Effect of Serum Interleukin-6 Concentration on the Prognosis After Radiotherapy for Patients With Hepatocellular Carcinoma
Abstract
Objective: The aim of this study was to explore the association between interleukin-6 (IL-6) concentration before radiotherapy (RT) and the prognosis after RT for patients with hepatocellular carcinoma (HCC).
Methods: The clinical data for 101 patients with HCC who received RT from October 2016 to June 2021 were retrospectively analyzed. In these patients, the tumors were confined to the liver, and IL-6 concentration was measured before RT. The survival rate was calculated using the Kaplan–Meier method, and the Cox proportional hazards regression model was used to explore the independent factors affecting the patients’ prognosis. X-tile software was used to obtain the optimal cut-off value of pre-RT IL-6 concentration (7.8 pg/mL) for overall survival (OS).
Results: The 1-, 2-, and 3-year OS rates were 84.4%, 55.8%, and 34.7%, respectively, for patients with a pre-RT IL-6 concentration > 7.8 pg/mL versus 96.0%, 80.1%, and 80.1%, respectively, for those with a pre-RT IL-6 concentration ≤ 7.8 pg/mL. The OS rates of the two groups were significantly different (p < 0.001). The median progression-free survival (PFS) time was 7.5 months versus 15.1 months for patients with pre-RT IL-6 concentrations > 7.8 pg/mL and ≤ 7.8 pg/mL, respectively (p = 0.001). Pre-RT IL-6 concentration was an independent prognostic factor of OS (hazard ratio [HR] = 3.421, 95% confidence interval [CI]: 1.477–7.927, p = 0.004). Pre-RT IL-6 concentration (HR = 2.235, 95% CI: 1.176–4.246, p = 0.014) and age (HR = 0.615, 95% CI: 0.383–0.987, p = 0.044) were independent prognostic factors for PFS.
Conclusions: The prognosis of HCC patients receiving RT was worse for those with a pre-RT serum IL-6 concentration > 7.8 pg/mL than those with a pre-RT serum IL-6 concentration ≤ 7.8 pg/mL. Pre-RT IL-6 concentrations may affect the prognosis of HCC patients.
1. Introduction
Primary liver cancer is one of the most common and fatal malignant tumors of the digestive system in humans, with the second and fourth highest incidence and mortality rates, respectively, among malignant tumors in China [1–3]. Due to the background of chronic hepatitis B virus infection, China has become a high-incidence area for hepatocellular carcinoma (HCC), which seriously threatens public health [4]. The development of precise radiotherapy (RT) technology, particularly image-guided technology, has greatly improved the prognosis of patients with HCC undergoing RT [5]. Stereotactic body RT (SBRT) is an effective alternative to radiofrequency ablation for small, unresectable HCC [6].
Some inflammatory factors in the tumor microenvironment, such as interleukin-6 (IL-6), play important roles in the occurrence, progression, invasion, and metastasis of tumors [7]. IL-6 promotes the formation of tumor blood vessels, accelerates tumor proliferation, and increases the ability of tumor cells to invade and metastasize [8]. Thus, it is an important factor contributing to resistance to antitumor therapy [8]. Liver tissue is rich in inflammatory immune cells in HCC patients. Inflammatory factors induced by the hepatitis virus cause damage to liver cells, leading to the occurrence of liver cancer. Therefore, it is particularly important to study the effects of inflammatory factors on the efficacy of RT in patients with HCC.
As previously reported, serum inflammatory factors in patients with HCC receiving conventional RT predict the effects of RT, especially the progression in the radiation field [9]. Wei et al. reported the effect of preoperative RT for HCC with tumor thrombus and found that an increase in the baseline serum IL-6 concentration also led to a poor effect of RT [10]. In their study, the patients had advanced disease and the radiation dose was relatively low due to preoperative neoadjuvant RT. However, the relationship between precise radical RT and IL-6 concentration in confined HCC without a tumor thrombus remains unknown, as there are few reports on this topic. In this study, we aimed to determine the predictive value of pre-RT IL-6 concentration on the prognosis after RT of patients with confined HCC without tumor thrombus.
2. Materials and Methods
2.1. General Information
From October 2016 to June 2021, 101 patients with confined HCC without tumor thrombus, who received RT at Zhongshan Hospital, Fudan University, were enrolled based on the inclusion and exclusion criteria for this study, and a retrospective analysis was conducted. The study protocol was reviewed and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No. B2021-386R). Informed consent was obtained from all patients.
Patients were included if they (1) had HCC confirmed by pathology or met the clinical diagnostic criteria [4]; (2) had a confined tumor in the liver (without tumor thrombus) and no extrahepatic metastasis, based on imaging examinations, and had received RT; (3) had their serum IL-6 concentration tested before RT; (4) had an estimated lifetime of more than 6 months; and (5) were aged 18–85 years.
Patients were excluded if they (1) did not complete RT as planned; (2) had diffuse HCC in the liver; (3) had an uncontrollable infection; (4) had a malignant tumor in another organ; (5) had a serious heart, lung, or kidney disease; (6) had a serious nervous system disease; or (7) were unable to respond to treatment clearly.
The median follow-up time was 22.0 months, ranging from 6.3 to 64.7 months. Of the 101 patients included in the study, 85 were male and 16 were female. The median age of the patients was 63 years (range: 28–83 years). The maximum diameter of the intrahepatic tumors ranged from 0.8 to 13.3 cm, and the cumulative maximum diameter of the intrahepatic tumors ranged from 0.8 to 13.3 cm. The patients’ characteristics are described in Table 1.
| General characteristics | n (%) |
|---|---|
| Gender | |
| Male | 85 (84.2%) |
| Female | 16 (15.8%) |
| Age (years) | |
| Median (range) | 63 (28–83) |
| ≤ 60 | 42 (41.6%) |
| > 60 | 59 (58.4%) |
| History of prior treatment | |
| Yes | 86 (85.1%) |
| No | 15 (14.9%) |
| HBV/HCV history | |
| Yes | 84 (83.2%) |
| No | 17 (16.8%) |
| AFP | |
| Positive | 47 (46.5%) |
| Negative | 54 (53.5%) |
| Number of intrahepatic tumors | |
| Single | 82 (81.2%) |
| Multiple | 19 (18.8%) |
| Cumulative maximum diameter | |
| ≤ 5 cm | 75 (74.3%) |
| > 5 cm | 26 (25.7%) |
| Child–Pugh class | |
| A | 97 (96.0%) |
| B | 4 (4.0%) |
| BED10 | |
| ≤ 80 Gy | 31 (30.7%) |
| > 80 Gy | 70 (69.3%) |
| Pre-RT IL-6 concentration | |
| ≤ 7.8 pg/mL | 81 (80.2%) |
| > 7.8 pg/mL | 20 (19.8%) |
- Note: An AFP concentration ≥ 20.0 ng/mL was considered positive and < 20.0 ng/mL was considered negative. Cumulative maximum diameter refers to the cumulative maximum diameter of the intrahepatic tumor. The biological effect dose (BED) was calculated based on the α/β value of hepatocellular carcinoma (10 Gy, BED10).
- Abbreviations: AFP, alpha-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; IL-6, interleukin-6; RT, radiotherapy.
2.2. Measures of Serum IL-6 Concentration
The serum samples collected from patients on an empty stomach were tested using the IMMULITE 1000 fully automatic chemiluminescence instrument from Siemens, Germany. The diagnostic reagents and supporting calibration materials were all prepared using Siemens’s matching reagents, and the measurement method was chemiluminescence.
2.3. RT Procedure
RT was performed using helical tomotherapy or an image-guided linear accelerator. The patients were placed in the supine position and immobilized with different devices based on the different treatment machines. Abdominal compression was performed to reduce the breath amplitude in patients who received helical tomotherapy treatment [11, 12]. Free shallow breathing after training was used to manage respiratory movement for patients who received static intensity–modulated RT (IMRT) or volume rotation intensity–modulated RT (VMAT) using an image-guided linear accelerator. Magnetic resonance or positron emission tomography/computed tomography images were used for image fusion to improve the accuracy of gross tumor volume (GTV) delineation when necessary. The GTV was contoured based on visible lesions. The clinical target volume (CTV) was created using different methods, based on the different technologies used; for example, the GTV was expanded by 5 mm to create the CTV for hypofractionated RT [13] but the CTV was exempted for SBRT. The internal target volume (ITV) was determined based on the respiratory movement amplitude of the intrahepatic tumors. A margin of 3–5 mm to the ITV was added to create the planning target volume (PTV) [5, 13]. Image-guided technology was used daily for patients undergoing helical tomotherapy. For patients who underwent IMRT or VMAT, image-guided technology was used before the first and second treatments, after which, it was used weekly.
2.4. Follow-Up
After treatment, the patients were followed up every 3 months, and they routinely underwent hematological and imaging examinations, such as routine blood tests, liver and kidney function tests, coagulation function tests, tumor marker measurements, inflammatory factor measurements, chest computed tomography, and abdominal enhanced magnetic resonance.
The modified response evaluation criteria in solid tumors were used to evaluate efficacy based on the following categories: complete remission (CR), partial response (PR), stable disease (SD), and progressive disease. The best response observed in the radiation treatment field was used to evaluate radiation treatment efficacy.
2.5. Statistical Analysis
X-tile software was used to determine the optimal cut-off value of the pre-RT IL-6 concentration (7.8 pg/mL) for overall survival (OS). SPSS 20.0 (IBM, Armonk, New York, USA) and Prism 8.3.0 (GraphPad, San Diego, California, USA) were used for data processing. Survival rates were evaluated using Kaplan–Meier analysis. The Cox proportional hazards regression model was used to explore independent factors affecting the patients’ prognosis. The variables of the Cox proportional hazards regression model p < 0.10 in the single-factor analysis were included in the multifactor analysis. Statistical significance was set at p < 0.05.
3. Results
3.1. Overall Treatment Effect
The best response observed in the radiation field was used to evaluate the field response to RT. The objective response rate (ORR) in the radiation field was 94.1%, and 78.2%, 15.8%, and 5.9% of the patients showed CR, PR, and SD, respectively. The median OS rate was not reached, and the 1-, 2-, and 3-year OS rates were 93.7%, 75.2%, and 69.8%, respectively. The median progression-free survival (PFS) time was 12.87 months, and the PFS rates at 1, 2, and 3 years were 51.3%, 33.2%, and 18.9%, respectively. The local control rates in the radiation fields for 1, 2, and 3 years were 91.1%, 89.6%, and 84.4%, respectively.
3.2. Relationship Between Pre-RT IL-6 Concentration and RT Treatment Response
There were no significant differences in best response ((p = 0.723) (Table 2) or ORR ((p = 0.742) between the groups with pre-RT IL-6 concentrations > 7.8 pg/mL and ≤ 7.8 pg/mL.
| Pre-RT IL-6 concentration (pg/mL) | Best response of the tumor in the radiation field | p value | ||
|---|---|---|---|---|
| CR | PR | SD | ||
| > 7.8 | 15 (75.0%) | 3 (15.0%) | 2 (10.0%) | 0.723 |
| ≤ 7.8 | 64 (79.0%) | 13 (16.0%) | 4 (4.9%) | |
- Abbreviations: CR, complete remission; IL-6, interleukin-6; PR, partial response; RT, radiotherapy; SD, stable disease.
3.3. Relationship Between Pre-RT IL-6 Concentration and Survival
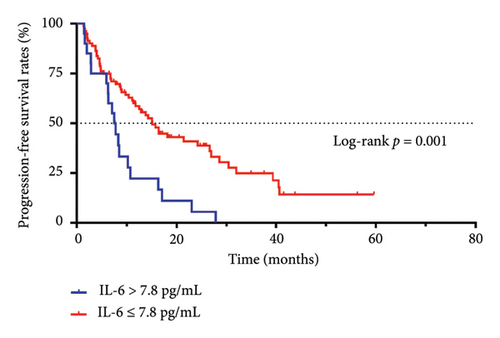
The median PFS time of patients with an IL-6 concentration > 7.8 pg/mL was 7.50 months, and the PFS rates at 1, 2, and 3 years were 22.2%, 5.6%, and 0%, respectively. The median PFS time of patients with an IL-6 concentration ≤ 7.8 pg/mL was 15.10 months, and the PFS rates at 1, 2, and 3 years were 58.6%, 40.9%, and 24.9%, respectively. There was a statistically significant difference between the two groups (p = 0.001, Figure 1(a)).
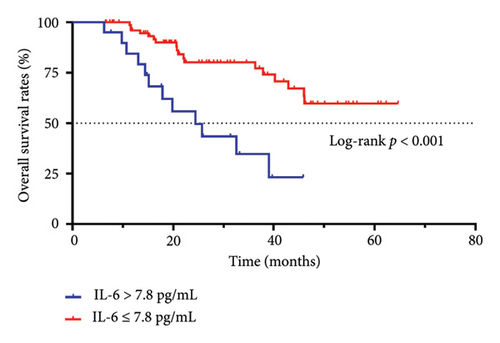
The median OS time of patients with an IL-6 concentration > 7.8 pg/mL was 24.4 months, and the OS rates at 1, 2, and 3 years were 84.4%, 55.8%, and 34.7%, respectively. The median OS time of patients with an IL-6 concentration ≤ 7.8 pg/mL was not reached, and the OS rates at 1, 2, and 3 years were 96.0%, 80.1%, and 80.1%, respectively. The OS time was significantly different between the two groups (p < 0.001, Figure 1(b)).
3.4. Univariate and Multivariate Cox Regression Analyses
As shown in Table 3, the BED10 and pre-RT IL-6 concentrations were statistically significant in the univariate analysis. These two factors were included in the multivariate COX proportional risk regression model analysis. In the Cox proportional hazards model, pre-RT IL-6 concentration was shown to be an independent prognostic factor for OS, with a hazard ratio (HR) of 3.421 and a 95% confidence interval (CI) of 1.477–7.927 ((p = 0.004, Table 3). The OS time was significantly greater for patients with a pre-RT IL-6 concentration ≤ 7.8 pg/mL than for those with a pre-RT IL-6 concentration > 7.8 pg/mL.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Gender (male vs. female) | 1.960 (0.594–6.471) | 0.269 | ||
| Age (years) (> 60 vs. ≤ 60) | 1.026 (0.499–2.109) | 0.944 | ||
| History of prior treatment (yes vs. no) | 0.991 (0.377–2.606) | 0.985 | ||
| HBV/HCV history (yes vs. no) | 1.312 (0.457–3.762) | 0.614 | ||
| AFP (positive vs. negative) | 1.786 (0.859–3.712) | 0.120 | ||
| Number of intrahepatic tumors (multiple vs. single) | 1.492 (0.660–3.370) | 0.336 | ||
| Cumulative maximum diameter (> 5 cm vs. ≤ 5 cm) | 1.675 (0.805–3.487) | 0.168 | ||
| Child–Pugh class (B vs. A) | 3.310 (0.774–14.153) | 0.106 | ||
| BED10 (> 80 Gy vs. ≤ 80 Gy) | 0.449 (0.218–0.925) | 0.030 | 0.684 (0.305–1.531) | 0.355 |
| Pre-RT IL-6 (> 7.8 pg/mL vs. ≤ 7.8 pg/mL) | 4.064 (1.901–8.691) | < 0.001 | 3.421 (1.477–7.927) | 0.004 |
- Note: An AFP concentration ≥ 20.0 ng/mL was considered positive and < 20.0 ng/mL was considered negative.
- Abbreviations: AFP, alpha-fetoprotein; BED, biological effect dose; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; IL-6, interleukin-6; RT, radiotherapy.
As shown in Table 4, four factors (p < 0.10) in the univariate analysis were included in the multivariate COX proportional hazards regression model. The results showed that pre-RT IL-6 concentration (HR = 2.235, 95% CI: 1.176–4.246, p = 0.014) and age (HR = 0.615, 95% CI: 0.383–0.987, p = 0.044) were independent prognostic factors for PFS. The PFS time was longer for patients with a pre-RT IL-6 concentration ≤ 7.8 pg/mL than for those with a pre-RT IL-6 concentration > 7.8 pg/mL. The PFS time was longer for patients older than 60 years than those younger than 60 years.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Gender (male vs. female) | 1.372 (0.700–2.686) | 0.357 | ||
| Age (years) (> 60 vs. ≤ 60) | 0.658 (0.413–1.049) | 0.078 | 0.615 (0.383–0.987) | 0.044 |
| History of prior treatment (yes vs. no) | 1.670 (0.844–3.303) | 0.140 | ||
| HBV/HCV history (yes vs. no) | 1.023 (0.547–1.911) | 0.944 | ||
| AFP (positive vs. negative) | 1.054 (0.658–1.689) | 0.826 | ||
| Number of intrahepatic tumors (multiple vs. single) | 1.465 (0.821–2.616) | 0.196 | ||
| Cumulative maximum diameter (> 5 cm vs. ≤ 5 cm) | 1.538 (0.925–2.558) | 0.097 | 0.771 (0.394–1.506) | 0.446 |
| Child–Pugh class (B vs. A) | 1.146 (0.359–3.661) | 0.818 | ||
| BED10 (> 80 Gy vs. ≤ 80 Gy) | 0.495 (0.301–0.814) | 0.006 | 0.576 (0.296–1.122) | 0.105 |
| Pre-RT IL-6 (> 7.8 pg/mL vs. ≤ 7.8 pg/mL) | 2.527 (1.468–4.350) | 0.001 | 2.235 (1.176–4.246) | 0.014 |
4. Discussion
HCC is considered to be an inflammation-related disease and the effects of inflammation range from chronic liver injury to the occurrence and development of HCC. Inflammatory factors, particularly IL-6, play important roles in the occurrence and development of HCC [14]. IL-6 binds to the IL-6 receptor; activates the receptor-related Janus kinase; stimulates phosphorylation; activates signal transducer and activator of transcription 3 (STAT3) to activate downstream signals; exerts antiapoptotic effects; and promotes angiogenesis, proliferation, invasion, and metastasis [15, 16].
Some previous studies have demonstrated that IL-6 may be used as a marker for the auxiliary diagnosis of HCC [17, 18]. The upper limit of the reference value for serum IL-6 concentrations is 3.4 pg/mL in our institution. Among the 101 patients with HCC included in this study, 69 patients (68.3%) had their serum IL-6 concentrations determined (range: 3.4–60.2 pg/mL) before RT and the values exceeded the upper limit of the reference value, and the percentage is similar to AFP [19]. Many previous studies have demonstrated that high IL-6 concentrations indicate poor treatment prognosis for patients with HCC [20–22]. Myojin et al. reported that the circulating IL-6 concentration is a prognostic biomarker for patients with advanced HCC undergoing combined immunotherapy [23]. Wu et al. reported that postintervention IL-6 concentration, rather than the pretreatment concentration or dynamic changes in IL-6 concentration, is a powerful predictor of the tumor response in HCC patients treated with transarterial chemoembolization [24].
The relationship between RT and IL-6 concentration has been the focus of research in the field of radiation immunity. The circulating IL-6 concentration is a predictor of radiation pneumonitis [25, 26]. Matsuoka et al. reported that the blockade of IL-6 signaling, combined with conventional RT, augments the treatment response and survival rate in patients with radioresistant oral squamous cell carcinoma [27]. In this study, we found that HCC patients with a high pre-RT IL-6 concentration were prone to tumor progression, with a reduced OS time, which suggested that IL-6 may weaken the effects of RT.
Increased IL-6 concentrations may lead to a decrease in RT sensitivity [28]. The mechanism for this phenomenon may be related to the fact that inflammatory factors, such as IL-6, promote the repair of DNA breaks after RT and reduce tumor cell apoptosis [29, 30]. DNA damage and repair are determinants of tumor cell sensitivity to radiation. High IL-6 concentrations promote tumor progression after treatment and may decrease RT sensitivity. RT induces the immunogenic death of tumor cells and the generation of injury-related molecules to promote the maturation of dendritic cells, sensitize and activate T cells through MHC-I, and activate the immune system to release a large number of inflammatory factors [31]. RT with concurrent IL-6 inhibition may be a potential therapeutic strategy to increase the radiation response in patients with HCC [32, 33]. In the clinical setting, for such patients, RT specialists also need to actively explore other interventions to improve the prognosis of patients, such as combination immunotherapy, the optimization and adjustment of the RT parameters, and proton or heavy ion therapy. IL-6/STAT3 is an important therapeutic target for HCC [15], and targeted drugs for HCC patients with elevated pre-RT IL-6 who experience relapse may become a promising treatment strategy. In addition, this study also found that patient’s age was an independent factor affecting PFS, and patients aged ≤ 60 years were more likely to develop tumors after RT than those aged > 60 years, which may be related to differences in metabolism in these patients.
This study has two main limitations. First, this was a single-center retrospective study and not a large-sample study. However, many patients in this study had participated in a prospective study. Second, this study only analyzed the relationship between pre-RT IL-6 concentration and the prognosis of HCC patients. The post-RT IL-6 concentration and dynamic changes (pre- and post-RT) in IL-6 concentrations were not assessed. It is important to further study the relationship between the dynamic changes in IL-6 concentration and the prognosis of patients with HCC who receive RT and to further explore the mechanism.
5. Conclusions
The prognosis of HCC patients receiving RT was worse for those with a pre-RT serum IL-6 concentration > 7.8 pg/mL than those with a pre-RT serum IL-6 concentration ≤ 7.8 pg/mL. Thus, the pre-RT IL-6 concentration may affect the prognosis of HCC patients.
Ethics Statement
The study protocol was reviewed and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No. B2021-386R).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the National Key R&D Program of China (2022YFC2503704 and 2022YFC2503700).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding authors on reasonable request.