Gut Microbiota Dysbiosis: A Neglected Risk Factor for Male and Female Fertility
Abstract
Infertility is a condition where a male or female is unable to achieve pregnancy through at least 1 year of regular, unprotected sexual intercourse. There are several known causes and risk factors associated with infertility. The gut microbiota is a complex community of trillions of microorganisms living in the gut. Due to modern lifestyle changes, such as dietary habits, physical inactivity, and increasing antibiotic use, the diversity and composition of these microbes may change in a detrimental manner. Dysbiosis or an imbalance of the gut microbiota compared to a normal composition can lead to various abnormalities, such as obesity, Alzheimer’s, metabolic disorders, and infertility. This review will cover the factors influencing gut microbiota composition, the mechanisms by which gut microbiota contributes to infertility in men and women, the effects of gut microbiota on problems that may arise during pregnancy, and therapeutic methods for diseases caused by dysbiosis of the gut microbiota.
1. Introduction
Infertility is a condition where a male or female is unable to achieve pregnancy after at least 1 year of unprotected sexual intercourse [1]. Infertility affects millions of people worldwide and can cause severe physical and mental problems that affect their families, relationships, and communities [2]. Difficulties in anxiety, sexual relationships, depression, and conjugal behavior issues may accompany it [3]. Exploring factors that affect the reproductive system and fertility helps prevent or treat infertility. Female infertility has been categorized into different types, such as ovulatory dysfunction, tubal infertility, endometriosis, diminished ovarian reserve (DOR), and uterine and cervical factors [4]. On the other hand, abnormal male physiology, reduced testosterone concentrations, and declined sperm count, are common lesions of male infertility [5].
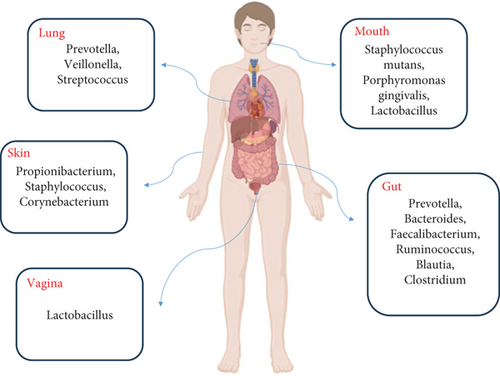
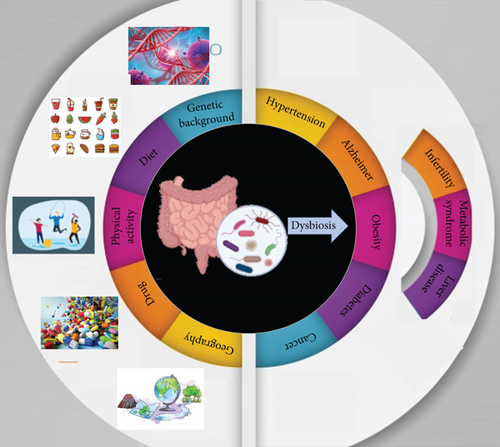
The human microbiota consists of archaea, bacteria, viruses, and eukaryotes located within and outside the body, impacting our health and disease. So that, from birth, there is a steady interplay (symbiosis) between the body and its domestic microbiota [6]. While all body sites are enriched by various microbes (Figure 1), the highest microbial numbers have been seen in the gut [7]. The gut microbiota is an assembly of microorganisms that live in the gastrointestinal tract and depend mutually on the host, providing essential signaling metabolites. Owing to improved knowledge in metabolomics, genomics, and other technologies, such as culturomics, our understanding of gut microbiota has improved [8]. Reports show that the gut microbiota includes almost 1015 microbial cells and at least 22 million microbial genes [9]. Indeed, the gut microbiota profile significantly influences human health and behavior. The deregulation of the proportion and composition of the gut microbiota has been associated with various health issues, including obesity, brain abnormalities, inflammatory bowel disease (IBD), cancer, infertility, and other anomalies (Figure 2). The metabolism of gut microbiota is closely linked to the types of microbes present in the gut. The normal composition of gut microbiota stimulates the host to release antimicrobial compounds. Competitive utilization of nutrients and attachment site occupation are essential for controlling bacterial composition [10]. The gut microbiota performs several functions, such as food fermentation, vitamin production, protection against pathogens, and modulation of the immune system [11]. The gut microbiota generally comprises six phyla, including Bacteroidetes, Firmicutes, Actinobacteria, Verrucomicrobia, Fusobacteria, and Proteobacteria. Among them, Bacteroidetes and Firmicutes are the most abundant types [12].
2. The Factors Affecting Gut Microbiota
Various factors including lifestyle, genetics, and environment affect the composition and diversity of gut microbiota (Figure 2). We will discuss some of these factors.
2.1. Nutrition
Nutrition is thought to influence all physiopathological events. The idea that diet affects the risk of initiation and progression of some diseases and also influences therapeutic effects has become widespread [13]. A growing body of research, mostly in animal models, has reported that the gut microbiota plays a critical role in host health. A number of the same diseases that have been reported to be associated with diet, such as metabolic syndrome, obesity, and cardiovascular disease (CVD), are also known to be associated with dysbiosis in the gut microbiota profile. The alterations in microbiota composition and associated microbial metabolites occur through both host physiology and diet. There is considerable evidence to support the view that diet plays a role in regulating host physiology and health. However, there are increasing reports supporting the concept that diet may have an indirect effect on human health by modulating the gut microbiota [14]. Unfortunately, human dietary habits have been greatly altered by rising incomes and urbanization [15]. An increasing number of studies have supported the issue that changes in dietary patterns in industrialized societies worldwide result in a significant reduction in bacterial diversity and composition, a decrease in some beneficial bacteria such as Lactobacillus spp., Roseburia spp., and Eubacterium rectale, and an increase in Alistipes spp., Bacteroides spp., and Bilophila spp. [16]. Short-term, dramatic dietary interventions have been shown to rapidly alter the composition of the microbiota. However, while these effects are transient, the long-term dietary pattern is associated with shaping the stable microbiota profile of each individual [17]. Ding et al. transplanted stool flora from mice fed a high-fat diet to ND (normal diet) mice and found increased Prevotella and Bacteroides in the intestinal tract of ND mice, followed by local inflammation and impaired sperm motility and spermatogenesis. They also indicated altered expression of testis genes as a result of HFD-induced dysbiosis of the gut microbiota [18]. Some investigators have examined whether the effects of a high-fiber diet on boar reproduction are mediated by changes in the gut flora. Although they observed insignificant increases in testicular volume and sexual desire in boars fed the high-fiber diet compared with those fed the basal diet, semen motility was significantly higher in the former group. They also showed an increased level of microbial diversity in the gut of boars fed the high-fiber diet. The higher abundance of beneficial bacteria such as unclassified gut microbiota group 005 (UCG-005), Rikenellaceae_RC9_gut_group, Ruminococcus, and Lactobacillus detected in the gut of boars fed with high fiber diet, while the frequency of Romboutsia, Clostridium_sensu_stricto_1, and Turicibacter were decreased in them. Finally, the study suggested that modulation of the gut microbiota profile is a possible underlying mechanism for the effects of high-fiber diets on reproductive activity [19].
2.2. Age
The process of aging is complex and has significant effects on various genetic, metabolic, immunological, and physiological functions. Studies suggest that differences in gut microbiota profile at different ages may mediate some effects of aging on health conditions. Elderly individuals exhibit distinct gut microbiota composition when compared to healthy adults. This discrepancy may be attributed to various factors, including senescence, lifestyle changes, weakened immune function, physical inactivity, diminished intestinal and overall function, alterations in intestinal physiology and morphology, repeated infections, hospitalizations, and the use of various medications. Elderly individuals generally exhibit a lower gut microbiota diversity, as well as decreased levels of beneficial Bifidobacteria, Bacteroides, and Lactobacilli, and elevated levels of opportunistic bacteria such as Perfringens, Enterobacteria, C. and C. difficile [20]. In old age, dysbiosis of microbial metabolic products is often observed, resulting in reduced levels of short-chain fatty acids (SCFAs) and increased risk of various diseases and abnormalities [21]. The reduced adhesion of bifidobacteria to the intestinal wall, due to alterations in the chemical composition and colon mucous membrane structure, may be related to the lower species and quantity of bifidobacteria in elderly humans. This may cause restricted functionality and immunological reactivity in the gut and elevate the susceptibility to gastrointestinal infections [22].
2.3. Drugs
There is a bidirectional relationship between drugs and gut microbiota. Drugs can alter the composition of gut microbiota, and conversely, gut microbiota can affect a person’s response to a drug by enzymatically modifying its structure and altering its bioactivity, bioavailability, or toxicity. This phenomenon is called pharmacomicrobiomics [23]. Antibiotics are one of the most well-studied classes of drugs regarding their impact on gut microbiota. They can reduce the diversity of gut bacteria and alter the relative abundance of specific microbial populations. The consequences may include the following: dysbiosis: a disruption in the balance of the microbiota, often leading to an overgrowth of pathogenic bacteria (e.g., Clostridium difficile); increased susceptibility to infections: Disruption of the normal microbiota may lead to increased risk of infections and gastrointestinal issues, recovery, and resistance: Some individuals may take a long time to recover their original microbiota composition after antibiotic treatment, while others may develop a stable dysbiotic state. [24] Clostridioides difficile (formerly known as Clostridium difficile) infection is a disease caused directly by antibiotic disruption of the gut microbiota. The illness spectrum is defined from mild diarrhea to death [25]. Research indicates that very preterm infants who underwent long-term antibiotic therapy exhibited less diverse bacterial communities and diminished species richness in their gut, along with an increased presence of antibiotic resistance genes [26]. Pérez-Cobas and Gosalbes et al. reported that the various types of antibiotics affect the gut microbiota in different ways. For example, a bacteriostatic drug, just inhibiting bacterial growth and reproduction, caused the flourishing of Gram-negative bacteria which was associated with an increase in the number of genes involved in lipopolysaccharide (LPS) synthesis, while the bactericidal drug, capable of killing bacteria, was followed by an increase in Gram-positive bacteria which was related to an over-representation of genes contributing to endospore formation [27].
Nonantibiotics can also alter the gut microbiome [28]. Evidence suggests that proton pump inhibitors (PPIs) suppress the production of stomach acid and metformin, a frequently used drug in type II diabetes, and alters the composition and activity of gut microbiota [29, 30]. By raising the pH in the stomach, PPIs can influence the microbial profiles in the gut, potentially leading to the overgrowth of certain bacteria. Patients receiving long-term PPIs may have a higher risk of gastrointestinal infections due to changes in microbial composition [31]. According to Imhann et al., fecal samples of patients who consumed PPI showed higher levels of the Enterobacteriaceae, Enterococcaceae, and Lactobacillaceae families and lower levels of the Ruminococcaceae and Bifidobacteriaceae families [32]. Furthermore, Vila et al. reported that while the use of antibiotics reduced the frequency of microbial pathways, such as biosynthesis of amino acids, metformin led to stimulated bacterial metabolic activity. Out of all antibiotic categories, tetracyclines displayed the strongest association with the modified pathways [33]. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics are also among those commonly used medications that have been reported to impact microbiota composition in both animals and humans. Mucosal inflammation, alterations in intestinal motility, luminal pH and bile acid metabolism, or direct drug–induced inhibitory effect on bacterial growth are all potential mechanisms by witch NSAIDs and opioid cause microbiota dysbiosis [34]. Certain classes of antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), have been shown to impact gut microbiota. Zhang et al. observed that there is an association between the administrations of SSRI fluoxetine (Flu) and the tricyclic antidepressant amitriptyline (Ami) and a low abundance of the phylum Firmicutes and a high frequency of the phylum Bacteroidetes [35]. Cancer treatments also could influence gut microbiota profile. Montassier et al. compared the composition of the gut microbiota of 28 patients with non-Hodgkin’s lymphoma before and after a 5-day myeloablative conditioning regimen with high-dose etoposide, carmustine, aracytine, and melphalan by 16S rRNA sequencing. They observed significantly decreased frequencies of Firmicutes and Actinobacteria in fecal samples, while an increased level of Proteobacteria after the chemotherapy. At the genus level, the relative frequencies of Oscillospira, Ruminococcus, Blautia, Lachnospira, Dorea, Roseburia, Clostridium, Adlercreutzia, Coprococcus, Anaerostipes, Collinsella, and Bifidobacterium reduced considerably compared to samples collected before chemotherapy [36].
Overall, the impact of drugs on gut microbiota composition can be significant, leading to a cascade of effects on physical and mental health. Understanding these interactions is crucial for developing strategies to mitigate adverse effects, particularly in vulnerable populations. Ongoing research continues to investigate the complexities of these relationships, potentially paving the way for personalized medical approaches that consider an individual’s gut microbiota profile in treating various ailments.
2.4. Exercise
There is growing evidence suggesting that exercise plays a significant role in modulating the gut microbiome. The alterations induced by exercise in the gut microbiota are accompanied by changes in human physiology, including modulation of immunity, metabolism, and behavior [37]. Estaki et al. have shown that individuals who regularly perform cardiorespiratory fitness exercises have higher gut bacterial diversity and a higher quantity of butyrate-producing bacteria [38]. Low-intensity exercise affects the gut microbiota, decreasing transient stool time and reducing the contiguity time between harmful microbes and the gastrointestinal mucus layer [39]. Prolonged exercise increases intestinal permeability, which leads to bacterial translocation from the colon. [40]. Evans et al. have reported a negative correlation between the total distance run and the Bacteroidetes/Firmicutes ratios [41]. Furthermore, it was found by Campbell et al. that Faecalibacterium prausnitzii was present in exercised mice, which protects the digestive tract by producing butyrate and lowering the oxygen tension in the lumen through a flavin/thiol electron shuttle. Research suggests that probiotics may enhance exercise performance by modifying the composition of gut microbiota, leading to improved health conditions in humans [40]. Thus, physical activity should be regarded as an effective means of preventing and treating diseases linked to gut microbiota composition.
2.5. Genetic Background
The relationship between the genetics of the host and the gut microbiota has been discovered. According to a study, the gut microbiomes of humans ranging from 27 to 94 years old on three different continents had more similarity to each other than to those of other mammals [42]. An evaluation of the phylogenies of great apes demonstrated that the composition of the gut microbiome conformed with the branching order of mitochondrial DNA phylogenies [43]. Recently, microbiome genome-wide association studies (GWAS) for almost 1500 individuals have demonstrated a strong relationship between human genetics and gut microbiota [44]. Furthermore, a higher similarity in the gut microbiota composition is observed between monozygotic twins compared to dizygotic. According to the suggestion, certain genes directly impact the composition of the gut microbiota, leading to the overgrowth of disease-causing species. Wang et al. identified 40 loci influencing the bacterial taxa including the lactase gene (LCT), FUT2, and NOD2 [45]. There is a strong association between SNPs in LCT and lactose intolerance as well as the frequency of lactose-metabolizing bacteria such as Bifidobacterium (from the phylum Actinobacteria) [46]. FUT2 has been reported to trigger a great variety of disorders such as celiac, IBD, and type 1 diabetes mellitus, as well as increase susceptibility to different bacteria and viruses [47]. The lack of terminal fucose residues on mucin glycans occurs in individuals with specific FUT2 variants called “nonsecretors,” who have lower gut microbiome diversity and a significant reduction in the frequency of Faecalibacterium-butyrate-producer and anti-inflammatory bacteria, as well as an increased abundance of Proteobacteria [48]. Indeed, fucose is known to be an important energy source for a variety of bacteria, while being a binding site for others. Some bacterial species, such as Bacteroides, require fucose for the biosynthesis of their bacterial capsule, colonization of the gut, and protection from the host immune system [47, 49, 50]. In addition, NOD2 is known to be an intracellular detector of muramyl dipeptide, present in the cell wall component of either Gram-positive or Gram-negative bacteria, which triggers an immune response via stimulation of mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) signaling pathways, followed by secretion of antimicrobial defensins, cytokines, and costimulatory agents required for T-cell activation. A hyperinflammatory response to commensal microorganisms occurs in the NOD-deficient state [51–53]. We mentioned some examples supporting the conformation of genetic background and gut microbiome composition.
2.6. Geography
There is increasing evidence supporting the existence of differences in the gut microbiota of people from different geographic conditions. For example, species of the Prevotella genus, with a positive impact on the glucose metabolism of Swedish adults are common in low- and middle-income countries [54]. He et al. observed that interindividual differences in the gut microbiota profile were justified by geographic location. Indeed, analyzing the relationship between gut microbiota composition and susceptibility to metabolic diseases without considering geography was followed by observation of a relatively weak association. However, when they experimented with partitioning patients’ data into geographic groups, the accuracy of analysis was extremely elevated [55].
Porras et al. were going to know if geographic differences in the human microbiome result in variation in susceptibility to intestinal infection. They exposed the mice from two populations of Guatemala and Fiji Islands with C. rodentium following colonization of their intestines with human-related microbiota. Afterward, they assessed C. rodentium of the stool and observed an infection peak at Day 5 for the Guatemala mice compared to Day 9 for both the US and FIJI mice. Although the FIJI mice had the highest levels of bacterial shedding within the course of the experiment, the Guatemala mice were found with remarkably reduced bacterial shedding compared to the FIJI and US mice beginning at Days 7 and 9 after infection, respectively [56].
2.7. Diseases
Some diseases can significantly alter gut microbiota composition, leading to profound effects on overall health. The human gut is home to trillions of microorganisms that play crucial roles in digestion, metabolism, and immune function. However, various diseases—ranging from gastrointestinal disorders like IBD and irritable bowel syndrome (IBS) to systemic conditions such as diabetes and obesity—can disrupt this delicate microbial balance. Various studies have depicted that the microbiota associated with IBD represents decreased diversity and an elevation in less common phyla, including Gammaproteobacteria. IBD-driven immune dysfunction and mucosal inflammation can considerably alter regional ecosystems, influencing intestinal permeability, mucus content and composition, mucosal gene expression, cellular content, and immune signals in the gut. These alterations also affect the composition, function, and interactions among gut microbial communities, influencing healthy host-microbe relationships and potentially leading to immune activation and inflammation in vulnerable individuals [57]. In addition, Celiac disease is an autoimmune disorder triggered by the ingestion of gluten in genetically predisposed individuals, leading to a range of gastrointestinal and systemic symptoms. At present, dietary gluten is known as the most important environmental factor involved in celiac disease pathogenesis. The high proline content of the gluten protein empairs its digestion by human gastrointestinal enzymes, which lack prolyl endopeptidase activity. Hampered gluten digestion causes the presence of immunogenic peptides that interact with the intestinal epithelium and mucosa resulting in altered gut permeability and an imbalanced microbial composition [58, 59]. An investigation on children, evaluating the gut Bifidobacteria composition found that the total numbers of Bifidobacterium and B. longum species in feces and duodenal biopsies were lower in patients with celiac disease compared with healthy children [60]. Current evidence on patients with celiac disease increasingly supports that dysbiosis occurs in celiac disease, but the issue of dysbiosis is a cause or consequence of diseases and whether the different intestinal diseases have specific patterns of change is still unclear. Factors other than gluten may modulate the intestinal microbiota in celiac disease, but this requires further investigation [58]. Liver diseases, such as cirrhosis and hepatitis, can remarkably alter the composition and function of gut microbiota. As liver function declines, the liver’s capability to metabolize and detoxify substances is impaired, causing accumulating toxins that can penetrate the intestinal barrier. This disruption makes intestinal permeability, called “leaky gut,” allowing unbeneficial bacteria and their metabolites to enter the bloodstream, thereby exacerbating liver inflammation and disease progression [61]. Interestingly, in patients with liver cirrhosis, the majority of identified bacterial species originate from the mouth, indicating a potential transmission of bacteria from the oral cavity to the intestines. Liver cirrhosis is associated with diminished innate immune system protection, characterized by lower gastric acid production, decreased bile secretion, and impaired production of antimicrobial peptides (AMPs). This weakened host defense may facilitate the transfer of bacteria from the mouth to the intestinal microbiome. Nevertheless, the extent to which each specific factor influences the changes in microbiota composition needs further investigation [62]. The alteration of the gut microbiota profile in the context of liver disease highlights the importance of the gut-liver axis in health and disease [63].
Overall, there is a bidirectional relationship between the gut microbiota and diseases. As the gut microbiota shifts, it can impact the progression of the disease itself, supporting the intricate relationship between microbial health and systemic disease [64].
3. Gut Microbiota and Male Fertility Behavior
A man who is unable to impregnate a fertile woman after 1 year of unprotected intercourse is defined as infertile. Defects in male reproductive behavior affect nearly half of infertile couples worldwide [65]. Several factors, including age, genetic background, lifestyle, and body mass index (BMI), affect sperm quality and number. Increasing evidence points to the role of the gut microbiota in male reproductive function. Alcohol has been shown to cause dysbiosis of the gut microbiota. Li et al. indicated that transplantation of fecal microbiota from the mice that had drunk alcohol into the intestines of normal mice was associated with attenuated sperm count, sperm concentration, and sperm motility [66]. Another study reported that the desirable effects of alginate oligosaccharides (AOS)—natural polymers from brown seaweed)—on sperm quality were mediated by modulation of the gut microbiota [67].
Various mechanisms could mediate the effects of gut microbiota on male fertility. In this section, we will briefly discuss some of them.
3.1. Gut Microbiota Could Modulate Inflammation and the Immune System
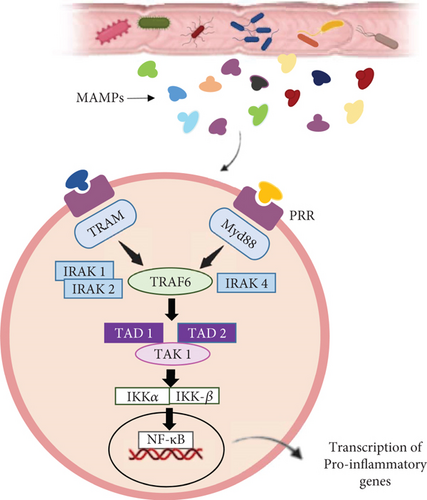
There is a proven crosstalk between the gut microbiota and immune activation and subsequent inflammation [68]. The gut microbiota may influence male fertility via immune activation. Microbial-associated molecular patterns (MAMPs) including lipoprotein acids, LPS, lipoproteins, and peptidoglycans enter the testis via the vas deferens and the testicular artery, leading to testicular damage. Pattern recognition receptors (PRRs) are expressed on immune cells that function as receptors for MAMPs, so MAMPs could activate innate immune cells that possess PRRs and cause inflammation in the testis. Indeed, LPSs induce NF-κB to translocate to the nucleus and upregulate MyD88 and TRAM pathways, leading to the expression of inflammatory cytokines (Figure 3) [69]. Cytokines, including interleukins (ILs) and tumor necrosis factors (TNFs), play an important role in signaling within the inflammatory process and contribute to the activation of innate immunity [70].
In general, the beneficial microbes in the gut prevent the growth of pathogenic microbiota through competition. The commensal microorganisms in the gut could inhibit or stimulate inflammation by interacting with immune system cells [71]. Some of the species can increase the permeability of the gut, causing MAMPs to leave the gut and enter the circulation, and consequently enter the testis through the testicular artery, followed by the production of mediators and cytokines and the initiation of inflammation leading to testicular damage [72].
In addition, intestinal epithelial cells transfer metabolites to cells of the immune system and stimulate inflammation, which is associated with reduced testosterone levels following inactivation of the Steroid Acute Regulatory (StAR) protein, a necessary regulator of steroidogenesis. Recent research has shown that inflammation can cause spermatogenesis failure and disrupt sperm maturation [73]. In addition, the prostate may be affected by inflammation by suppressing the gland’s secretion of citric acid and gamma-glutamyl transpeptidase [74].
3.2. The Gut Microbiota Contributes to the Production of Antioxidant Agents
The excessive oxidative modification of macromolecules, lipids, proteins, and nucleic acids occurs as a result of oxidative stress caused by the generation of oxidants such as free radicals and reactive oxygen species (ROS). As a result, key cellular functions and processes are disrupted, leading to a variety of diseases, including diabetes, cancer, hepatitis, neurological disorders, lung disease, and male reproductive dysfunction [75, 76]. Oxidative stress exerts undesirable effects on the structure and function of spermatozoa by damaging both mitochondrial and nuclear DNA and also by affecting the sperm epigenome, leading to infertility and recurrent stillbirths [75]. Disruption of spermatogenesis is the major cause of male infertility. Increased levels of ROS and oxidative stress contribute to reduced semen quality and spermatogenesis failure [77]. Recent evidence indicates the existence of a crosstalk between oxidative stress in the testis and inflammation [78].
Increasing studies focused on the capacity of gut microbiota to produce antioxidants. For example, Uchiyama et al. identified reactive sulfur species (RSS) as molecules with high antioxidant capacity derived from gut microbiota [76]. Additionally, hydroxytyrosol (HT), a polyphenol, has received much attention for its antioxidant, anti-inflammatory, and antibacterial functions [79]. Indeed, HT leads to increased permeabilization of bacterial membranes and binds to DNA followed by inhibited bacterial growth. Modulation of the bacterial profile is one way that HT improves sperm motility and quality. An investigation illustrated that HT caused increased beneficial bacteria such as Lactobacillus, Bifidobacterium, Intestinimonas, Eubacterium, Butyricicoccus, and Coprococcus, while, reduced undesirable microbes, such as Escherichia, Oscillibacter, Streptococcus, Barnesiella Clostridium_sensu_stricto, and Phascolarctobacterium. Improving the bacterial composition leads to an improvement in plasma metabolites, which promotes spermatogenesis and improves semen quality. The study evaluated the correlation between different bacteria with metabolome production and the products with sperm quality. For example, fecal Lactobacillus and Bifidobacterium were found to have a positive correlation with plasma naringenin 7-O-glucuronide, although Streptococcus and Escherichia had an inverse correlation with this metabolite [80]. It has been reported that plasma naringenin 7-O-glucuronide acts as an antioxidant that inhibits membrane damage of spermatozoa, prevents excessive DNA damage, and upregulates enzymes involved in sperm DNA repair [81]. There are also some reports describing its beneficial effects on genes necessary for DNA-associated apoptotic events of spermatozoa [82].
3.3. The Gut Microbiota Regulates Signaling Pathways
Various microbes can regulate signaling pathways. Zhao et al. showed that Akkermansia has a positive correlation with protein kinase A (PKA) expression, and Prevotella and some other bacteria have a positive correlation with p-AKT, which is required for sperm maturation and sperm motility [80, 83]. Mohseni et al. discussed the influences of various microbes on the PI3K/Akt/mTOR signaling pathway [84]. This pathway contributes to various stages of male fertility, including the regulation of the hypothalamic-pituitary-gonad (HPG) axis during spermatogenesis, the proliferation and differentiation of spermatogonia and somatic cells, and the modulation of sperm autophagy and testicular endocrine function in the presence of environmental pollutants, particularly endocrine disrupting chemicals (EDCs) [85]. EDCs are highly heterogeneous environmental contaminants that may be natural or synthetic molecules [86]. These agents could enter the human body through the diet and cause unwanted interference with hormone-regulated functions. There are reports supporting the idea that EDCs play a role in altering the composition of the gut microbiota. Interestingly, Fabozzi et al. suggested that the negative effects of these agents on spermatogenesis and sperm viability may be mediated by dysbiosis of the gut microbiota [87].
In addition, the retinoic acid pathway is recognized as essential for spermatogenesis because of its role in the initiation of miosis. It is reported that this pathway is influenced by microbiota and specific microbes could stimulate retinoic acid production. Induction of these microbes increased the expression of the spermatogonial cell marker genes PLZF and DAZL, representing the onset of spermatogenesis [88]. Furthermore, there is evidence supporting the role of gut microbiota in modulating the canonical Wnt signaling pathway [89]. Several studies have demonstrated that Wnt signaling is strongly involved in male reproductive physiology, such that deregulation of the pathway would result in pathological dysfunction of the genital organs and spermatozoa [90]. For example, Basu et al. proposed the Wnt3 ligand as a stabilizing protein for sperm adhesion to Sertoli cells; in fact, Wnt3 interacts with connexin 43, a protein involved in the gap junction complex that establishes cellular connections between Sertoli cells and germ cells. Knockdown of Wnt3 in transgenic male mice was associated with severe germ cell impairment and decreased testicular weight [91].
3.4. The Gut Microbiota Plays a Role in the Correct Development of Testis
Proper development of the testes is essential for male reproductive function. Stress caused by environmental factors in germ cells or pathogenic microorganisms is suppressed by the testis-blood barrier. The testis-blood barrier is established by Day 15–16 after birth in mice, the period of transformation of the testis cords into seminiferous tubules with lumens. Al-Asmakh et al. showed that some tubules are still closed at this time in control mice compared to mice that had been exposed to Clostridium tyrobutyricum. The presence of the microbe caused the expression of genes involved in cell-cell adhesion of Sertoli cells in the testis-blood barrier such as occludin, ZO2, and E-cadherin. The occurrence of delay in the formation of the testis-blood barrier makes damaging of germ cells and reduces spermatogenesis. Thus, the investigation suggested the importance of gut microbiota composition in testis development and consequently male reproductive behavior [92].
3.5. The Gut Microbiota Impact Sexual Behavior and Sexual Hormone Production
Normal sexual intercourse is a necessary factor of the health of reproductive function, which is affected by various factors, including sex hormone levels, psychogenic, neurogenic, and hemodynamic factors. Considering the issue that gut microbiota could influence various causes of sexual function, it should be a logical target for the treatment of infertility in patients suffering from obesity, hypertension, anxiety, diabetes, and sex hormone disorders [93].
The influence of gut microbiota on the production of sex hormones has been reported. Prevotella has been shown to have a strong correlation with the hormone testosterone. In a study conducted by Markle et al. provoked testosterone levels and metabolomic changes were observed following the transplantation of microbiota from adult male mice to immature female mice [94]. Ridlon et al. have shown that Clostridium scindens and Ruminococcus gnavus are human gut microbes with the ability to convert glucocorticoids, pregnenolone, and hydroxypregnenolone to androgens via side chain cleavage [95]. Different microbes may have positive or negative effects on sex hormone production, and this may be determined by host physiology, strain, and other factors [93]. The gut microbiota may express enzymes that play a role in the steroid process and produce steroid hormones. Diviccaro et al. reported higher levels of testosterone-related metabolites, such as dihydrotestosterone (DHT), 3α-diol, and 17β-E, in the colon than in plasma [69]. Leptin and ghrelin may also be important factors in reproductive function. Elevated leptin levels negatively regulate steroid production. The secretion of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone in the testes is also suppressed by ghrelin. These hormones are affected by gut microbiota dysbiosis [96].
3.6. Gut Microbiota Influences Erectile Dysfunction (ED)
ED is described as the inability of a man to achieve or maintain an erect penis sufficient for desirable sexual intercourse [97]. It has been suggested that dysbiosis of the gut microbiota may be followed by elevated serum trimethylamine-N-oxide (TMAO) levels, resulting in cavernous endothelial and smooth muscle cell damage and consequently the occurrence of ED [69]. A review article by Leelani et al. also suggested that gut microbiota may influence ED by playing a role in the occurrence of known risk factors for ED, such as hypertension, obesity, diabetes, and metabolic syndrome. In addition, they reported a lower Bacteroidetes-to-Firmicutes ratio for ED, especially in obese individuals [98].
The study by Geng et al. supported the issue that the diversity of the gut microbiota is lower in patients with ED compared to normal subjects [99]. Additionally, a significant difference in the relative abundance of Alistipes and Clostridium XVIII is observed between the men with ED and the healthy group [100]. Also, Li et al. showed a decreased abundance of Anaerotruncus, Bifidobacterium, Allobaculum, and Eubacterium in a diabetic mouse model with ED compared to diabetic mice without ED, while they observed an increased abundance of LPS, TMAO, and inflammatory factors [101].
The relationship between gut microbiota and male infertility is complex, and various mechanisms have been proposed for the impact of the gut microbiota profile on reproductive behavior. Therefore, altering the gut microbiota composition may be a logical approach to treating infertility.
4. Gut Microbiota and Female Infertility
There is increasing evidence that gut microbiota composition and function may influence the female reproductive system and fertility [102]. This part has collected several underlying mechanisms that have been suggested for the link between the gut microbiota and female reproductive function.
4.1. Gut Microbiota Regulates Steroid Hormones
Estrogen plays a major role in the female’s reproductive function, controlling various fertility-related events including estrus status, elevated serum gonadotropin concentration, ovulation, uterine proliferation, and secretion of the endometrial gland. Aromatase is an enzyme encoded by the gene CYP19A1 that catalyzes the production of estrogen from androgens. Estrogen functions in key aspects of female fertility including the formation of the ovulatory follicle(s) and stimulating the midcycle preovulatory surge of gonadotropins and facilitating implantation by preparing the endometrial lining of the uterus. Disturbed estrogen production may be associated with infertility disorders [103]. Gut microbiota has been shown to regulate estrogen levels. First, Adlercreutz et al. found decreased estrogen levels after antimicrobial supplementation [104]. β-Glucuronidase is an enzyme with the ability to deconjugate estrogen, binding it to its receptors with subsequent physiological effects. This protein is secreted by the intestinal microbiota. Reduced production of β-glucuronidase due to deregulated gut microbiota composition results in reduced activated estrogen-causing pathologies such as obesity and CVD; however, increased production of β-glucuronidase associated with elevated levels of activated estrogen may also result in disorders such as endometriosis and cancer [105]. The optimal amount of β-glucuronidase is required to maintain desirable estrogen levels in women. In addition, the gut microbiota can influence sex steroid hormone levels by producing SCFAs, of which acetate, propionate, and butyrate are the most abundant. Lu et al. found that butyrate regulates progesterone and estradiol synthesis in porcine granulosa cells (PGCs) via the cAMP signaling pathway [106]. The significance of progesterone in pregnancies has been established for some time. It was discovered in the early 1970s that administering progesterone can prevent abortion by decreasing the likelihood of sweeping the corpus luteum before the 7th week of pregnancy [107, 108]. Insufficient endogenous progesterone is linked to a reduced likelihood of successful pregnancy attributable to inadequate preparation of the endometrium for implantation and an unfavorable immune response to fetal antigens [109]. It has been reported that immune responses in normal pregnant women are reliant on T-helper 2 (Th2) cells, while women with recurrent miscarriage have an increased Th1/Th2 ratio that is negatively linked to progesterone levels. Progesterone has positive effects on the regulation of the immune system during pregnancy. Moreover, there is growing evidence to support the crucial role of progesterone in the successful implantation and maintenance of pregnancy in Assisted Reproduction Technology (ART) [110]. It is well-established that a higher diversity of gut microbiota is associated with increased production of steroid hormones, including estrogen and progesterone [111].
4.2. Gut Microbiota Impacts the Trace Element Concentrations
Recent studies have provided evidence supporting the correlation between gut microbiota and endocrine and metabolism-related ailments, particularly those that affect the female reproductive endocrine system [112]. Trace elements are essential for basic metabolic processes and play an indispensable role in the human body [113]. Research has determined that human trace elements, specifically Cu, Zn, Ca, Mg, and Fe, are necessary for the normal reproductive behavior of females. Carl et al. reported that maternal copper deficiency is associated with intrauterine growth retardation, teratogenicity, fetal death, and ongoing postpartum issues [114]. Additionally, research has found that impaired quality of oocytes and a lack of ability to develop into fertilized eggs can occur in cases of dietary zinc deficiency [115]. Moreover, calcium deficiency in pregnant women results in the disturbed epigenetic regulation of gene expression and subsequent undesirable effects on the fetus [116]. Also, iron deficiency is associated with infertility, miscarriages, reduced birth weight, and preterm birth [117]. Furthermore, Stuefer et al. suggested a correlation between higher magnesium concentrations and an increased likelihood of pregnancy [118]. Several studies support the significant role of gut microbiota in regulating these blood elements in humans. For example, gut microbiota can enhance calcium absorption by improving the function of the protein channels responsible for calcium exchange. Moreover, certain genera including Oscillibacter, Bacteroides, Dialister, and Butyricicoccus can enhance calcium solubility by fermenting soluble corn fiber, which produces specific SCFAs that lower the pH of the gut [119]. Hence, modulating the composition of human gut microbiota may improve female reproductive function by regulating significant blood trace elements.
4.3. Gut Microbiota and Endometriosis
Endometriosis is a chronic inflammatory illness that is estrogen-dependent. The World Health Organization (WHO) has reported that it affects approximately 10% (190 million) of women and girls of reproductive age worldwide. According to Bulletti et al., between 30% and 50% of patients with endometriosis experience infertility [120]. The ovary is the most common location for endometriosis, and ovarian endometriosis is associated with the disqualification of oocytes, which can cause infertility [121]. Evidence exists to support the role of gut microbiota in the pathogenesis of endometriosis. The impact of gut microbiota on endometriosis is mediated by estrogen, immunity, inflammation, and tumor characteristics [122]. Yu et al. found that patients with endometriosis manifested reduced diversity of gut microbiota and an elevated ratio of Firmicutes/Bacteroidetes. Furthermore, there was a significant increase in the amount of Actinobacteria, Cyanobacteria, Saccharibacteria, Fusobacteria, and Acidobacteria. Therefore, modulating the gut microbiota could be a logical approach to inhibit endometriosis and subsequently, prevent infertility [123].
4.4. Microbiota and Polycystic Ovary Syndrome (PCOS)
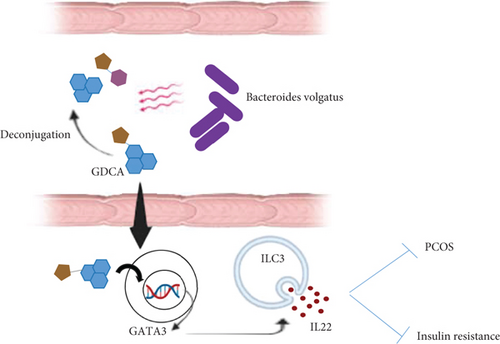
PCOS is the most common endocrine disorder, affecting roughly 15% of women of childbearing age [124]. PCOS patients typically exhibit ovulatory dysfunction, androgen excess, hypothalamic neuronal dysregulation, and polycystic-appearing ovaries [125]. PCOS is the primary cause of hyperandrogenism and oligoanovulation in reproductive-age females, leading to infertility in the majority of cases. Reportedly, 70-80% of females with PCOS are infertile [126]. Recent studies have demonstrated an association between the composition of gut microbiota and the onset of PCOS. Additionally, several studies have reported a decrease in gut microbiota diversity [127, 128]. An investigation stated that the augmentation of gamma-aminobutyric acid-producing bacteria, including Parabacteroides distasonis and Bacteroides fragilis, was observed in PCOS patients, and Escherichia coli had a positive relationship with serum LH levels which is associated with PCOS occurrence [129]. In women with PCOS, the overproduction of LH causes ovarian theca cells to produce excess androgen, and decreased secretion of FSH is associated with impaired folliculogenesis and anovulation, which is the main cause of infertility due to ovulatory dysfunction [130]. Qi et al. conducted a study in which fecal transplantation from PCOS women to adult mice was performed to evaluate the effects of gut microbiota on the appearance of PCOS phenotype. The female recipient mice showed the most significant PCOS deficiencies, including hyperandrogenism, elevated secretion of LH, defective reproductive cycles, ovarian dysfunction, reduced levels of tauroursodeoxycholic acid (TUDCA), and insulin resistance. Subsequently, the investigators could recapitulate all PCOS-related traits in mice administered with B. vulgatus, proving that this bacterium is responsible for the occurrence of PCOS symptoms [131]. B. vulgatus alters bile acid metabolism, mediating its impact on the pathogenesis of PCOS [132]. B. vulgatus deconjugates the liver-synthesized conjugated bile acids such as TUDCA and glycodeoxycholic acid (GDCA). This explains the reduced abundance of GDCA observed in PCOS patients with higher levels of B. vulgatus [133]. GDCA prompts the secretion of IL22 from intestinal group 3 innate lymphoid cells (ILC3s) by promoting the expression of GATA binding protein 3 (GATA3). The secretion of IL22 is crucial in suppressing insulin resistance and ovarian dysfunction in PCOS (Figure 4). These findings reveal the existence of crosstalk between gut microbiota and female infertility. The study suggests targeting the gut microbiota-bile acids-IL-22 axis as a beneficial treatment option for PCOS [131].
4.5. Gut Microbiota and Thyroid Dysfunction
The most important function of the thyroid gland is to secrete the iodine-containing thyroid hormones triiodothyronine (T3), thyroxine (T4), and the peptide hormone calcitonin [134]. Diseases related to the thyroid are commonly found in females of childbearing age. Women suffering from thyroid dysfunction frequently experience infertility or miscarriage [135]. Researchers have revealed that the intestinal microbiota has a considerable impact on thyroid disorders [136]. Zhang et al. reported differences in the diversity and composition of the gut microbiota in patients with thyroid cancer and healthy individuals. They observed elevated amounts of Neisseria and Streptococcus in the patients suffering from thyroid cancer, and diminished abundance of Butyricimonas and Lactobacillus [137]. Bile acids mediate the main influences of the gut microbiota on thyroid diseases, with variation occurring between patients with hyperthyroidism and hypothyroidism. For instance, secondary bile acid deoxycholic acid is the most common bile acid in hypothyroid patients, while chenodeoxycholic acid is the most frequent bile acid in hyperthyroid patients. The production of thyroid hormones is affected by the profile of bile acids [138]. SCFAs cross-talk with the thyroid hormone triiodothyronine and modulate hormone secretion by regulating enterocyte gene transcription. Thyroid hormones significantly affect reproductive performance. There is a well-established relationship between hypothyroidism and infertility. Animal experiments have shown that hypothyroidism induction leads to menstrual cycle dysfunctions [139]. The effects of hypothyroidism on female fertility may be mediated by upregulated anovulatory cycles, increased prolactin (PRL) levels, impaired luteal phase, and disturbed sex hormones, as suggested by research. Therefore, it is imperative to evaluate thyroid function during pregnancy and in women planning to conceive. By modulating the gut microbiota profile, it may be possible to improve thyroid performance and, thus, reduce the risk of infertility or subfertility [140]. Also, it is suggested that the effects of hypothyroidism on female fertility are mediated by upregulated anovulatory cycles, PRL levels, impaired luteal phase, and disturbed sex hormones. Hence, correct thyroid function must be assessed during pregnancy and in women who are intended to get pregnant. Modulating the gut microbiota profile could improve thyroid performance to reduce the possibility of infertility or subfertility [141, 142].
4.6. Gut Microbiota and Obesity
Growing evidence suggests that gut microbiota contributes to the onset of obesity, which is reported as a risk factor for infertility. A. muciniphila or the pasteurized bacterium has been shown to prevent obesity and its complications [143]. The genus Bifidobacterium has also been found to cause weight loss in humans [144]. These data support the role of the gut microbiota in regulating host metabolism. The production of bile acids from cholesterol takes place in the liver, and their metabolism is carried out by the gut microbiota in the intestine [145]. In addition, their important role in nutrient absorption and biliary secretion of cholesterol is known. SCFAs may protect the body against HFD-induced obesity by switching lipogenesis to fat oxidation [146]. Dysbiosis of the gut microbiota in obesity is associated with several endocrine complications, including reproductive dysfunction. Indeed, obesity can negatively affect various aspects of female fertility, including the secretion of sex hormones, oocyte differentiation and maturation, endometrial implantation, and other processes required for normal fertility [147, 148]. Alteration of the gut microbiome in obese women results in increased LPS, endotoxemia, IL-6, and IL-1β, followed by the onset of inflammation, which leads to reduced oocyte quality, impaired meiotic and cytoplasmic maturation, and the development of reproductive disorders [149]. In conclusion, the composition of the gut microbiota and associated metabolites regulate host metabolism, stimulate or prevent obesity, and influence reproductive-related processes.
5. Gut Microbiota and Pregnancy Complications
Dysbiosis of the gut microbiota can lead to some gastrointestinal diseases and physiological problems in childbearing women. Obese women have an increased risk of various pregnancy complications such as recurrent miscarriage. Ghimire et al. reported that the likelihood of miscarriage in obese women was 45% higher than in normal-weight women [150]. Bellver observed a higher pregnancy loss rate after in vitro fertilization (IVF) in women with elevated BMI which is caused by metabolomic, epigenetic, or mitochondrial disorders of the oocyte and embryo, or by the unusual endocrine, metabolic, and inflammatory uterine environment induced by obesity [151]. It is found that the diversity of the gut microbiota is reduced in obesity [152]. The higher frequency of Firmicutes and increased Firmicutes/Bacteroidetes (F/B) ratio were also found in overweight and obese childbearing women [153]. The underlying mechanism of the association of the bacterial profile with obesity is related to SCFAs, which have been found to play an important role in host glucose metabolism via G protein-coupled receptors (GPR) 41 and GPR43. One study reported that the abundance of the butyric acid-producing bacteria Lachnospira and Ruminococcus was decreased in obese pregnant mice. In contrast, the secretion of LPS is higher in obese pregnant women caused of increased gram-negative bacteria [154].
Preterm birth is also a pregnancy complication accounting for 85% of perinatal morbidity and mortality. Gershuni et al. showed that the alpha diversity of the gut microbiota in the second trimester was greatly reduced in spontaneous preterm births [155]. Other studies reported an increased abundance of Bifidobacterium, Streptococcus, Clostridium, and Bacteroides and a decreased abundance of Lactobacillales in pregnant women with preterm delivery [156, 157]. Streptococcus and Bifidobacterium are able to produce lactic acid as well as SCFAs, which play a significant role in preventing preterm birth by inhibiting muscle layer contraction and membrane rupture. The expression of enzymes involved in myometrial remodeling and fetal membrane degradation is also suppressed by SCFAs [158]. Clostridium and Bacteroides have been reported to stimulate Treg cells in mice. These cells inhibit preterm labor caused by their role in suppressing inflammation by secretion of interleukin-10. In the absence of IL-10, a very small amount of LPS can promote preterm birth [159].
Acute fatty liver of pregnancy (AFLP) is a serious pregnancy problem that can lead to maternal and fetal morbidity, manifested in the mother by symptoms such as anorexia, nausea, vomiting, jaundice, and fever [160]. The insufficient function of mitochondria in the oxidation of fatty acids resulting in an accumulation in hepatocytes leads to AFLP [161]. Recent evidence supports the existence of bidirectional crosstalk between the gut microbiota and mitochondria performance [162]. The positive correlation between increased alpha-diversity of the gut microbiota and AFLP occurrence was recently uncovered by Jin et al. They showed that Acinetobacter, Enterococcus, Weissella, and Lysinibacillus are potential disease-causing bacteria that are increased in AFLP patients [163].
Anemia in pregnancy is common in childbearing women and has adverse consequences for both mother and fetus, including preterm delivery, low birth weight, and even maternal and fetal morbidity [164]. The gut microbiota is one of the factors that has been found to play a role in the occurrence of anemia in pregnant women. Jin et al. reported reduced alpha diversity of the gut microbiota in gestational anemia (GA) within the third trimester. Also, both F. prausnitzii and Ruminococcus, which have been shown to play a role in butyrate production and inflammation prevention, were found to be decreased in GA [165].
In summary, several serious life-threatening complications can occur during pregnancy, and reduced alpha-diversity of the gut microbiota, decreased beneficial microbes, and increased harmful microbes have been found to be associated with them. The major role of gut microbiota in the pathogenesis of these diseases is mediated by SCFAs and LPS.
6. Therapeutic Agents of Gut Microbiota Dysbiosis
The use of probiotics and fecal microbiota transplantation (FMT) are presented as effective ways to regulate the composition of the gut microbiota. The word “probiotic” is derived from the Greek word “pro bios” which means “life” as opposed to the word “antibiotic” which means “against life”. Probiotics are live microorganisms that have a positive effect on the human body. Probiotics are beneficial microbes that act by playing a competitive role with harmful species and suppressing their growth, improving the performance of the intestinal barrier, regulating the immune system, and modulating pain perception. Therefore, the use of probiotics can be a prevention or treatment method for a variety of diseases. There is also growing evidence to support the role of probiotics in promoting male reproduction. Stimulation of sperm motility and reduced sperm DNA fragmentation by reducing semen oxidative stress levels, as well as effects on testicular histopathology and regulation of testosterone levels, are proposed mechanisms for the beneficial influence of probiotics on male fertility. Dardmeh et al. observed improved morphological parameters and motility of mouse spermatozoa 4 weeks after adding a probiotic to the diet of a high-fat diet-induced obese mouse model [166].
The gut microbiota and genital tract microbiota have strong communication with each other; gut microbes could translocate to the vagina and alter the composition and diversity of the microbiota [167]. Probiotics may also affect female reproduction by modulating the bacterial balance in the vagina, ameliorating bacterial vaginosis, and improving inflammation; furthermore, positive outcomes of probiotics for ART, avoiding pregnancy complications, and menopausal infections are uncovered [168]. The healthiest vaginas have the highest abundance of Lactobacillus; therefore, determining the abundance of these bacteria is considered an approach to assessing healthy vaginal microbiota [169]. Hereby, exogenous administration of Lactobacillus probiotics is a plausible way to rebalance the vaginal microbiome and subsequently improve fertility, ameliorate pregnancy complications, and treat menopausal infections [168].
FMT is defined as the transfer of fecal matter from a desirable, previously screened donor to a patient suffering from a disease rooted in microbiota dysbiosis [170]. There are reports supporting the sufficiency of FMT in the treatment of human reproductive disorders. Hao et al. demonstrated that FMT increased the ratio of Bacteroides/Firmicutes in the cecum and colon, resulted in improvement of liver function, stimulated production of polyunsaturated fatty acids (PUFA), increased testosterone levels, leading to promoted expression of proteins required for spermatogenesis [88]. After FMT, bacteria and metabolites can influence both systemic immunity and the local microbial community, thereby balancing other areas, such as the female genital tract. FMT could reverse female infertility by treating some diseases that are reasons for reproductive dysfunction, such as PCOS, endometriosis, and bacterial vaginosis (BV) [170]. Guo et al. found an increase in Lactobacillus and Clostridium and a decrease in Prevotella in the intestine of PCOS rats that had received FMT, followed by an improvement in estrous cycles with reduced androgen biosynthesis and normalized ovarian morphology [171].
To conclude, the use of probiotics and the application of FMT are two introduced approaches with the ability to normalize the composition and diversity of the gut microbiota and genital tract microbiota, followed by the improvement of reproductive function.
7. Conclusions, Limitations, and Future Perspectives
According to the latest reports, the human gut microbiota composition influences almost all aspects of human health, and the generation of various disorders follows the imbalance of it and also causes reproductive dysfunction in men and women as well as the appearance of various pregnancy complications leading to increased risk of stillbirth. This article has collected complete information about various factors impacting gut microbiota composition and the consequences of events affecting male and female fertility and also pregnancy duration. However, there is limited information on the exact underlying mechanisms by which gut microbiota or derived metabolites could affect fertility behavior. Further studies are needed to elucidate these mechanisms and to determine whether gut microbiota dysbiosis is a cause or a consequence of these disorders. Nevertheless, modulation of the gut microbiota through the use of probiotics and high-fiber diets, microbial supplementation, physical activation, and FMT has attracted considerable attention as prevention or treatment strategies for reproductive disorders. Finally, it is possible that assessment of the gut microbiota profile could be considered a useful approach for noninvasive early diagnosis of reproductive disorders.
Nomenclature
-
- AFLP
-
- acute fatty liver of pregnancy
-
- AOS
-
- alginate oligosaccharides
-
- ART
-
- assisted reproduction technology
-
- BMI
-
- body mass index
-
- BV
-
- Bacterial vaginosis
-
- CVD
-
- cardiovascular disease
-
- DHT
-
- dihydrotestosterone
-
- DOR
-
- diminished ovarian reserve
-
- ED
-
- erectile dysfunction
-
- EDCs
-
- endocrine-disrupting chemicals
-
- FMT
-
- fecal microbiota transplantation
-
- FSH
-
- follicle-stimulating hormone
-
- GA
-
- gestational anemia
-
- GATA3
-
- GATA-binding protein 3
-
- GDCA
-
- glycodeoxycholic acid
-
- GPR
-
- G protein-coupled receptors
-
- GWAS
-
- genome-wide association studies
-
- HFD
-
- high-fiber diet
-
- HPG
-
- hypothalamic-pituitary-gonad
-
- HT
-
- hydroxytyrosol
-
- IBD
-
- inflammatory bowel disease
-
- IL
-
- interleukin
-
- ILC
-
- innate lymphoid cells
-
- IVF
-
- in vitro fertilization
-
- LH
-
- luteinizing hormone
-
- LPS
-
- lipopolysaccharide
-
- MAMP
-
- microbial-associated molecular pattern
-
- MAPK
-
- mitogen-activated protein kinase
-
- ND
-
- normal diet
-
- NF-Κb
-
- nuclear factor-kappa B
-
- PCOS
-
- polycystic ovary syndrome
-
- PGCs
-
- porcine granulosa cells
-
- PKA
-
- protein kinase A
-
- PPI
-
- proton pump inhibitor
-
- PRL
-
- prolactin
-
- PRR
-
- pattern recognition receptor
-
- PUFA
-
- polyunsaturated fatty acids
-
- ROS
-
- reactive oxygen species
-
- RSS
-
- reactive sulfur species
-
- SCFA
-
- short-chain fatty acid
-
- StAR
-
- steroid acute regulatory
-
- T3
-
- triiodothyronine
-
- T4
-
- thyroxine
-
- Th
-
- T-helper
-
- TMAO
-
- trimethylamine-N-oxide
-
- TNF
-
- tumor necrosis factor
-
- TUDCA
-
- tauroursodeoxycholic acid
-
- WHO
-
- World Health Organization
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Faeze Ahmadi Beni: conceptualization, investigation, visualization, writing—original draft. Hossein Saffarfar and Anis Elhami: investigation, writing—revised draft, editing. Mohammad Kazemi: writing—review and editing, visualization, supervision, and project administration. All coauthors approved the final version of the manuscript.
Funding
This research was supported by Isfahan University of Medical Sciences.
Open Research
Data Availability Statement
The authors have nothing to report.