Antitumor Effect of Apilactobacillus kosoi 10H by Inducing Immunostimulatory Macrophages
Abstract
Apilactobacillus kosoi (A. kosoi) 10H is a fructophilic lactic acid bacterium found in vegetable sugar fermentation liquid. It has been shown to possess high intestinal immunostimulatory activity. In this study, we investigated the potential for A. kosoi 10H heat-killed cells and their components to convert the phenotype of immunosuppressive M2 macrophages to immunostimulatory M1 macrophages. We further investigated the ability of induction of apoptosis of cancer cell lines by macrophage culture medium in the presence of A. kosoi 10H heat-killed cells. After induction of macrophage differentiation by phorbol 12-myristate 13-acetate in a THP-1 human monocyte–derived cell line, immunosuppressive M2 macrophages were induced by interleukin-6 (IL-6). When A. kosoi 10H heat-killed cells were brought into contact with IL-6-induced M2 macrophages, the expression of CD163 and CD209, which are M2 macrophage markers, markedly decreased, and the expression of CD80, human leukocyte antigen-DR isotype (HLA-DR), and tumor necrosis factor-α (TNF-α), markers of M1 macrophages, markedly increased. A similar effect was observed with a water-soluble extract and a hydrophobic liquid extract from A. kosoi 10H heat-killed cells. In addition, when M2 macrophage culture medium that had been conditioned with A. kosoi 10H heat-killed cells was added to the MCF-7 breast cancer cell line, we found that apoptosis was induced. TNF-α in the medium conditioned with macrophages cultured in the presence of A. kosoi 10H heat-killed cells was found to be partially responsible for MCF-7 cell death. This study presents basic data on the potential to use lactic acid bacteria to improve the cancer microenvironment.
1. Introduction
There is a growing awareness of the need for health promotion and preventive medicine through dietary improvement. In this regard, there is notably increasing interest in the functionality of lactic acid bacteria. These play a major role in improving the intestinal environment, which is important for immunity [1]. Of the lifestyle-related diseases that affect a high percentage of modern people, cancer is one of the most important to control due to its damaging and fatal effects [2].
The cancer microenvironment is the key to investigating cancer. Cancer tissues usually contain large numbers of immunosuppressive M2 macrophages called TAMs (tumor-associated macrophages). One strategy to confront cancer is to convert these immunosuppressive M2 macrophages to immunostimulatory M1 macrophages [3]. The present study centers on the ability of lactic acid bacteria to affect this conversion.
Applied research on lactobacilli in the antitumor field has so far focused on the direct action of lactobacilli on cancer cells [4] and on their immune adjuvant effect [5]. There are, however, few studies on the potential to use lactobacilli to improve the cancer microenvironment and stimulate cancer immunity.
Fructophilic lactobacilli, including Apilactobacillus kosoi (A. kosoi) 10H, are a group of recently discovered lactic acid bacteria that have a preference for fructose as a sugar source [6]. They are known to inhabit fructose-rich environments such as bee intestinal tracts and nectar and have a symbiotic relationship with bees. There have so far been few studies on the physiological activity of fructophilic lactobacilli and their potential for industrial use, but they appear to have future potential for application to the medical field and in food products [7].
A. kosoi 10H was found as an uncultured fructophilic lactic acid bacterium in vegetable brown sugar fermentation solution [8, 9]. It has high intestinal immunostimulatory capacity. The lipoteichoic acid molecules on its cell surface, which are responsible for immunostimulation, have been shown to have a unique structure [10].
The present study shows that A. kosoi 10H heat-killed cells and its extracts can induce phenotypic conversion of macrophages from the immunosuppressive M2 type to the immunostimulatory M1 type and that macrophage-conditioned medium cultured in the presence of A. kosoi 10H heat-killed cells induces apoptosis in MCF-7 breast cancer cell line.
2. Materials and Methods
2.1. Culture of A. kosoi 10H
A. kosoi 10H, a strain preserved at the Kitami Institute of Technology, was used. The genome sequence of A. kosoi 10H (NBRC 113063) has been deposited in GenBank under Accession Number BEXE00000000. The version used in the project (01) has the Accession Number BEXE01000000 and consists of sequences BEXE01000001–BEXE01000067 [8]. The strain was incubated in de Man, Rogosa, and Sharpe (MRS) medium (BD Difco Lactobacilli MRS Broth, Fisher Scientific, Roskilde, Denmark) supplemented with 10% (w/v) D-(–)-fructose (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) in a tightly sealed 500-mL medium bottle (AGC Techno Glass Co. Ltd., Shizuoka, Japan). After culture was complete, the MRS medium was removed using a centrifuge (Model 6800, Kubota Shoji Co. Ltd., Tokyo, Japan) at 13,000 g for 20 min to obtain a microbial precipitate. This was suspended in Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific Inc., Massachusetts, United States) and centrifuged (13,000 g, 20 min) (Model 6800, Kubota Shoji Co. Ltd.) three times to obtain a washed microbial precipitate. A portion of this precipitate was heat-treated at 70°C for 30 min to give heat-killed cells and then freeze-dried using a lyophilizer (FDU-2200, Tokyo Rika Kikai Co. Ltd., Tokyo, Japan). In the experiment described in Sections 2.4 and 2.7, a heat-killed cell suspension of A. kosoi 10H was made by suspending the A. kosoi 10H heat-killed cells in DPBS at a density of 40 mg/mL.
2.2. Preparation of Extracts of A. kosoi 10H
Nineteen milligrams of the washed microbial lyophilized powder of A. kosoi 10H heat-killed cells obtained in Section 2.1 was suspended in 100 μL of 0.1 M sodium acetate buffer (Fujifilm Wako Pure Chemical Corporation, pH = 4.7), transferred to an Easy Beads tube (76813M, AMR Corporation, Gifu, Japan) and subjected to vortex mixing (M&S Instruments Inc., Osaka, Japan) for 2 min and 30 s. Then, 100 μL of n-butanol (Fujifilm Wako Pure Chemical Corporation) was added and subjected to vortex mixing for 30 min. Next, 100 μL of 0.1 M sodium acetate buffer (pH = 4.7) and 100 μL of n-butanol were added and subjected to vortex mixing for 30 min. The two layers were then separated by centrifugation at 5300 g (MX-301, Tomy Seiko Corporation, Tokyo, Japan) for 5 min. The upper layer (the n-butanol phase) and the lower layer (the aqueous phase) were collected in separate tubes and lyophilized. The extract from the upper layer (the n-butanol phase) is hereinafter described as the “hydrophobic liquid extract” and the extract from the lower layer (the aqueous phase) as the “water-soluble extract.” In the following experiments described in Sections 2.4 and 2.7, the hydrophobic liquid extract and water-soluble extract were dissolved in methanol (Fujifilm Wako Pure Chemical Corporation) and DPBS, respectively, to a concentration of 10 mg/mL.
2.3. Preparation of Immunosuppressive M2 Macrophages
The THP-1 human monocytic leukemia cell line (Japanese Collection of Research Bioresources (JCRB) cell bank) was inoculated into 24-well plates (Nunc cell-culture treated plates, Fisher Scientific) to achieve 2 × 105 cells/well using Roswell Park Memorial Institute (RPMI) 1640 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (shown as complete RPMI 1640 medium).
Next, phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), dissolved in complete RPMI 1640 medium, was added to make a final concentration of 100 ng/mL and incubated in a CO2 incubator (WKN-MC35, WakenBtech Co. Ltd., Kyoto, Japan) at 37°C and 5% CO2 for 72 h to induce macrophages. The obtained macrophages were washed three times with complete RPMI 1640 medium and cultured in complete RPMI 1640 medium supplemented with human interleukin-6 (IL-6) recombinant protein (Proteintech Group Inc., Illinois, United States) at a final concentration of 12.5 ng/mL for 72 h to induce immunosuppressive M2 macrophages [11, 12]. The obtained M2 macrophages were washed three times with complete RPMI 1640 medium.
2.4. Induction of Immunostimulatory M1 Macrophages From Immunosuppressive M2 Macrophages
Immunosuppressive M2 macrophages adhered to 24-well plates were cultured in complete RPMI 1640 medium for 24 h after the addition of A. kosoi 10H heat-killed cells, water-soluble extract or hydrophobic liquid extract, to a final concentration of 2 or 20 μg/mL. After incubation, the culture medium was removed and the cells were rinsed with DPBS and then detached from the plate by adding 0.3 mL of 0.5 μM EDTA (Nacalai Tesque Co. Ltd., Kyoto, Japan) and holding for 10 min. Next, 0.3 mL of complete RPMI 1640 medium was added and the cells were collected into 1.5-mL microtubes (Violamo, As One Corporation, Osaka, Japan) by pipetting. The wells were washed again with 0.3 mL of DPBS, and the washing solution was collected in the same tubes. The supernatant was then removed by centrifugation (MX-301, Tomy Seiko Corporation) at 2300 g for 5 min at 4°C to obtain cell pellets.
2.5. Preparation of Antibody Solutions for Flow Cytometry
First, a solution of DPBS was prepared by the addition of Fc Blocker (BioLegend Co. Ltd., California, United States) at 100-fold dilution, followed by 7-aminoactinomycin D (7-AAD, Beckman Coulter Co. Ltd., California, United States) at 50-fold dilution and FITC-conjugated anti-CD11b antibody (BioLegend Co. Ltd.) at 50-fold dilution.
The antibody solutions were prepared by adding each fluorescent-labeled antibody to the above solution at 50-fold dilution, plus a combination of PE-conjugated anti-CD163 antibody (BioLegend Co. Ltd.) and APC-conjugated anti-CD209 antibody (BioLegend Co. Ltd.) or PE-conjugated anti-CD80 antibody (BioLegend Co. Ltd.) and APC-conjugated anti-HLA-DR antibody (BioLegend Co. Ltd.).
2.6. Measurement of CD163, CD209, CD80, and HLA-DR Expression by Flow Cytometry
The prepared antibody solution was added to the macrophage cell pellets and homogenized using a vortex mixer. Cell pellets were stained with fluorescent-labeled antibodies at 4°C for 15 min and then analyzed using a flow cytometer (CytoFlex B53015, Beckman Coulter Co. Ltd.). For flow cytometric analysis, 7-AAD-negative cells were gated and the expression rates of CD163, CD209, CD80, and HLA-DR were quantified within the gate of CD11b-positive cells. The increase or decrease in the expression rate of CD80 and HLA-DR was used as markers for immunostimulatory M1 macrophages (hereinafter referred to as M1 markers), and CD163 and CD209 were used as markers for immunosuppressive M2 macrophages (hereinafter referred to as M2 markers).
2.7. Measurement of TNF-α by Enzyme-Linked Immunosorbent Assay (ELISA)
After the addition of 2 or 20 μg/mL of A. kosoi 10H heat-killed cells, water-soluble extract or hydrophobic liquid extract to differentiated immunosuppressive M2 macrophages, the culture supernatant was collected after 24 h of incubation. A DuoSet ELISA kit (R&D Systems Inc., Minnesota, United States) was used to measure TNF-α production in the culture supernatant. All measurements were performed according to the protocol recommended by the kit.
2.8. Preparation of Macrophage-Conditioned Medium
First, immunosuppressive M2 macrophages were induced by the same method as in Section 2.3. Immunosuppressive M2 macrophages were incubated with A. kosoi 10H heat-killed cells (2 μg/mL) for 24 h. The culture supernatant was centrifuged at 5800 g (MX-301, Tomy Seiko Corporation) for 5 min at 4°C to remove the microbial cells, and the supernatant was collected as conditioned medium. The immunosuppressive M2 macrophage culture medium without A. kosoi 10H cells treated in the same manner as above was used as the nonadditive control.
2.9. Culture of MCF-7 Breast Cancer Cell Line
For this set of experiments, we employed the MCF-7 breast cancer cell line (JCRB cell bank), cultured in Dulbecco’s modified Eagle medium (DMEM) (Nissui Pharmaceutical Co. Ltd.) supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cancer cell line was seeded at 1.2 × 104 cells/well and allowed to adhere to the bottom of the 24-well plate by culturing in a CO2 incubator (WKN-MC35, WakenBtech Co. Ltd.) at 37°C and 5% CO2 for 24 h. The culture medium of the cancer cell line was then replaced with a medium in which 50% of the culture medium had been replaced with the macrophage-conditioned medium. The cells were then cultured for a further 72 h. They were then rinsed with DPBS, and 0.3 mL of TrypLE express (Thermo Fisher Scientific Inc.) diluted twofold in DPBS was added, and the cells were detached from the plate by holding for 5 min. Next, 0.3 mL of the medium containing 10% fetal bovine serum (v/v) was added, the enzymatic reaction was stopped, and the cells were collected in 1.5-mL microtubes (Violamo, As One Corporation). The plate was further rinsed with 0.3-mL DPBS, and the cells were collected and centrifuged at 2300 g for 5 min at 4°C (MX-301, Tomy Seiko Corporation) to obtain cell pellets.
2.10. Detection of Cell Apoptosis
7-AAD (Beckman Coulter Co. Ltd.) solution diluted fivefold in DPBS and FITC-labeled Annexin V (BioLegend Co. Ltd.) solution diluted 25-fold in DPBS were prepared. Thirteen microliters of 7-AAD diluted solution was added to the cells and stained at 4°C in the dark for 15 min. Next, DPBS was added, and the cell pellet was obtained using a centrifuge (MX-301, Tomy Seiko Corporation) at 2300 g, 5 min, and 4°C. After addition of 20 μL of FITC-labeled Annexin V diluted solution to the cell pellet, the cells were stained at room temperature in the dark for 15 min. Then, 200 μL of Annexin V Binding Buffer (BioLegend Co. Ltd.) was added, and the percentage of apoptotic cells was analyzed by a flow cytometer (CytoFlex B53015, Beckman Coulter Co. Ltd.). The apoptotic percentage was calculated as the percentage of both 7-AAD and Annexin V double-positive cells to total cells taken up.
2.11. Analysis of a Factor Causing MCF-7 Cell Death Using Recombinant TNF-α and Anti-TNF-α Antibody
The MCF-7 breast cancer cell line was cultured as described in Section 2.9 except for using 96-well plate and the seeded cell density of 0.6 × 104 cells/well. Anti-human-TNF-α antibody (100 ng/mL, Proteintech Group Inc.) or control IgG antibody (100 ng/mL, Proteintech Group Inc.) was added to the macrophage-conditioned medium and allowed to stand for 1 h (37°C and 5% CO2). The culture medium of the cancer cell line was then replaced with the abovementioned macrophage-conditioned medium containing antibody, and the cells were cultured for a further 72 h. As the positive control, human recombinant TNF-α (10 ng/mL, Proteintech Group Inc.) dissolved in complete RPMI 1640 medium was used, followed by culturing for 72 h.
2.12. MTT (3-(4,5-Di-methylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) Assay
The viability of MCF-7 cells was assayed using the MTT method. At the end of incubation, the culture medium was removed and 100 μL of MTT (Dojindo, Kumamoto, Japan) solution (final concentration 0.5 mg/mL), dissolved in complete RPMI 1640 medium, was added to each well. The microplates were incubated in a high-humidity environment (37°C, 5% CO2) for 2 h. After removal of the MTT reagent, 100 μL of 2-propanol solubilizing solution (Fujifilm Wako Pure Chemical Corporation) was added to each well and the microplate was shaken (NS-P, As One Corporation) for 30 min. The absorbance of the samples was measured using a microplate reader (Model 1500, Thermo Fisher Scientific Inc.) at a wavelength of 570 nm against the reference wavelength of 650 nm.
2.13. Statistical Analyses
Each result is expressed as the mean and standard deviation of the mean (SDM). Data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey test for Figures 1 and 2, using t test for Figures 3, 4, and 5(a), and using Tukey test for Figure 5(b). All statistical analyses were performed using EZR (R Version 4.0.3, R Commander 2.7-1, [13]).
3. Results
3.1. Induction of Immunostimulatory M1 Macrophages by A. kosoi 10H Heat-Killed Cells
We examined the effects of the A. kosoi 10H heat-killed cells, water-soluble extract, and hydrophobic liquid extract of A. kosoi 10H heat-killed cells on immunosuppressive M2 macrophages derived from THP-1 cells, focusing on whether A. kosoi 10H cells and the above extracts possess the ability to induce immunostimulatory M1 macrophages from immunosuppressive M2 macrophages.
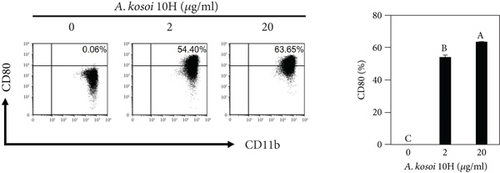
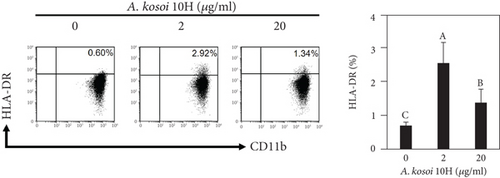
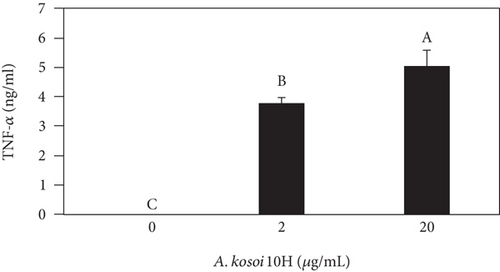
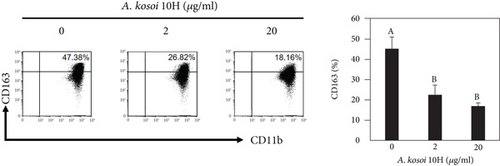
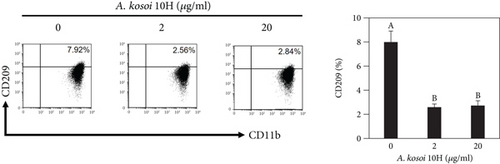
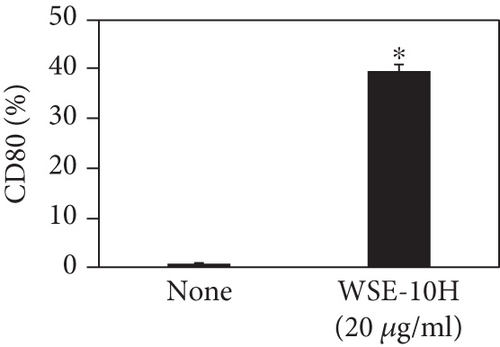
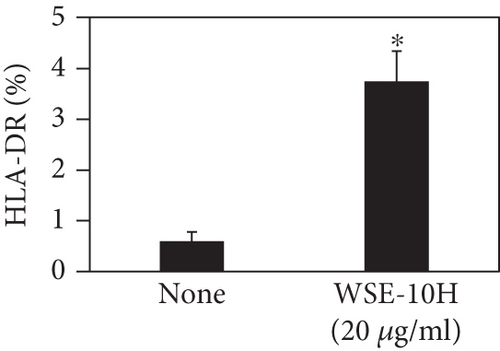
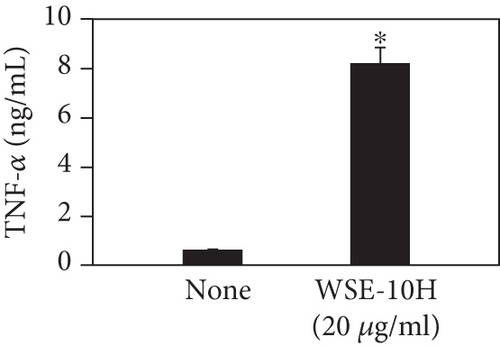
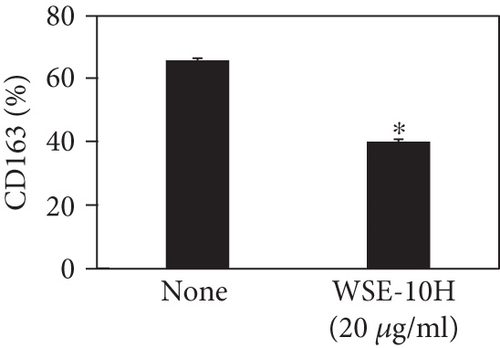
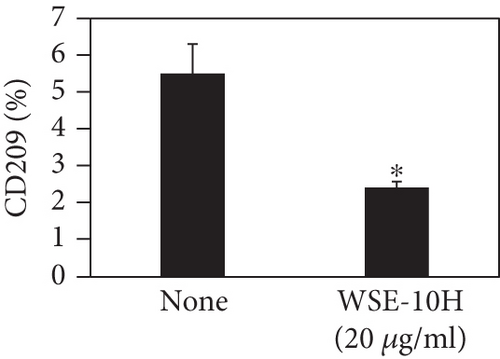
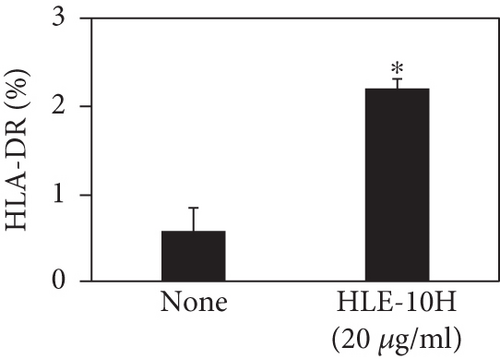
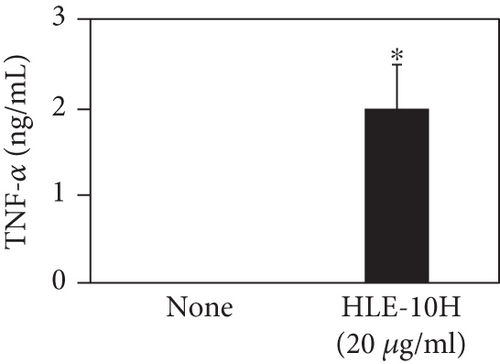
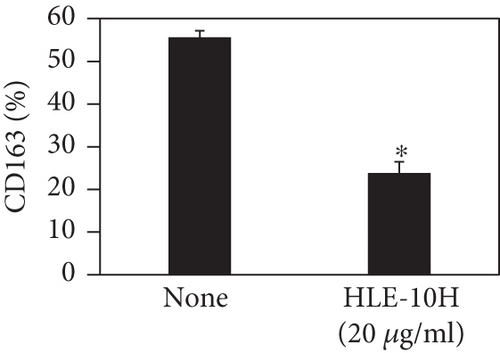
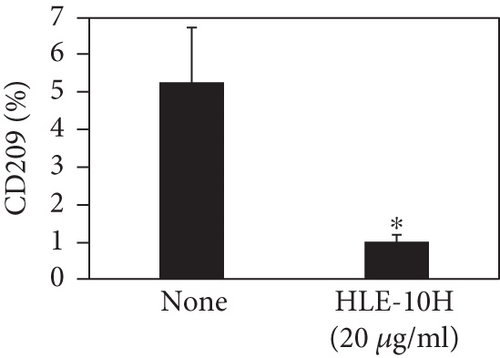
We investigated the phenotype conversion ability of A. kosoi 10H heat-killed cells for macrophages from M2 type to M1 type. As shown in Figure 1, the rates of expression of CD80 and HLA-DR and the production of TNF-α, which are M1 macrophage markers, increased as a result of adding A. kosoi 10H heat-killed cells to the M2 macrophage culture medium in comparison with those without added A. kosoi 10H cells. On the other hand, as shown in Figure 2, the expression rates of CD163 and CD209, which are M2 macrophage markers, decreased on the addition of heat-killed A. kosoi 10H cells to the M2 macrophage culture medium in comparison with those without added A. kosoi 10H cells. These results suggest that heat-killed A. kosoi 10H cells have the ability to convert the phenotype of M2 macrophages to that of M1 macrophages.
3.2. Induction of Immunostimulatory M1 Macrophages by the Water-Soluble Extract of A. kosoi 10H
Next, to clarify which of these active compounds has the ability to convert the phenotypes of macrophages, we decided to evaluate the A. kosoi 10H cell-derived extracts by dividing them into water-soluble and hydrophobic fractions using two-phase solvent fractionation, employing the purification process of lipoteichoic acids described in Morath et al. [14].
We describe below the phenotype-converting effects of the water-soluble extract of A. kosoi 10H heat-killed cells on macrophages from the M2 type to the M1 type. As shown in Figure 3, the expression rate of CD80 and HLA-DR and the production of TNF-α, which are M1 macrophage markers, increased on addition of the water-soluble extract of A. kosoi 10H heat-killed cells to the M2 macrophage culture medium compared to those without the extract. Conversely, as shown in Figure 3, the expression rates of CD163 and CD209, which are M2 macrophage markers, decreased on the addition of the water-soluble extract of A. kosoi 10H heat-killed cells to the M2 macrophage culture medium compared to those without the extract. From these results, it was elucidated that the water-soluble extract of A. kosoi 10H heat-killed cells has the ability to convert the phenotype of M2 macrophages to that of M1 macrophages.
3.3. Induction of Immunostimulatory M1 Macrophages by the Hydrophobic Liquid Extract of A. kosoi 10H
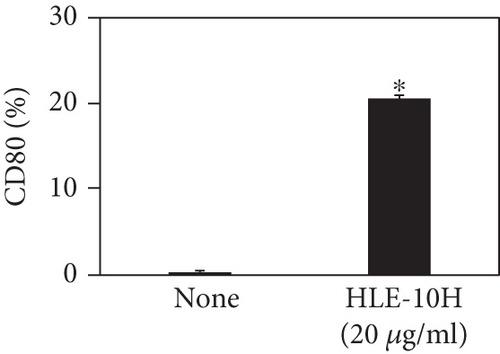
Next, we describe the phenotype conversion effects of the hydrophobic liquid extract of A. kosoi 10H heat-killed cells on macrophages from the M2 type to the M1 type. As shown in Figure 4, the expression rate of CD80 and HLA-DR and the production of TNF-α, which are M1 macrophage markers, increased on addition of the hydrophobic liquid extract of A. kosoi 10H heat-killed cells to the M2 macrophage culture medium compared to those without the extract. As shown in Figure 4, the expression rates of CD163 and CD209, which are M2 macrophage markers, decreased on the addition of the hydrophobic liquid extract of A. kosoi 10H heat-killed cells to the M2 macrophage culture medium compared to those without the extract. These results reveal that the hydrophobic liquid extract of A. kosoi 10H heat-killed cells has the ability to convert the phenotype of M2 macrophages to that of M1 macrophages.
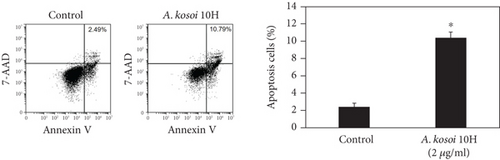
3.4. Induction of Apoptosis of MCF-7 Cells by the Conditioned Medium of Immunosuppressive M2 Macrophages Stimulated With A. kosoi 10H
In light of these results, we conclude that the A. kosoi 10H heat-killed cells, water-soluble extract, and hydrophobic liquid extract of A. kosoi 10H heat-killed cells all possess activity that induces immunosuppressive M2 macrophages to convert to immunostimulatory M1 macrophages. It appears that immunostimulatory M1 macrophages produce factors that inhibit the growth of cancer cells [3, 15]. We next examined the effects of the macrophage-conditioned medium on the MCF-7 breast cancer cell line. Figure 5(a) shows induction of apoptosis in this cell line on addition of the medium conditioned by immunosuppressive M2 macrophages that had been cultured in the presence of A. kosoi 10H heat-killed cells. The apoptosis ratio of MCF-7 cells was significantly raised after treatment with the immunosuppressive M2 macrophage-conditioned medium that had previously been incubated with A. kosoi 10H heat-killed cells, in contrast to those not incubated in the A. kosoi 10H cell-conditioned medium.
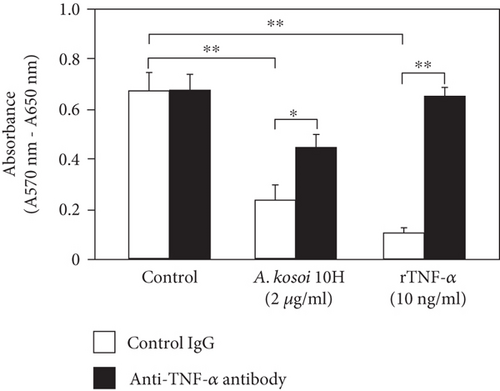
3.5. Effect of Anti-TNF-α Antibody on MCF-7 Cell Death Caused by the Medium Conditioned With Immunosuppressive M2 Macrophages Stimulated With A. kosoi 10H Cells
When MCF-7 was cultured in macrophage-conditioned medium cultured in the presence of A. kosoi 10H heat-killed cells, the viable MCF-7 cells after 72 h of culture were significantly lower than those in the macrophage-conditioned medium in the absence of A. kosoi 10H heat-killed cells. This decrease in the viable MCF-7 cells was partially counteracted by the addition of anti-TNF-α antibody. In the control experiment, MCF-7 cells were cultured in the presence of recombinant TNF-α. The viable MCF-7 cells after 72 h of culture were significantly lower than those in the macrophage-conditioned medium without A. kosoi 10H heat-killed cells. This decrease in the viable MCF-7 cells was completely counteracted by the addition of anti-TNF-α antibody (Figure 5(b)).
4. Discussion
In cancer research, it is important to take into account the cancer microenvironment. M2-like TAMs predominate and maintain an immunosuppressive environment [3, 15]. One promising strategy to improve the cancer microenvironment is to induce immunostimulatory M1 macrophages and suppress immunosuppressive M2 macrophages, namely, TAMs. The aim of this study was to elucidate the potential of a lactic acid bacterial species to improve the cancer microenvironment. We measured the ability of lactic acid bacteria to polarize macrophages from the immunosuppressive phenotype to the immunostimulatory phenotype. To better recreate the cancer microenvironment, we opted to experiment with the THP-1 cell line rather than TAMs in our in vitro experiments [16].
THP-1 cells are a human-derived monocytic cell line that can be induced to become dendritic cells or macrophages depending on the conditions [17]. THP-1 cells can be induced to become macrophages by the action of phorbol esters. They can also be induced to become immunosuppressive M2 macrophages by the action of cytokines such as interleukin-4 (IL-4), IL-6, and interleukin-10 (IL-10) [18, 19]. In our experiments, we ascertained that IL-6 can induce immunosuppressive M2 macrophages from M0 macrophages induced from THP-1 cells by the action of phorbol esters.
IL-6 is known to be associated with diseases such as cancer [20], obesity [21], rheumatoid arthritis [22], and Alzheimer’s disease [23, 24]. On the other hand, IL-6 is also known to induce follicular helper T cells and promote IgA production from B cells to promote intestinal immunity [25, 26]. IL-6 is therefore a cytokine with dual function [27, 28]. In relation to cancer, IL-6 is produced by TAMs and cancer cells, induces TAMs by autocrine and paracrine signaling, and maintains the cancer microenvironment in an immunosuppressive state [29, 30]. Several studies using human cells, including that of Fu et al. [11], have shown that IL-6 alone induces M2 macrophages. They also note that enriched IL-6 and M2 macrophages are closely correlated with the tumor stage and survival in patients with gastric cancer. Roca et al. [12] have shown that IL-6 alone induces M2 macrophages. M2 macrophage populations are also enhanced in human prostate cancer metastatic lesions. The use of IL-6 to induce immunosuppressive M2 macrophages in vitro thus models the in vivo cancer microenvironment [11, 12].
CD80 and HLA-DR are surface markers on immunostimulatory M1 macrophages [31]. TNF-α production is also used as a marker of immunostimulatory M1 macrophages, as it is produced by M1 macrophages and has the ability to induce M1 macrophages via autocrine signaling [32]. CD163 [31] and CD209 [33–35] are surface markers of immunosuppressive M2 macrophages. Wang et al. [35] found that M2 macrophages coexpress four markers (CD163, CSF1, MMD, and CD209) and reported its association with the negative regulation of immune system processes and prognosis in bladder cancer. Hu et al. [33] reported that CD209 (DC-SIGN), which is expressed on TAMs, is a therapeutic target in patients with muscle-invasive bladder cancer (MIBC). CD209 is considered to be an important marker of M2 macrophages in therapeutic and clinical practice. The results of the experiments shown in Figures 1, 2, 3, and 4 suggest that A. kosoi 10H heat-killed cells, water-soluble extract, and hydrophobic liquid extract of A. kosoi 10H heat-killed cells can all induce immunostimulatory M1 macrophages from immunosuppressive M2 macrophages, as evidenced by the increases in M1 markers (CD80, HLA-DR, TNF-α) and the decreases in M2 markers (CD163 and CD209), compared to those without A. kosoi 10H cells and the extracts.
Previous studies have shown that the aqueous phase of the water/n-butanol extraction contains lipoteichoic acid [14]; and it is assumed that this cell wall component, lipoteichoic acid, is responsible for at least a part of the immunostimulatory activity of water-soluble extracts [10]. This hypothesis is supported by a report that lipoteichoic acids from lactobacilli activate macrophage functions [36]. Lipoteichoic acid from A. kosoi 10H has been shown to be a novel lipoteichoic acid that contains L-lysine, which possesses high IgA-inducing activity [37]. Lipoproteins have also been shown to be involved in the immunomodulatory effects of lactic acid bacteria such as Lactiplantibacillus plantarum [38]. Furthermore, A. kosoi 10H retains the genes required to synthesize the lipoproteins, Lgt (preprolipoprotein diacylglyceryl transferase) and Lsp (lipoprotein signal peptidase) [39], and is therefore likely to express lipoproteins. Lipoproteins are extractable with n-butanol [40] and have the property of transferring to the organic phase [41], so it is possible that lipoproteins are one of the immunostimulatory active components in the hydrophobic liquid extracts.
Contrary to a report that an intact cell wall structure is important for stimulation of macrophages by lactobacilli [42], multiple extracts from A. kosoi 10H have been shown to have the ability to convert macrophage phenotypes. It appears that the balance of actions brought about by the different signaling pathways in macrophages via the different immune-modulating molecules of microbes results in different phenotypes, that is, for immunostimulation or immunosuppression. This hypothesis is supported by Kaji et al. [43], who report that the diverse immunomodulatory functions of probiotics result from costimulation with microbial components. The above considerations suggest that A. kosoi 10H has the potential to convert the phenotypes of macrophages from the immunosuppressive to the immunostimulatory phenotype by the action of more than two components of A. kosoi 10H cells via different signal transduction pathways. The focus of future research will be to identify active components of A. kosoi 10H that affect host–cell signal transduction pathways and induce an immunostimulatory phase.
Other natural products also have the ability to convert the phenotypes of macrophages, including plant-derived materials [44] such as curcumin [45], polysaccharide isolated from Brassica rapa L. [46], and ginseng [47]. Lactic acid bacteria that have the ability to convert the phenotypes of macrophages have also been studied. Enterococcus faecalis [48], Lactobacillus delbrueckii subsp. bulgaricus [49], and a combination of Lacticaseibacillus casei and Limosilactobacillus reuteri [50] are lactic acid bacteria that are reported to possess the ability to induce immunostimulatory M1 macrophages; and Leuconostoc lactis [51], Lactobacillus acidophilus [52], and Lactobacillus johnsonii [53] are lactic acid bacteria that are reported to possess the ability to induce immunosuppressive M2 macrophages. On the other hand, Levilactobacillus brevis is a probiotic that is reported by Song et al. [54] to possess M1 macrophage-inducing activity and, in other reports, M2 macrophage-inducing activity [55]. Lacticaseibacillus rhamnosus GG is a probiotic that is reported by Wang et al., [56] to possess M1 macrophage-inducing activity and, in other reports, M2 macrophage-inducing activity [57, 58]. Zhao et al. [59] and Duan et al. [60] report Lactiplantibacillus plantarum, a probiotic, to possess M1 macrophage-inducing activity and, in other reports [61, 62], to have M2 macrophage-inducing activity. The diversity of immunomodulatory effects from a wide range of probiotic microbes is thought to be caused by the different characteristics of species and strains or the biochemical characteristics of the microbe–host cell interaction under conditions such as infection or disease. There is some information regarding the signal transduction pathways involved in M1 macrophage induction. Toll-like receptor (TLR)2, TLR4 [46, 63], nuclear factor-kappa B (NFκB) [64, 65], and signal transducer and activator of transcription (STAT)1 [66] are reported to be involved in M1 macrophage polarization. Srivastava et al. [67] report that activation of TLR4–nitric oxide synthase (NOS)1–adaptor protein complex (AP)1 signaling is related to M1 macrophage induction, and Arranz et al. [68] report that Akt1 ablation results in the M1 macrophage polarization. There are several reports on the signal transduction pathways involved in M2 macrophage induction, such as STAT3 [69], STAT6 [70], suppressor of cytokine signaling (SOCS)3 [58], and phosphatidylinositol 3-kinase (PI3K)/Akt [71] as positive regulators and Akt2 [68], NFκB, and AP1 [61] as negative regulators. Furthermore, there is limited research on the active components possessed by the microorganisms that induce macrophage polarization. The mechanisms behind these differences that occur in immune-modulating activity caused by probiotics are therefore not fully understood. One focus of further interest will be to elucidate the mechanisms by which different species or strains of lactic acid bacteria exert different effects on host immunity in relation to its physiological and pathological state.
Attempts are being made to employ the immunostimulatory effects of lactic acid bacteria in cancer immunotherapy [72]. There are two possible mechanisms by which lactic acid bacteria induce apoptosis in cancer cells: direct action on cancer cells and immune-mediated action. The direct action of probiotics on cancer cells in inducing apoptosis is typically due to exopolysaccharides, inosine, conjugated linoleic acid, short-chain fatty acids such as butylate, urolithin A, and bacteriocins such as nisin A [72–74]. These are active compounds that are produced by probiotics such as lactic acid bacteria and Bifidobacterium. There are two possible immune-mediating actions: the innate immune system, which consists of immune cells such as natural killer cells and macrophages, and the adaptive immune system, which includes lymphocytes such as T cells and B cells [75]. The effects of lactic acid bacteria on the adaptive immune system [76] appear to influence each step of the cancer immune cycle [77], that is, cancer antigen presentation, T cell priming and activation, T cell trafficking to tumors, CD8+ T cell infiltration into tumors, and the killing of cancer cells. The active ingredients of lactic acid bacteria include the live bacterial cells, heat-killed bacterial cells, bacterial components, and products derived from the bacteria. In this study, we focused on immune-mediated action and investigated the apoptosis-inducing effect of macrophage-conditioned medium cultured in the presence of A. kosoi 10H heat-killed cells on the MCF-7 breast cancer cell line. Because the macrophage-conditioned medium had been centrifuged to remove bacterial cells, there were no A. kosoi 10H heat-killed cells in the cancer cell line culture medium to which the macrophage-conditioned medium had been added. Thus, in our experiment, we examined the immune-mediated induction of apoptosis in MCF-7 breast cancer cell line brought about by the immunostimulatory M1 macrophage product induced by A. kosoi 10H heat-killed cells.
It has been shown that a culture medium of immunostimulatory M1 macrophages can induce apoptosis of cancer cell lines such as colon cancer cell lines [78] and breast cancer cell lines [79]. It has been shown that a probiotic cocktail of the L. delbrueckii sp. bulgaricus DWT1 strain and the Streptococcus thermophilus DWT4 strain can induce apoptosis of cancer cells through proinflammatory activation of macrophages [49]. Our experiments show that the conditioned medium of immunosuppressive M2 macrophages cultured in the presence of A. kosoi 10H heat-killed cells has the potential to induce apoptosis in the MCF-7 breast cancer cell line. These results suggest that A. kosoi 10H has the potential to induce M2 macrophages to produce apoptosis-inducing materials against the MCF-7 breast cancer cell line, by inducing immunosuppressive M2 macrophages to convert to immunostimulatory M1 macrophages.
The possible involvement of TNF-α was examined with the aim of elucidating the mechanism by which macrophage-conditioned medium caused MCF-7 cell death (Figure 5(b)). TNF-α is produced by M1 macrophages and has the ability to induce M1 macrophages [31, 32]. The physiological activities of TNF-α include activation of NFκB and induction of apoptosis in cancer cells [80, 81]. TNF-α is considered to be one of the important factors secreted from macrophages, causing cell death in cancer cells [46]. Furthermore, although attempts have been made to apply TNF-α to cancer therapy [82], it has been pointed out that TNF-α has dual effects in cancer biology [83]. In this study, TNF-α in the conditioned medium of macrophages cultured in the presence of A. kosoi 10H heat-killed cells was shown to be partially involved as a factor causing cell death of the MCF-7 breast cancer cell line (Figure 5(b)).
5. Conclusion
Our experiments show that the A. kosoi 10H heat-killed cells, water-soluble extract, and hydrophobic liquid extract of A. kosoi 10H heat-killed cells all possess activity that induces immunostimulatory M1 macrophages from immunosuppressive M2 macrophages and show A. kosoi 10H to have the potential to induce apoptotic cell death in cancer cells by activating antitumor immunity. This suggests the possibility of using A. kosoi 10H to ameliorate the cancer microenvironment.
Nomenclature
-
- A. kosoi
-
- Apilactobacillus kosoi
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- IL-6
-
- interleukin-6
-
- HLA-DR
-
- human leukocyte antigen-DR isotype
-
- TNF-α
-
- tumor necrosis factor-α
-
- TAMs
-
- tumor-associated macrophages
-
- MRS
-
- de Man, Rogosa, and Sharpe
-
- DPBS
-
- Dulbecco’s phosphate-buffered saline
-
- RPMI
-
- Roswell Park Memorial Institute
-
- 7-AAD
-
- 7-aminoactinomycin D
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- DMEM
-
- Dulbecco’s modified Eagle medium
-
- MTT
-
- 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- IL-4
-
- interleukin-4
-
- IL-10
-
- interleukin-10
-
- TLR
-
- Toll-like receptor
-
- NFκB
-
- nuclear factor-kappa B
-
- STAT
-
- signal transducer and activator of transcription
-
- NOS
-
- nitric oxide synthase
-
- AP
-
- adaptor protein complex
-
- SOCS
-
- suppressor of cytokine signaling
-
- PI3K
-
- phosphatidylinositol 3-kinase
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Japan Environ and Organic Farming Foundation.
Open Research
Data Availability Statement
Data will be made available on request.