Variations in Lorica Morphology and Element Composition in the Euglenoid Trachelomonas hispida var. coronata Under the Influence of Fe and Mn Supply
Abstract
Trachelomonas hispida var. coronata is one of the most widely reported varieties of T. hispida from water bodies worldwide. The specimens of this variety, apart from their species-specific features, such as an ellipsoidal lorica covered with strong, sharp spines, have a crown consisting of long spines surrounding the apical pore opening. The process of lorica formation is poorly understood, and in the few studies dealing with this topic, results indicate that these taxa and the entire species can produce lorica completely devoid of spines, a diacritic feature of the species. In our study, we observed in detail the formation process of the lorica in this taxon under different chemical conditions in relation to the concentration of the basic elements, Fe and Mn, which are saturated in trachelomonad lorica. The results showed that in the Fe-enriched medium, monads formed delicate, porous, spiny envelopes, whereas in the Mn-enriched medium, the loricae were more solid and less porous and had weaker developed spines; rather, they were in the form of short papillae. Differences were also observed in the structure surrounding the apical pore, which was developed differently in the two sets of media modifications (Fe- or Mn-supplemented media). We also observed different elemental compositions and colouration of loricae of cells growing in different media. This revealed that the features considered during the process of species identification are very unstable making the entire exercise highly complicated. Our research also shows that a broad discussion of the problem should be undertaken, and modern methods must be developed to unravel the complexities not only within the species but also within the entire Trachelomonas.
1. Introduction
Among trachelomonads, Trachelomonas hispida and its few varieties such as coronata and crenulatocollis, besides Trachelomonas volvocinopsis and Trachelomonas volvocina, are one of the most frequently recorded taxa worldwide [1–8]. The typical lorica (envelope, case)—a structure formed by a young monad and constituting a complete whole unicellular organism with it—has an oval shape, and the surface is uniformly covered with sharp spines. Such a description, complete with information about monad characteristics, can be found in sources for the taxonomic identification of the species [9–12]. However, there are a limited number of research studies, showing the influence of various factors on the crucial features of trachelomonad loricae, such as its morphology and colouration [13–16]. Therefore, the outer ornamentation (spines, warts, ribs, and pores) and colour (hyaline, yellowish, reddish, brown, or even almost black) of the lorica among different species of Trachelomonas are known to vary and are often influenced by different environmental and culture conditions, such as the composition of the medium, especially of Fe and Mn amounts [17]. The presence of Fe was first described by Klebs [18], who used a cytochemical test to demonstrate the presence of Fe compounds in the lorica. Subsequently, it was suggested that Fe compounds were mainly responsible for the dark colour of some envelopes [19–21]. Pringsheim [22] suggested that Fe and Mn are deposited on the walls of euglenoids that form loricae and that the addition of Fe and Mn salts to the environment affects the development and morphology of trachelomonad loricae. In turn, Deflandre [19] studied T. hispida loricae and stressed that different lorica appearance was the result of differences in the age of cells, indicating that colourless loricae belonged to young cells, whereas deep brown loricae were likely to be old cells that could have been influenced by the duration of exposure to compounds provided in the medium used. However, this issue is not as simple as it seems. In research conducted by Pringsheim [17] in a genetically homogeneous population, specimens varied in colour and ornamentation. A part of the population never reached the point when adult/mature envelopes would have been deep in colour and had the most elaborate ornamentation. Why this occurred was never fully explained. Pringsheim [17] suggested that lorica morphology and colour are strongly influenced by changes in the composition of the culture medium, particularly the presence or absence of Fe and Mn ions. In that study, he showed that the lorica walls of several species of Trachelomonas remained thin and lacked ornamentation when the culture was grown in Fe-poor medium and indicated that the loricae were brightly coloured in the absence of Mn in the medium. Subsequent chemical analyses consistently showed that Fe and/or Mn were the dominant elements in the majority of surveys to date concerning the examination of trachelomonad envelopes [13, 16–18, 23–26]. In contrast, the first direct demonstration of Mn content in the euglenoid envelope did not appear until 1977, in a study carried out by West and Walne [27]. Later studies [14, 16, 25, 28, 29] suggested that the colour of the lorica wall may be related to its elemental composition, and there may often be differences in the amounts of Fe and Mn as the lorica darkens. More recent studies using scanning electron microscopy (SEM) and transmission electron microscopy have observed that in Trachelomonas loricae, Fe and Mn usually occur together in different proportions, showing a spatial segregation that is evident in the ultrastructure of loricae [25]. Although there is some knowledge about the elemental composition of loricae and how the main ions influence lorica appearance, little is known regarding how exactly the increase or decrease in the amount of a particular component of the medium used reflects the change in lorica colour and/or ornamentation. It is worth mentioning that the lorica, with its characteristics, is still the main criterion for the identification of the species level of specimens in water samples. However, whether it is a good criterion is questionable. Pringsheim [17] previously emphasised that owing to the fact that lorica shape, colour, and ornamentation can vary according to the conditions, identification of species becomes difficult. Little has changed in this regard over the following decades with regard to hydrobiological research.
In this study, we present the results of an initial study focusing on the effect of medium composition on the appearance of T. hispida var. coronata loricae. We aimed to check the effect of a series of increasing Fe and Mn ion concentrations on the (1) sculpture of the surface, (2) colour, and (3) chemical composition of the lorica of the studied species.
2. Material and Methods
In this study, T. hispida var. coronata Lemmerm. culture CCAP 1283/2, obtained from the Culture Collection of Algae and Protozoa, Dunstaffnage Marine Laboratory, Oban, Scotland, UK, was used. The cells were grown in Jaworski’s medium enriched with soil extract (JM:SE) [30]. Cultures were incubated at 22°C ± 2°C on a 12:12 h light–dark cycle with illumination at 35–36 μm m−1 s−1 provided by 40 W cool white fluorescent bulbs. The cultures were grown in the following concentrations of FeNa-EDTA and MnCl2·4H2O as the sources of Fe and Mn, respectively: 1.4, 1.9, and 2.2 mL in comparison with 1 mL per 1 L of the JM in the control sample. In this manner, we obtained final concentrations of 0.315%, 0.428%, and 0.495% of FeNa-EDTA and 0.195%, 0.264%, and 0.306% of MnCl2·4H2O in the experimental set of samples (comparing to 0.225 and 0.139 in the control sample, respectively). All the samples were mixed daily using a rotary shaker (100 rpm) for 15 min. The cultures were supplemented weekly with 1 mL of fresh medium to avoid mineral depletion. The experiment duration was 12 weeks, and every 4 weeks, we checked the cultures. Light microscopy of trachelomonad cells was performed using a Nikon Eclipse E600 microscope equipped with Nomarski differential interference contrast optics. Light microscopy photographs were taken using a Nikon NIS-Elements digital camera. Samples for SEM analysis were prepared according to the procedures described by Bozzola and Russel [31]. The fixed material was washed several times with distilled water to remove buffer salts, and then, the samples were dehydrated using a graded ethanol series. Afterwards, drops of dehydrated material were transferred to slides mounted on aluminium stubs (Ø 10.0 mm) with graphite coating and air-dried overnight at approximately 20°C. After drying, the stubs were sputter-coated with a 20-nm cover of carbon using a Cressington Sputter Coater (Cressington Scientific Instruments Ltd.). SEM observations of the carbon-coated material were performed using a Hitachi S-4700 microscope. Microscopic images (culture samples) were obtained using a Zeiss Ultra Plus field-emission scanning electron microscope. The samples were placed in a chamber on 12.5 mm aluminium stubs, and a pressure of 5 × 10–4 Pa was applied. Images were recorded at an electron beam energy of 20 keV, aperture of 30 μm, and working distance of 9 mm. An energy-dispersive x-ray (EDX) detector was used for quantitative, qualitative, and elemental mapping. Characteristic radiation signals of elements were collected from the detector over 100 s real time (spectra) and over 10 min real time (maps) using Quantax software to visualise and interpret the qualitative, quantitative, and map data. The EDX spectra and elemental maps were recorded for selected fragments of the samples, and SEM images of their surfaces were taken. Quantitative analysis of EDX spectra was carried out with the use of a “nonstandard” method. Statistica software (Version 13.0) was used to draw box-whisker plots for presenting the differences in the length of loricae in the studied samples. The Wilcoxon signed-rank test was used to compare the change in length of T. hispida var. coronata lorica growing in Fe- or Mn-enriched media with respect to a control sample.
3. Results
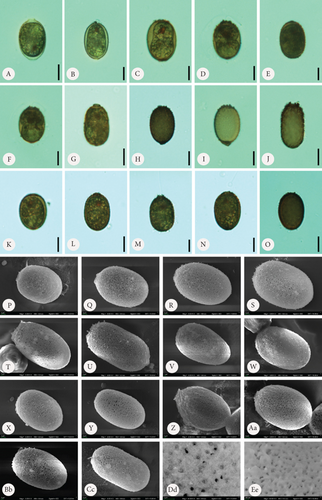
3.1. Morphology and Colour of T. hispida var. coronata Lorica
Loricae in the control sample were usually elliptical in shape, although differently shaped envelopes were also observed (Figure 1). Some observed shapes included longitudinally oval (Figure 1 C, K, and P), almost round (Figure 1 F and T), lemon-shaped (Figure 1 U), or conically tapered toward the posterior end (Figure 1 V). The loricae possessed apical pores that were smooth (Figure 1 D and I) or surrounded by spirally arranged folds (Figure 1 Q and R) and only occasionally by small spines (Figure 1 P and S). There were also cells that formed loricae with short collars or, in contrast, relatively long collars (Figure 1 F–H and J and T and K and N–O, respectively). All these variations also applied, to some extent, to loricae from the experimental sets, both with the addition of Fe or Mn. A common feature of all the observed loricae from the control sample was the lack of spines on the surface, which are the main diagnostic features of the taxon (and the entire species) and are typical of specimens found in the field. There were only small warts/papillae regularly distributed, but they rarely spread on the entire lorica and were visible in the SEM microphotographs (Figure 1 P–W). In the control sample, there were many monads that did not form loricae at all, and there were also monads with a very thin and hyaline lorica hardly visible at first sight (Figure 1 A and B).
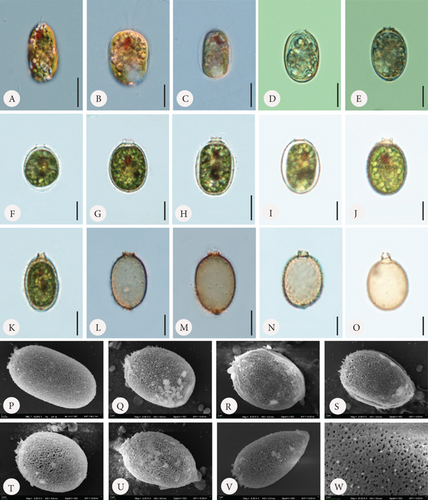
In the samples to which we provided Fe at three different concentrations, we observed changes on the surface of the lorica, which mainly consisted of spine formation (Figures 2 and 3). First, the lorica surfaces were densely covered with spines. The spines were visible and prominent under SEM. The spines developed the most with the lowest added amount of FeNa-EDTA (+1.4 mL) (Figure 3 A–H) and the least in the medium amount of FeNa-EDTA (+1.9 mL) (Figure 3 Q–T). Regardless of the concentration, we observed that the lorica wall was very porous, and the pores were large, which gave the impression that the wall had a highly reticulate structure (Figure 3). The development of the collar also differed from that of the control sample. The lorica apical pore was surrounded by numerous and sharp spines forming a crown (which is typical of the variety coronata); in some cases, there were also short collars with spines at the rim (Figure 3 B and J). It is worth mentioning that in samples with Fe supplementation, the loricae were of a little different shapes, from elliptical to round, and the number of oval-round loricae was significant. The colouration differed such that in the sample with the least amount of added Fe, the coverings produced by the monads were the brightest, ranging from hyaline to slightly greenish-brownish (Figure 2 A–E). In the two subsequent higher concentrations, envelopes were darker with more red colour, and this was particularly evident in the empty envelopes, which may demonstrate that Fe deposition in the lorica walls increases with cell aging and/or the duration of exposure (Figure 2 F–O).
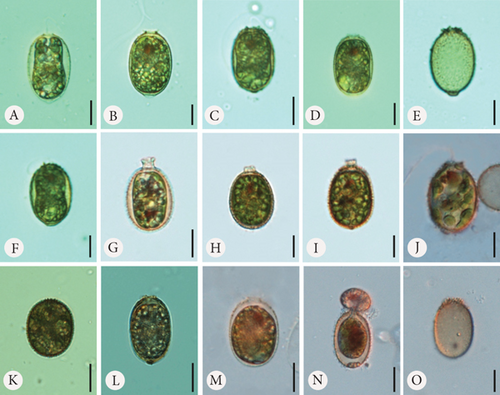
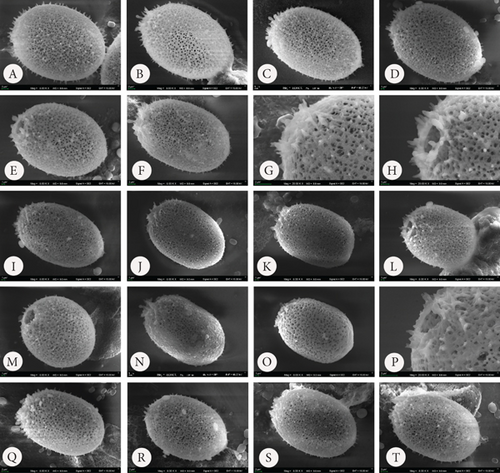
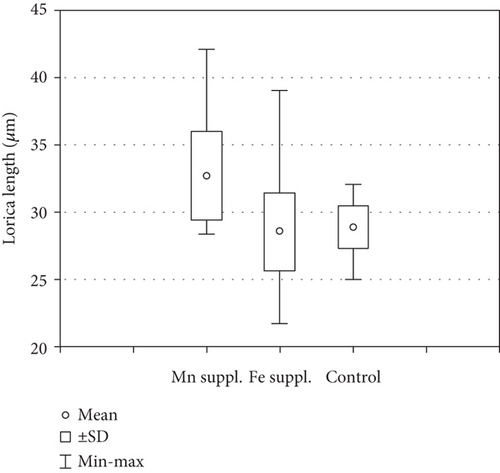
In the samples with Mn supplementation, the envelopes were elliptical and, in most cases, more elongated than the structures formed under Fe supplementation conditions. The walls of the loricae were also porous, but the pores were smaller, and the structure was more densely arranged; therefore, the loricae appeared solid and thick, especially at higher concentrations of Mn in the sample (Figure 4 A–Ee). Spines on the surface of the envelopes were developed but were sparsely distributed on the walls and were often reduced to small warts (Figure 4 P–S, U, V, and Cc). This difference was most visible in the samples with the highest concentration of MnCl2·4H2O in the medium (+2.2 mL). Another element of the lorica is a usually well-developed collar. We found different stages of collar development. In the vast majority, the collar was very low but distinctly formed and uneven at the rim. This feature was observed in all samples, regardless of the Mn concentration (Figure 4). One observation that should be highlighted is that the loricae from the samples supplemented with Mn were longer and more elongated than those from the Fe-supplemented samples. A comparative analysis of the length of the envelopes showed that there were statistically significant differences in the dimension tested in specimens growing in a manganese-enriched medium (Wilcoxon test, Z = 4.52, p ≤ 0.001). However, no difference was observed in lorica size from the Fe-enriched medium (Wilcoxon test, Z = 0.88, p > 0.05). The box-whisker plot analysis showed the length distribution of the lorica T. hispida var. coronata indicating a clear increase in the parameter under study in individuals cultivated in the medium supplemented by Mn ions (Figure 5). The colouration of the envelopes was darker than that in the samples with Fe supply. They were brown or even deep brown (Figure 4 C–O), although there were also specimens, likely young ones, with brighter loricae (Figure 4 A and B). The percentage of the brightest envelopes was the highest in the sample with +1.4 mL MnCl2·4H2O; in the remaining two experimental conditions with higher concentrations, the envelopes were darker in most of the specimens.
3.2. Element Composition
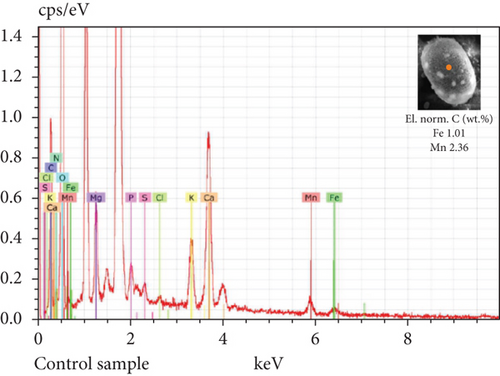
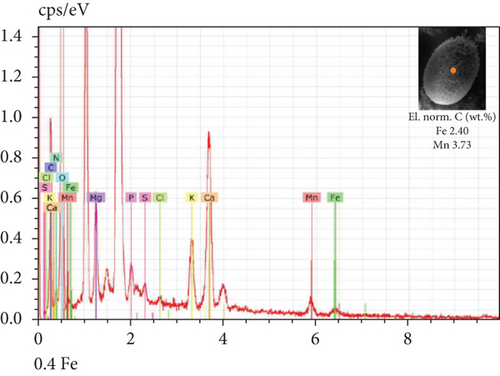
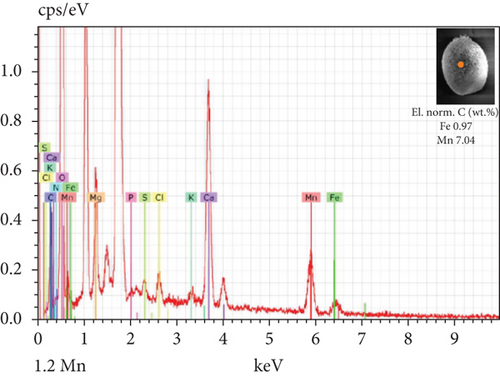
The SEM-EDX spectroscopy analysis revealed differences in the elemental composition of T. hispida var. coronata loricae. In the envelopes from the control sample, there was a higher percentage share of Mn than of Fe compounds, 2.36% and 1.01%, respectively (Figure 6a). In our study, we observed that both ions were incorporated into the walls of loricae, regardless of their concentration. The increasing supplementation of any ion and its incorporation manifested as a high percentage in lorica composition, resulting in the other ion also being incorporated in a high amount. However, with the increasing content of Mn in the medium and in the wall of the envelopes, the Fe content was small, usually constituting no more than 15% of the Mn content (Figure 6e, 6f, and 6g). In the case of Fe supplementation, Mn still constituted more than 50% of the total amount of Fe in the studied loricae (Figure 6b, 6c, and 6d). It should be noted that with increasing concentrations of a given ion, its higher content was also manifested in the chemical composition of the lorica walls. Only at the lowest experimental concentration of the ferric ion was the concentration of manganese in the lorica wall higher than that of iron (Figure 6b). The remaining constituent elements of the studied envelopes were Ca, Mg, and K, as well as small amounts of P, S, and Cl (Figure 6). All the results obtained during the study are summarized in Table 1.
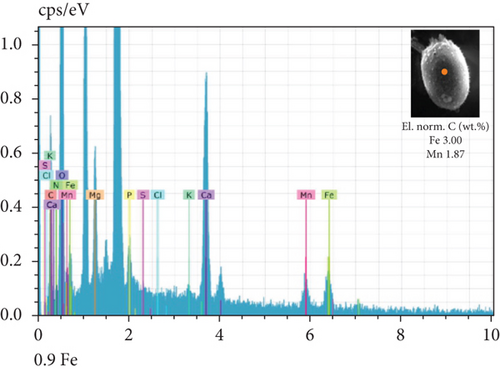
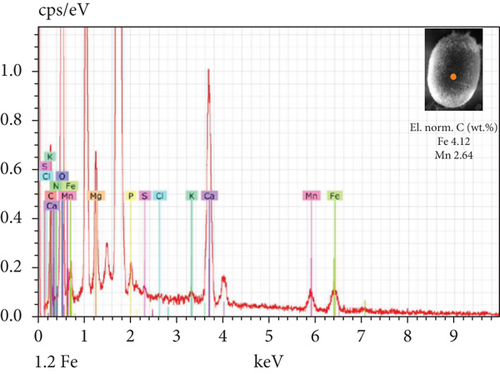
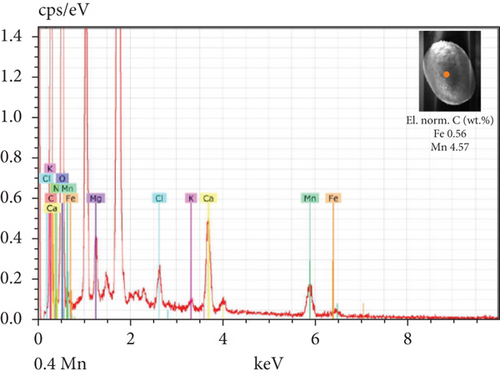
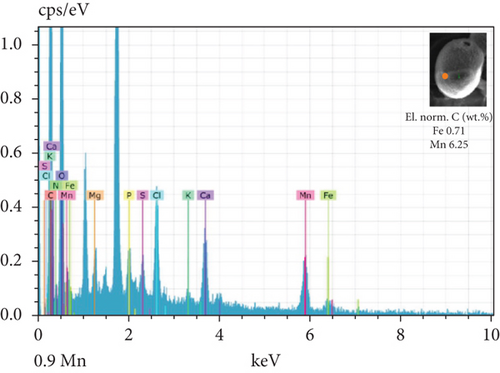
| Control sample | Fe supplementation | Mn supplementation | |
|---|---|---|---|
| Shape | Elliptical, longitudinally oval, round, lemon-shaped, conically tapered to the posterior | Elliptical to round, oval-round | Elongated elliptical |
| Spines | No spines on the lorica wall, small warts/papillae instead | Present, densely distributed on the lorica wall | Present, sparsely distributed on the wall and often reduced to small warts |
| Apical pore | Smooth, with spirally arranged folds, occasionally with small spines, short or long collars | Surrounded by numerous and sharp spines forming a crown or by a short collar with spines at the rim | Equipped with well-developed collar, uneven at the rim |
| Pores on lorica wall | Rather small, the wall less porous than in Fe-enriched medium and more porous than in Mn-enriched medium | Large and numerous, highly reticulate structure of the wall | Small, densely arranged structure of the wall |
| Colouration | Often hyaline | Hyaline to slightly greenish-brownish, even red | Brown, deep brown |
| Content of Fe and Mn | Higher share of Mn (57%) than Fe (43%) | High content of Fe, pretty high content of Mn (> 50%) | High content of Mn, small content of Fe (< 15%) |
4. Discussion
4.1. Lorica Morphology
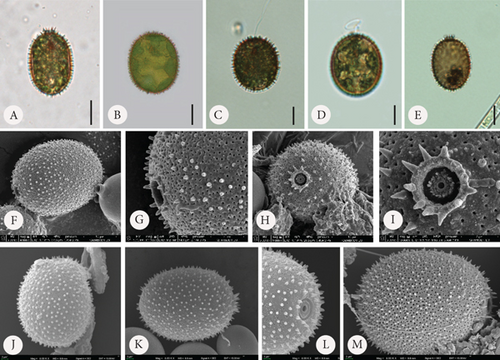
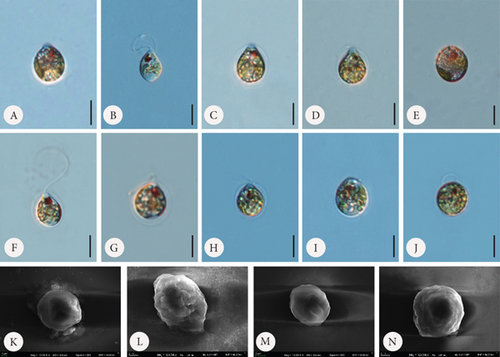
According to available sources for taxon identification, the typical T. hispida var. coronata forms a crown around the apical pore. This crown consists of sharp, conical spines, usually longer (according to Sena et al. [32] up to even 3.5 μm long) than on the rest of the lorica, or sometimes a short collar equipped with distinct spines [1, 4, 6, 15, 33, 34]. This structure is the feature that distinguishes the variety from the nominative variety hispida, which has spines around the apical pore that are as long as those on the lorica surface and from other varieties such as the most commonly recorded, crenulatocollis, (which means collar around apical pore with some sort of crenulated [toothed] rim) or spinulosa (with a distinct dentate collar) [9, 10, 35]. Our specimens formed either crowns of spines, especially in the samples enriched with Fe or short collars, without spines, mainly in samples with Mn, or they did not form any structured element around the apical pore. Thus, these characteristics are highly variable and dependent, at least to some extent, on the chemical composition of the medium. Our observations concurred with those of Rosowski et al. [36], who, studying the same taxon under laboratory conditions, found differently developed collars from inconspicuous to distinct. This is not different from other diagnostic features. The presence of spines on the surface is an important factor. Using the available key guides, one can see that the lorica surface of specimens belonging to the species T. hispida is covered with spines or can only be punctuated without spines (nominative variety, var. crenulatocollis, var. volicensis). For the remaining varieties, the available information mentions that they have spines or warts, or information on the ornamentation is absent; thus, implicitly, these specimens have spines similar to those of the nominative variety [9–11, 37]. Nevertheless, it is very difficult to find any record of T. hispida from natural ecosystems without spines (alternatively warts) on the envelope surface, and they would have only been punctuated (e.g., [1, 4, 6, 8, 13, 26, 32, 38, 39]). For comparison, we presented microphotographs (LM and SEM) of T. hispida cells from environmental samples (Figure 7). In our study, we observed loricae, which were always punctuated to a greater or lesser extent and were completely spineless, with small warts, delicate papillae, or spines on the lorica surface. It must be highlighted, however, that the spines did not look like those on loricae observed in natural collections found in freshwater. However, there are only a few records showing cells of spiny Trachelomonas species in culture conditions that might not form spines. The first evidence, in the form of LM and SEM pictures, was provided by West and Walne [13], who reported spineless loricae of T. hispida var. coronata in the majority of the samples under culture conditions. Kim, Shin, and Boo [15] transferred T. hispida cells from Korean ponds and lakes into the laboratory and observed both spiny and spineless loricae during the research conducted. Similarly, Poniewozik, Sajnaga, and Zięba [40] used T. hispida in an experimental study that investigated the development of loricated monads in water from various natural water bodies, filtered through a GF/C and a 0.22-μm Millipore syringe filter. After the experiment, they did not observe loricae that formed conical sharp spines covering the envelopes. Rosowski et al. [36] provided surprising insights into Trachelomonas oblonga var. punctata. This taxon is known from the taxonomic description as having punctuated loricae, being placed in Pringsheim’s T. volvocina group of “never spiny” lorica, and not having a collar around the apical pore. Under the culture conditions, the cells formed heavily ornamented envelopes, equipped with short spines and/or extremely polymorphic papillae [36]. Our research shows that the availability of basic ions with which the lorica of trachelomonads saturates is essential for the formation of a robust structure, such as a collar, and for the development of spines on the wall surface. This was particularly evident in cultures grown in the presence of increasing amounts of Fe chelate and less so in cultures grown with an additional pool of Mn. In the latter, the lorica formed shorter spines, had a smaller number of pores, and appeared to have a denser and more rigid internal architecture. This suggests that the presence of increased amounts of these two elements in the water or medium affects lorica formation differently in T. hispida var. coronata. The structures surrounding the apical pore were also differently developed in specimens grown in Fe- or Mn-supplemented media (long crown-forming spines vs. short collars with fine serrations at the edge). We must acknowledge, however, that although individuals from Fe-enriched cultures, regardless of the ion concentration, had numerous and long spines on the surface of the lorica, these spines were not as strong and thick as those reported and documented from environmental samples [6, 8, 25, 34, 41, 42]. To investigate the influence of different medium compositions on the lorica formation process, we also attempted to test another species, assuming that supplementation with the basic components of lorica, which Fe and Mn are considered to be, would allow the development of additional structures in individuals that, in the natural environment, produce smooth lorica without any ornamentation on the surface. For this purpose, we used the strain T. volvocina (Ehrenb.) Perty (CCAP 1283/4B) and placed samples under the same conditions as T. hispida var. coronata. However, during the course of observation, we noticed that the lorica formation process was almost completely stopped. Most cells functioned as naked monads, devoid of lorica. If this structure was formed, it was only by a few cells, and the lorica was very thin, delicate, transparent, and completely colourless, as observed under LM (Figure 8). The lorica was such a delicate structure that despite several attempts, we failed in observing it under SEM by preparing the material using the method commonly used for this type of structure. Therefore, we believe that the studies showing spineless “spiny” trachelomonads from culture conditions and the present study on T. hispida var. coronata provide evidence regarding triggers of the process of lorica formation as a solid structure equipped with strong spines. Additional drivers besides chemical composition are needed or, more likely, the entire environmental complexity must occur to cause an effect of distinct spines on the surface of the lorica. The absence of a lorica, or a different model for the formation of spines that are consequently usually thin and fragile under laboratory conditions, may suggest a role for a well-developed, rigid structure, such as lorica, in protection against predators in the natural environment, as has been suggested by Poniewozik, Sajnaga, and Zięba [40].
4.2. Element Composition and Lorica Colour
The trachelomonad lorica mainly comprises Ca, Mg, K, P, S, Cl, Fe, and Mn, of which the last two are the most important depositional elements providing colour and determining the type of internal microarchitecture of the envelopes (granular or microcrystalline) [14, 25, 43–46]. Sometimes, the amount of some elements is higher or lower. Poniewozik, Sajnaga, and Zięba [40] observed a lack of Mn in young loricae and a lack of Fe in old loricae in studies conducted on T. hispida. West and Walne [13] demonstrated special cell selectivity for Mn, which was incorporated faster than Fe was in the envelopes of T. hispida var. coronata. Our results are in accordance with the results [13, 40] as we observed that Mn was incorporated in a significant amount, even in the samples with Fe supplementation, whereas in the samples with Mn supplementation, its amount was considerably high with a concurrent low amount of Fe. Another aspect is the colour of loricae. We observed differently coloured envelopes of T. hispida var. coronata at different stages of lorica development. In the control sample and in both sets of experiments, young, bright, and thin loricae formed by the monads shortly after cell division were observed. We also observed older loricae with a reddish-brown colour (in samples with Fe chelate) and those that were brown or even brownish-black (in samples with Mn in the form of hydrated manganese chloride). Generally, we observed that cells growing in Mn-supplemented medium were darker than those grown in Fe-rich medium, which took on a reddish colour, with the colour becoming increasingly intense for cells growing in increasing ion concentrations in the medium and over time. Therefore, the change in colour was due to both the age of the cell and the duration of exposure to the chemical element. Similar previous observations suggest that the aging of cells is a main factor of envelope darkening [13, 40] or the chemical composition of the medium [17]. In general, in trachelomonads in nature, the formation of very thin and transparent loricae is a rare phenomenon. Of the widely distributed species, only T. oblonga is described as having a thin, bright lorica [9–11], but it still appears different from the specimens we observed in culture. These tended to be yellowish, slightly reddish, and, above all, considerably thicker. There was no difficulty in answering the question of whether a found individual likely has or does not have a lorica, a question we sometimes asked ourselves when conducting our observations. However, during our research, we had the opportunity to identify young cells that were in the process of forming a new lorica. A question that remains unanswered is why cells with hyaline and thin lorica are rarely found in environmental samples. Further research in this area is required to identify the chemical composition and microarchitecture of the envelopes of different taxa. The range of the amounts of different elements present in loricae and how various physical and chemical parameters affect lorica composition should also be investigated.
4.3. Taxonomic Ambiguity
In light of the research presented, we consider that trachelomonad loricae, although still a basic taxonomic criterion, are not an entirely good criterion. We are not in a position to claim definitively whether from among specimens that are found in environmental samples, those that do not have spines on the surface of the lorica would not be T. hispida (having the other taxonomic features of the species, i.e., a more or less elliptical/oval lorica, several chloroplasts with double-sheathed pyrenoids, and within the size range for the species). Hypothetically, it could then be labelled as, for example, T. oblonga or T. botanica, which differ from T. hispida primarily in the absence of spines. It is also impossible to transfer the specimens to laboratory conditions and observe how the lorica will form after new monads have been produced under other laboratory conditions. Regarding T. hispida, we suppose that most of the functioning varieties or forms in which this species is unusually rich—there are close to 50 of them—are merely within phenotypic plasticity. The differences in the keys to identification, later reported in scientific studies from different parts of the world, are subtle. An example is the variety caudata [47] and acuminata [19], described several decades ago, of which descriptions are very brief, coinciding with each other and not showing the differences between the taxa. Another example is the varieties crenulatocollis and coronata, both frequently reported globally, where the differences are only in the form of the structure surrounding the apical pore; as shown in the present study, this feature is highly variable, and individuals of one strain (CCAP 1283/2) can produce both types of this structure. Many of these varieties have not been reported for several years, and the differences between them are so subtle that different individuals may represent different varieties in the subjective judgement of researchers. The existence of many taxa, especially those distinguished by Skvortzov [48], who described new varieties of T. hispida: papillata, punctulosa, simplex, charkoviensis, hyalina, or spinopunctulosa, disagreed with the description of Van Oye [49], who considered them to be within the variation of the species. There are many examples of the introduction of multiple varieties based on minor differences in cell morphology. Another example is the work of Dreżepolski [50], who, in a study of euglenoids of water bodies from selected regions of Poland, Lithuania, and Ukraine, in one paper alone described as many as five new varieties of T. hispida: granulo-spina, irregularis, niezabitowskii, setosa, verrucosa, and volicensis. These taxa were, in most cases (an exception being variety volicensis), not subsequently found in other studies by other researchers. The same doubts concerning the overestimated number of species within the genus have been raised and published in papers on two other species within Trachelomonas: the cosmopolitan Trachelomonas caudata, which is morphologically very similar to several other species [51] and the morphologically convergent Trachelomonas volzii and Trachelomonas dubia, for which many varieties and forms have been described from various ecosystems in different parts of the world [16]. This type of research on Trachelomonas loricae, the process of its formation, and the drivers triggering its development is not common, and there is very little data on the subject; hence, there are many areas on this topic that should be developed to propose a different or improved method of identification within Trachelomonas. In the recent 20 years, advanced molecular studies altering taxonomy within the euglenoid genera Euglena, Lepocinclis, Phacus, or Monomorphina have been published [52–58]. These have reordered the taxonomic position of many species, although it must be acknowledged that their value, though undoubtedly great, in environmental studies makes the identification process even more complex. For correct identification of many species within these genera, it becomes indispensable to transfer the cells found to laboratory conditions and carry out molecular studies. Only then can it be said with certainty which species we are dealing with. A similar path probably awaits Trachelomonas (and Strombomonas), especially considering what we believe to be too many taxa introduced into the taxonomy, mainly based on the description of lorica morphology. Pringsheim [17] suggested reducing the number of taxa described based on lorica morphology. We believe that we should proceed in this direction in the taxonomy of Trachelomonas.
Disclosure
Mateusz Kudlak is a former student of biotechnology at the John Paul II Catholic University of Lublin and his contribution to conducting experiments on Trachelomonas hispida and taking LM microphotographs relates to when he was a student.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
M.P. designed experiments and prepared all the manuscript. M.K. conducted experiments and took LM microphotographs. E.Z. performed SEM and SEM-EDS analysis.
Funding
This research was financially supported by the National Science Centre (NSC, Poland) (Project No. DEC-2020-04/X/NZ8/00292). Part of the research was also funded by the statutory fund of the John Paul II Catholic University of Lublin.
Acknowledgments
This research was financially supported by the National Science Centre (NSC, Poland) (Project No. DEC-2020-04/X/NZ8/00292). Part of the research was also funded by the statutory fund of the John Paul II Catholic University of Lublin. We would also like to thank our colleague Dr. Tomasz Lenard (Department of Physiology and Toxicology, The John Paul II Catholic University of Lublin) for a very interesting discussion and valuable guidance on statistical methods used in biology/ecology.
Open Research
Data Availability Statement
Data for this study is available from the corresponding author upon reasonable request.