in the literature. Other radiographic features may include tooth displacement, interdental bone loss, loss of lamina dura, and peripheral reactive cortical bone formation [26]. Conventional osteosarcoma often displays a characteristic “sunburst” or “sunray” appearance on radiographs due to the formation of thin, irregular spicules of new bone extending from the lesion as the tumour invades the periosteum [27]. Although this appearance is classic for long bone osteosarcomas, it is not pathognomonic for OSJ [7, 28] but was seen in three of the cases we highlighted in the literature [11, 12, 20]. Other features may include Codman’s triangle, resulting from the elevation of the bony cortex due to new periosteal bone formation over the expanding tumour. This was not seen in any of the 12 cases presented except for our case. However, radiographic features can vary significantly due to the destruction of normal bone and the degree of mineralization of the newly formed bone [10].
Computed tomography (CT) and magnetic resonance imaging (MRI) are useful for assessing the bone destruction pattern in OSJ, which can present as lytic, sclerotic, or mixed. The osteolytic variant appears completely radiolucent, while the mixed pattern shows radiopaque spicules within a radiolucent mass, and the osteosclerotic pattern shows high-density new bone formation [6]. From the information gathered on the 10 patients who had either a CT or MRI, the most common pattern encountered was the mixed variant [11, 12, 15, 17, 19, 20, 22], which coincided with our case.
Histopathologically, the diagnosis of osteosarcoma is based on identifying osteoid production by malignant mesenchymal cells, though this alone is insufficient. Osteosarcoma can be classified into central and peripheral subtypes. Central osteosarcoma histotypes may include conventional, high-grade central, low-grade central, epithelioid, and telangiectatic [1]. The main types of cellular differentiation in conventional osteosarcoma are osteoblastic, chondroblastic, and fibroblastic, with the final diagnosis depending on the type of extracellular matrix produced by the tumour cells [8]. Typically, one histologically predominant pattern is observed, although two patterns may be seen in some lesions [26]. Peripheral osteosarcoma histotypes may include parosteal (juxtacortical), low-grade, and high-grade, among others [1].
As per our review, two patients had epithelioid osteosarcoma [14, 18], two had telangiectatic osteosarcoma [15, 19], three patients had conventional osteosarcoma [11, 12, 22], one had high-grade osteosarcoma [23], two had low-grade osteosarcoma [20, 21], one had parosteal (juxtacortical) osteosarcoama [17], and one case was unspecified [16]. Our patient’s diagnosis aligned with the most common type of osteosarcoma we found in the literature—the conventional type.
However, diagnosing OSJ can be complex on a histological level, often requiring multiple sequential biopsies to confirm the diagnosis [29]. Therefore, a combination of clinical, radiographic, and histopathologic features is needed to arrive at a definitive diagnosis.
Primary treatment for OSJ involves radical surgical removal with clear margins of 1.5–2 cm [4]. A hemimandibulectomy is usually the treatment of choice for mandibular OSJ, while complete resection is challenging for maxillary OSJ, leading to a higher likelihood of local recurrence [26]. Therefore, routine CT scans are important for maxillary OSJ patients to assess for soft tissue extension, particularly into the nasal cavity, antral floor, and floor of the orbit [5].
Chemotherapy, typically recommended before surgery for osteosarcomas in long bones, remains a point of debate for OSJ [9]. OSJ exhibits lower likelihood of distant metastasis, and patients generally achieve a 5-year survival rate of 77% if the disease is localized and fully resected [4, 9]. In our review, three patients [15, 18, 21], including our patient, received chemotherapy either before surgery, after surgery, or both and have shown no signs of recurrence for over a year, with one patient [21] remaining disease-free for 5 years. However, patients who did not receive chemotherapy and were not palliative or unspecified [12, 17, 20] also responded well to treatment, with one patient [17] being disease-free for 3.5 years.
Histologic subtypes have been found not to significantly impact prognosis. Therefore, regular follow-up is essential for all osteosarcoma variants due to their tendency to spread along the PDL, inferior alveolar nerve, mandibular foramen, mandibular canal, and marrow spaces when located in the jaw [5].
4. Conclusion
Since OSJ lacks definitive clinical and histopathological features, careful evaluation is essential for each case presenting with unusual radiographic characteristics. This emphasizes the importance of a thorough, multidisciplinary approach in diagnosing and managing such lesions. By integrating clinical observations, radiographic findings, and pathological analysis, healthcare professionals can achieve a more accurate diagnosis and develop an effective treatment plan. This comprehensive correlation is crucial for identifying the nature of the tumour and ensuring optimal patient outcomes.
Consent
Since the patient was a minor, her mother provided consent for the use of her information.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Open Research
Data Availability Statement
The data supporting this case report are available from the corresponding author upon reasonable request.