Antibacterial Activities and Time-Kill Analysis of Leaf Extracts of Combretum adenogonium Steud. Ex A. Rich In Vitro
Abstract
Combretum adenogonium Steud. Ex A. Rich is a shrub tree native to tropical Africa. Its various parts are used in traditional medicine to treat both infectious and noninfectious diseases. This study investigated the activities and the killing rate of the plant leaf extracted with aqueous and ethanol (70% v/v) against Staphylococcus aureus (American Type Culture Collection (ATCC) 29213), Staphylococcus epidermidis (ATCC 14990), Enterococcus faecalis (ATCC 29212), Pseudomonas aeruginosa (Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSM) 1117), Escherichia coli (DSM 25944), and Salmonella typhi (ATCC 6539). The synergistic effect of each extract in combination with the standard antibiotics, ampicillin, ciprofloxacin, chloramphenicol, and tetracycline, was also investigated in vitro. It was revealed that both extracts were active against all test bacteria, with a mean zone of growth inhibition (ZoI) ranging from 23.50 ± 0.50 to 11.00 ± 0.00 mm at a concentration of 100 mg/mL. The mean ZoI of the ethanol extract (100 mg/mL) against E. faecalis (23.50 ± 0.50 mm) was significantly (p ≤ 0.05) higher when compared with tetracycline (64 μg/mL) (18.67 ± 0.29 mm). The minimum inhibitory concentrations of the extracts were between 0.39 and 12.50 mg/mL, while the minimum bactericidal concentrations ranged from 0.78 to > 25 mg/mL. Time-kill kinetics analysis revealed that both extracts were bacteriostatic against all Gram-negative bacteria. The extract–antibiotic combinations resulted in synergy, additive, or indifferent outcomes, with none resulting in an antagonistic effect. The findings of the current study confirm the relevance of Combretum adenogonium in traditional medicine for the treatment of infections. For the first time, the time-kill kinetics analysis and the interaction between the extracts of C. adenogonium in combination with some commonly used antibiotics against bacteria have been established. The activity could be attributed to the presence of saponins, phenolic compounds and tannins, flavonoids, phytosterols, and terpenes which were detected in the extracts by phytochemical screening.
1. Introduction
Infectious diseases remain a substantial cause of morbidity and mortality, especially in the developing regions of the world, and are considered as one of the greatest threats to the existence of man, apart from war and famine [1]. Prior to the COVID-19 pandemic, it was revealed that about 50,000 people died each day globally from infectious diseases [2]. Although there has been an improvement in the health status and quality of life of humankind due to the advances in medicine over the years, a new challenge confronting the world is the emergence and re-emergence of antimicrobial resistance (AMR) in pathogens threatening the treatment of infectious diseases. This has resulted in prolonged illness, disability, and death and an increase in the cost of healthcare [3].
The global annual death rate due to AMR is projected to hit 10 million by 2050, with a global economic cost of one hundred trillion US dollars [4]. Of particular interest is the phenomenon of bacterial AMR, which has become an increasingly important global public health concern, a situation described as a latent and overlooked pandemic [5]. The Antimicrobial Resistance Collaborators [6] established that 1.27 million deaths in 2019 were directly attributable to bacterial AMR, with the western sub-Saharan Africa region having the highest all-age death rates attributable to and associated with bacterial AMR. The phenomenon of bacterial AMR is attributed to a conglomeration of factors such as poor healthcare amenities, over-the-counter accessibility, misuse of antimicrobial agents as a result of self-medication, inadequate access to inexpensive drugs such as penicillin, and excessive use of antibiotics causing the microbes to mutate to form strains that are multidrug-resistant (MDR) [5, 7].
The menaces of bacterial AMR have profound impact on human health and well-being, political stability, social cohesion, and economic development [8], thus the need for alternative strategies [9, 10]. Plant-based medicines have played a significant role in the healthcare delivery system of many communities and cultures for centuries. Several studies have demonstrated that plants represent a highly promising reservoir for the acquisition of natural compounds that are capable of functioning as potent antimicrobial agents. The use of such naturally derived products has witnessed considerable success in the realm of novel drug discovery. According to estimates, a significant proportion of the population living in developing countries, specifically ranging from 75% to 80%, rely on herbal medicines as their primary source of healthcare [11]. Medicinal plant products are attractive because they are found to be cheaper and more accessible to most people compared to allopathic medicine. Moreover, the use of herbal medicines is deeply ingrained in the culture of most communities in developing countries, making them culturally acceptable. Added advantages of exploring herbal antimicrobials include their low production costs, low side effects, and no environmental problems [12]. According to Gharbani et al., employing plant and natural material products in producing antimicrobials also fits into the ideals of sustainable development [13]. Furthermore, it is rare for bacteria and other microorganisms to develop resistance to herbal medicines, a phenomenon attributed to the presence of multiple bioactive components in the herbal mixture [14]. These compounds act in synergy, each with different mechanisms to bring about a delayed evolution of resistance by the pathogenic organism to the individual components in combination [15].
Combretum adenogonium Steud. Ex A. Rich (syn: Combretum fragrans F. Hoffm or Combretum ghasalense Engl. and Diels), belonging to the Combretaceae family, is a shrub or small tree native to tropical Africa [16]. Known in English as “four-leaved bushwillow,” the plant has several vernacular names in Ghana including Atena (Akan-Bono), Kwahinkwagya (Akan-Twi), Pamara (Dagari), Kpamara (Wale), Tachale (Dagbani), and Kamagiri (Grusi-Kasena). Traditional medicine healers use different plant parts of C. adenogonium for the treatment of leprosy, cough, syphilis, snakebite, diabetes, diarrhea, new and chronic wounds including diabetic wounds, inflammation, malaria and malarial fever, fungal infections of the scalp, hypertension, gonorrhea, jaundice, ulcers, and cancers [16–20]. In Ghana, the leaf decoction or infusion is used in conjunction with other herbs in the treatment of stomach aches, fever, and malaria [21, 22]. The leaf is also used as a steam bath to manage body pain. Different studies have documented the pharmacological and biological activities of different solvent extracts of the root, leaf, and stem bark of C. adenogonium, including antiproliferative and cytotoxic [23], anticancer [19], antioxidant [24], antimicrobial and anti-HIV-1 protease [16, 25], and antiplasmodial [26] activities. The study, therefore, sought to investigate the antibacterial activities of ethanol and aqueous leaf extracts of C. adenogonium alone and in combination with some commonly used conventional antibiotics. Furthermore, the time-kill kinetics of the antibacterial activities of the extracts was investigated.
2. Materials and Methods
2.1. Collection of Plant Material
Fresh leaves of Combretum adenogonium Steud. Ex A. Rich. were collected from Saboro (latitude 10°54′7″ N, longitude 1°5′12″ W), Navrongo Municipality, Upper East Region, Ghana, in September 2019. The plant (Figure 1) was authenticated in the herbarium of Ghana Herbaria, Northern Savannah Biodiversity, Savannah Herbarium, Tamale. A voucher specimen (SH/2019/MD/L001) was prepared and deposited in the same herbarium.

2.2. Preparation of Plant Extracts and Conventional Antibiotics
Fresh leaves of C. adenogonium were washed under running tap water, air-dried for 2 weeks under shade, and then ground into powder using a blender. One hundred grams of the pulverized sample was added to distilled water (700 mL) in a 2-L beaker and boiled for 1 h. The mixture was strained through a double-layered sterile white cotton wool after cooling. The marc was re-extracted with 300 mL distilled water. The filtrates were combined and subsequently filtered with sterile Whatman Number 1 filter paper. The resultant filtrate was lyophilized using a freeze dryer (Telstar LyoQuest, Telstar Life Sciences, Spain) fixed to a vacuum pump (ULVAC GLD 137CC, Ulvac GmbH, Germany) to obtain a semisolid matter (percentage yield of 6.85%) and designated CAA (C. adenogonium leaf aqueous extract). Another portion of the pulverized sample (50 g) was extracted with 500 mL ethanol (70% v/v) with shaking (KS501 Digital, IKA Labortechnick, Germany) at 180–190 rpm for 72 h at room temperature. The mixture was then strained through a double-layered sterile white cotton wool, and the marc was then re-extracted with 300 mL ethanol (70% v/v) for 24 h. The filtrates were combined and subsequently filtered with sterile Whatman Number 1 filter paper. The resultant filtrate was then concentrated on a Rotavapor (Laborota 4001 ENcient, Heidolph Instruments GMBH, Germany) at 40°C under reduced pressure. The concentrate was further placed in a water bath at 40°C to remove excess solvent to obtain a semisolid substance of constant mass of 3.75 g. It was labeled as CAE (C. adenogonium leaf ethanol extract). Both extracts were kept in a refrigerator at 4°C until further use. A stock solution (400 mg/mL) of each extract for antibacterial analysis was prepared by reconstituting the crude extract in 1% (v/v) dimethyl sulfoxide (DMSO). The reconstituted extract solution was further sterilized by filtering through a 0.2-μm membrane filter (PET0225100, Sterlitech, United States). An aliquot (2 mL) of the sterile filtrate was then added to 10 mL of sterile nutrient broth (Biomark Laboratories, Pune, India) and incubated at 37°C for 24 h. The absence of turbidity in the broth after the incubation period indicated the sterility of the stock solutions.
2.3. Preparation of Different Concentrations of Conventional Antibiotics
Standard antibiotics that were employed in the study were ampicillin sodium salt, representing β-lactams (penicillin); ciprofloxacin, representing fluoroquinolones; tetracycline hydrochloride, representing tetracycline; and chloramphenicol, representing the amphenicol family. They were all obtained from Sigma-Aldrich, Saint-Quentin-Fallavier, France. The guidelines recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) were used to prepare solutions of these conventional antibiotics [27, 28] (Supporting Information 1).
2.4. Qualitative Phytochemical Screening
Qualitative phytochemical analysis was assessed on the crude aqueous and ethanol extracts of C. adenogonium using standard methods [29–31] with little modification. The phytochemicals that were screened included alkaloids, saponins, tannins, reducing sugars, cyanogenic glycosides, anthracene glycosides, flavonoids, phytosterols, and triterpenes.
2.5. Test Bacteria and Preparation of Inocula
The bacteria used for the study were Staphylococcus aureus (American Type Culture Collection (ATCC) 29213), Staphylococcus epidermidis (ATCC 14990), Enterococcus faecalis (ATCC 29212), Pseudomonas aeruginosa (Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSM) 1117), Escherichia coli (DSM 25944), and Salmonella typhi (ATCC 6539). Bacterial inoculum was prepared using the Clinical and Laboratory Standards Institute (CLSI)–recommended direct colony suspension method described by [32–34]. A loopful of a 24-h bacterial suspension was streaked on nutrient agar (Biomark Laboratories, Pune, India) and incubated for 18–24 h at 35 ± 2°C. About two to three well-isolated colonies of similar morphology were picked from the 18–24-h agar plate culture using a sterile inoculating loop, then suspended in 10 mL Mueller–Hinton broth (MHB) (Oxoid Ltd, Basingstoke, United Kingdom), vortexed, and incubated for about 2–4 h at 35 ± 2°C. The density of the bacterial suspension was adjusted to 0.5 McFarland Turbidity Scale, equivalent to a bacterial inoculum size of 1–2 × 108 colony-forming units (CFU)/mL.
2.6. Agar Well Diffusion Assay
The initial susceptibility of the test bacteria to the crude extracts was determined using the agar well diffusion assay described by Balouiri et al. [33]. A volume of the adjusted bacterial suspension (100 μL) was transferred onto a sterile Mueller–Hinton agar (MHA) (Oxoid Ltd, Basingstoke, United Kingdom) plate and evenly spread on the entire surface of the plate using a sterile cotton swab. With a sterile cork borer (diameter = 6 mm), four equidistant wells were aseptically created on the plate and left to dry for about 5 min. Each extract (100 μL), of varying concentrations (200, 100, and 50 mg/mL prepared in 1% v/v DMSO), was transferred into the respective well and allowed to stand at room temperature for 2 h to allow the extract to diffuse into the medium. The plates were then incubated at 35 ± 2°C for 18–24 h, and the zones of inhibition were measured in millimeters. DMSO (1% v/v) and sterile MHB without any extract/antibiotic were used as negative quality controls, while ciprofloxacin (4.00 μg/mL) and tetracycline (64.00 μg/mL) were used as positive quality controls. Determinations were done in duplicates and repeated on three separate occasions.
2.7. Determination of Minimum Inhibition Concentration (MIC)
The broth macrodilution technique previously described [33, 34] was used to determine the MIC of the crude extracts. Twofold serial dilution of each crude extract was prepared in sterile MHB from a stock solution of 50 mg/mL to obtain varying concentrations ranging from 25.00 to 0.05 mg/mL. An aliquot (3 mL) of each extract solution was pipetted into sterile test tubes and inoculated with 100 μL of a 24 h bacterial suspension (prepared in sterile MHB and adjusted to 0.5 McFarland Scale). After mixing, the inoculated tubes were incubated for 24 h at 35 ± 2°C. After the 24-h incubation period, 20 μL of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 1.25 mg/mL, was added to each test tube and incubated for 30 min at 35 ± 2°C. The lowest concentration of the extract at which there was no purple coloration upon the addition of MTT indicated that there was no bacterial growth and was regarded as the MIC. Extracts only without inoculum, sterile MHB without extract and inoculum, and inoculum without extract were used as quality controls. The procedure was performed in triplicate. The same procedure was used for the standard antibiotics using their respective concentration range (Supporting Information 1).
2.8. Determination of Minimum Bactericidal Concentration of Extracts
The broth macrodilution technique was used to determine the minimum bactericidal concentration (MBC) of the crude extracts according to previously described method with slight modifications [35–37]. Twofold serial dilutions of each extract were prepared in fresh sterile MHB from the stock solution (50 mg/mL) to obtain concentrations corresponding to those in the test tubes that did not show any bacterial growth during the MIC determination described in the previous section. Then, 2 mL of each solution was transferred into sterile test tubes and inoculated with 100 μL of 24 h bacterial suspension (prepared in MHB and adjusted to 0.5 McFarland Scale). After mixing, the inoculated tubes were incubated for 24 h at 35 ± 2°C. After the 24-h incubation period, aliquots of 100 μL from each test tube were aseptically plated on sterile MHA and incubated for 24 h at 35 ± 2°C. The lowest concentration of the extract that produced < 10 colonies was considered as MBC value.
2.9. Determination of Bactericidal or Bacteriostatic Effect of Extracts
The type of antibacterial activity of each extract was determined using the MBC/MIC ratio as described earlier [35]. The activity was classified as bactericidal if the MBC/MIC ratio is ≤ 4 and bacteriostatic if the MIC ratio is > 4.
2.10. Time-Kill Kinetics Analysis of Extracts
Time-kill kinetics analysis of the extracts was performed in accordance with previously described methods with slight modifications to time and concentration, and partly reproducing their wording [37, 38]. A loopful of a 24 h bacterial suspension was transferred to freshly prepared 5 mL sterile MHB and incubated for 2–3 h at 35 ± 2°C. The culture was adjusted to a 0.5 McFarland Scale and further diluted with sterile MHB so that the starting inoculum was approximately between 1 × 105 and 1 × 106 CFU/mL. Concentrations of each extract equivalent to ×1, ×2, and ×4 MIC were prepared in sterile MHB (10 mL), and each was then seeded with 100 μL inoculum, followed by incubation on a water bath at 35 ± 2°C. Aliquot (100 μL) was taken from each tube at time intervals 0, 2, 4, 6, 8, 12, and 24 h and transferred to a tube containing 900 μL sterile MHB. A 10-fold serial dilution was then performed, and then, 100 μL of each dilution was plated on a sterile MHA plate and incubated for 24 h at 35 ± 2°C. Bacterial CFU were determined after the 24-h incubation using a plate counter. Plates yielding six to 60 colonies were selected for colony counts. Bacterial suspension without any extract served as the growth control, while MHB and extract only were used as quality control checks. The log10 CFU/mL of viable bacterial cells were plotted against time for each bacterial strain to obtain the time-kill kinetics curve.
2.11. Determination of Synergistic Interaction Between Conventional Antibiotics and Extracts in Combination
A combination was characterized as synergy if FICI ≤ 0.5, additive if 0.5 < FICI ≤ 1.0, indifferent if 1.0 < FICI ≤ 4.0, and antagonistic if FICI > 4.0 [40].
2.12. Statistical Analysis
All data were presented as mean ± standard deviation (SD) of at least three independent experiments, where each experiment had at least a duplicate for each sample. Experimental data were analyzed using GraphPad Prism (version 9.02, GraphPad Inc, San Diego, California, United States).
3. Results
3.1. Phytochemical Screening
Qualitative phytochemical screening showed the presence of phenolic compounds and tannins, and flavonoids in both CAA and CAE (Table 1). Saponins and reducing sugars were present only in the aqueous extract, while phytosterols and terpenes were present in only the ethanol extract. Alkaloids and cyanogenic glycosides were, however, not detected in either extract.
| Phytochemical | Extract | |
|---|---|---|
| CAA | CAE | |
| Alkaloids | − | − |
| Saponins | + | − |
| Phenolic compounds and tannins | + | + |
| Anthracene glycosides | − | − |
| Reducing sugars | + | − |
| Cyanogenic glycosides | − | − |
| Flavonoids | + | + |
| Phytosterols | − | + |
| Terpenes | − | + |
- Note: + = detected; − = not detected; CAA = C. adenogonium leaf aqueous extract; CAE = C. adenogonium leaf ethanol extract.
3.2. Bacterial Susceptibility Testing
The susceptibility of the test bacteria to the extracts was initially determined using the agar well diffusion method by measuring the zones of inhibition. The results are displayed in Table 2. All extracts showed varying growth inhibition against all bacterial strains in a concentration-dependent order. At the highest concentration of 100 mg/mL, CAA showed significantly (p ≤ 0.05) the highest mean zone of growth inhibition (20.17 ± 0.58 mm) against S. aureus, a Gram-positive bacterium, while the least (11.83 ± 1.53 mm) was exhibited against the Gram-negative bacterium, E. coli. However, the inhibition on E. coli was not significantly different (p > 0.05) from the other Gram-negative bacteria, S. typhi and P. aeruginosa, when compared. It was also observed that the activity of CAA against P. aeruginosa was not significantly different (p > 0.05) from all bacterial strains except for S. aureus.
| Conc. (mg/mL) | Test bacteria | |||||
|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. faecalis | E. coli | S. typhi | P. aeruginosa | |
| CAA | ||||||
| 100 | 20.17 ± 0.58a | 14.50 ± 0.87bcef | 15.17 ± 0.76bcf | 11.83 ± 1.53def | 12.33 ± 0.29bdef | 13.00 ± 0.87bcdef |
| 50 | 16.83 ± 0.76a | 8.67 ± 0.76bdef | 11.33 ± 1.76c | 7.00 ± 1.32bdef | 7.67 ± 0.76bdef | 8.33 ± 0.29bdef |
| 25 | 11.33 ± 1.89a | 7.00 ± 1.32bcdef | 8.17 ± 0.58bce | 5.50 ± 0.50bdef | 6.17 ± 1.61bcdef | 5.00 ± 0.50bdef |
| CAE | ||||||
| 100 | 21.00 ± 0.87a | 18.50 ± 0.50b | 23.50 ± 0.50c¥ | 11.00 ± 0.87def | 11.00 ± 0.00def | 11.67 ± 0.76def |
| 50 | 14.00 ± 0.50ab | 14.17 ± 1.26ab | 18.50 ± 1.00c | 6.67 ± 1.04def | 7.00 ± 0.50def | 6.50 ± 0.50def |
| 25 | 8.67 ± 0.58a | 10.83 ± 0.58b | 13.00 ± 0.50c | 4.17 ± 0.58def | 4.17 ± 0.76def | 4.50 ± 0.87def |
| Control | ||||||
| CIP (4 × 10−3) | 30.50 ± 1.00ade | 26.50 ± 0.87b | 23.50 ± 0.00cf | 28.83 ± 0.58ade | 30.83 ± 058ade | 21.33 ± 0.29cf |
| TET (64 × 10−3) | 25.17 ± 0.58ad | 28.17 ± 0.76bd¥ | 18.67 ± 0.29cef | 26.17 ± 1.26abd | 20.00 ± 0.50cef | 20.83 ± 0.76cef¥ |
| DMSO (1% v/v) | nz | nz | nz | nz | nz | nz |
- Note: Values are mean ± standard deviation (n = 3); different letters in superscript indicate significance (p ≤ 0.05) when different bacterial strains are compared by column order for the same concentration of each extract/standard drug using two-way ANOVA followed by Tukey’s post hoc test.
- Abbreviations: CAA = C. adenogonium leaf aqueous extract; CAE = C. adenogonium leaf ethanol extract; CIP = ciprofloxacin; DMSO = dimethyl sulphoxide; TET = tetracycline; nz = no growth inhibition zone (0.00 ± 0.00 mm).
- ¥Significantly not different (p > 0.05) when each concentration of extract/TET is compared with CIP for the same bacterial strain using two-way ANOVA followed by Tukey’s post hoc test.
CAE at the highest concentration of 100 mg/mL displayed the highest mean zone of growth inhibition (23.50 ± 0.50 mm) against E. faecalis, while the least was displayed against S. typhi (11.00 ± 0.00 mm). The activity against S. typhi was, however, not significantly different (p > 0.05) from the other Gram-negative bacterial strains when compared. The susceptibility of two test Gram-positive bacteria, S. aureus and E. faecalis, to CAE was significantly higher (p ≤ 0.05) than all test Gram-negative bacteria. Both standard antibiotics, ciprofloxacin and tetracycline, used as positive controls, presented significantly (p ≤ 0.05) higher zone of growth inhibition compared to the extracts against all test bacteria. The exception was seen in tetracycline, which presented a lower growth inhibition against E. faecalis (18.67 ± 0.29 mm) when compared to CAE (23.50 ± 0.50 mm). With the same organism, the zones of growth inhibition of CAE and ciprofloxacin (23.50 ± 0.00 mm) were significantly not different (p > 0.05).
3.3. MICs, MBCs, and Type of Antibacterial Activity of the Extracts
The MICs of CAE (0.39 to 6.25 mg/mL) were comparatively lower than those of CAA (0.78 to 12.50 mg/mL). It was also observed that the Gram-positive bacteria relatively showed lower MICs compared with the Gram-negative bacteria (Table 3). The MBCs were observed to range from 0.78 to > 25 mg/mL. Based on the MBC/MIC ratios, the type of antibacterial activity of CAA was bactericidal against S. aureus and S. epidermidis, bacteriostatic against E. faecalis, and uncertain against all the Gram-negative bacteria. On the other hand, the activity of CAE was bactericidal against all the Gram-positive bacteria, bacteriostatic against E. coli and P. aeruginosa, and uncertain against S. typhi.
| Bacteria | CAA | CAE | ||||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MBC/MIC ratio | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC ratio | |
| S. aureus | 3.13 | 12.50 | 4 (+) | 0.78 | 3.13 | 4 (+) |
| S. epidermidis | 1.56 | 6.25 | 4 (+) | 1.56 | 3.13 | 2 (+) |
| E. faecalis | 0.78 | 6.25 | 8 (−) | 0.39 | 0.78 | 2 (+) |
| E. coli | 12.50 | > 25 | na (nc) | 3.13 | 25 | 8 (−) |
| S. typhi | 12.50 | > 25 | na (nc) | 6.25 | > 25 | na (nc) |
| P. aeruginosa | 6.25 | > 25 | na (nc) | 3.13 | 25 | 8 (−) |
- Note: (+) = bactericidal; (−) = bacteriostatic.
- Abbreviations: CAA = C. adenogonium leaf aqueous extract; CAE = C. adenogonium leaf ethanol extract; na = not available; (nc) = not certain.
3.4. Time-Kill Kinetics Analysis of Extracts
3.4.1. Time-Kill Kinetics of CAA
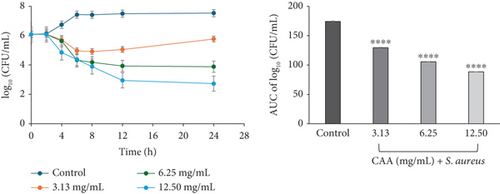
Exposure of S. aureus (ATCC 29213) to CAA at 3.13 mg/mL (×1 MIC) showed a decline in the bacterial cell count over the first 8 h, followed by a gradual regrowth up to the 24th hour. In contrast, the bacteria on exposure to 6.25 (×2 MIC) and 12.50 mg/mL (×4 MIC) CAA showed a decline in bacterial cell count from 0 to the 24th hour. A concentration-independent profile was observed in CAA against S. aureus (Figure 2a).
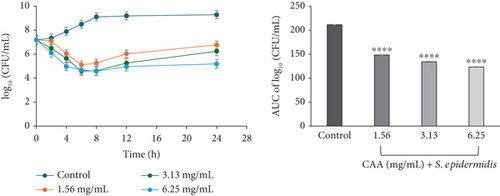
A concentration-independent rate of kill was observed for CAA against S. epidermidis, characterized by a rapid reduction in bacterial cell count over the first 6 h at 1.56 (×1 MIC) and 3.13 mg/mL (×2 MIC) CAA, followed by a regrowth up to 24 h incubation. At 6.25 mg/mL (×4 MIC) CAA, the bacterial cell count rapidly reduced over the first 8 h, followed by a regrowth at the 24th hour of exposure. The time-kill profile suggested the course of antimicrobial activity of CAA against S. epidermidis to be bacteriostatic at all concentrations studied (Figure 2b).
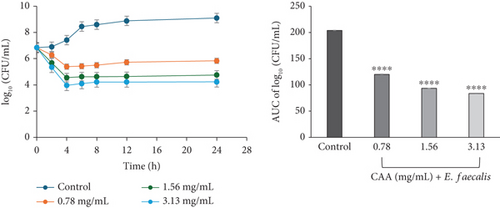
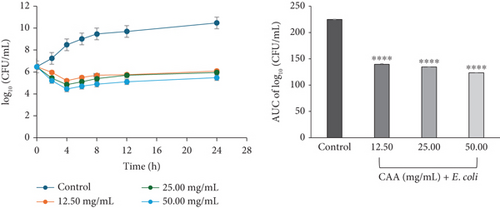
The time-kill kinetics curves of E. faecalis (Figure 2c) and E. coli (Figure 2d) on exposure to CAA were concentration-dependent, each characterized by a rapid decline in the bacterial cell count over the first 4 h at all studied MICs, followed by a slight rise up to the 24th hour of incubation. The profile suggested the course of antibacterial activity at the concentrations studied to be bacteriostatic.
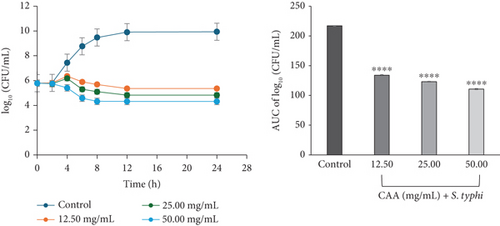
The time-kill kinetics profile of CAA against S. typhi (ATCC 6539) exhibited a concentration-dependent activity at all concentrations studied. At 12.50 (×1 MIC) and 25.00 mg/mL (×2 MIC), the bacterial cell count slightly increased over the first 4 h, before reducing up to the 24-h incubation period. However, at 50.00 mg/mL (×4 MIC) CAA, a steady decline in the number of bacterial cell count was observed over the 24-h incubation period (Figure 2e).
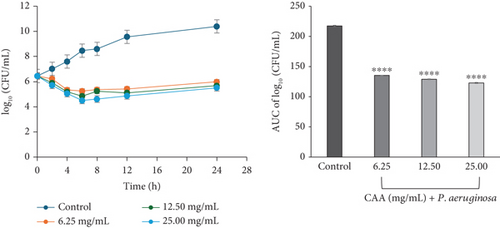
The time-kill curve of exposure of P. aeruginosa to CAA exhibited a bacteriostatic and concentration-dependent activity at all multiples of MICs studied. This was characterized by a decline in the bacterial cell count for the first 6 h at 6.25 (×1 MIC), 12.50 (×2 MIC), and 25 mg/mL (×4 MIC). This was followed by a slight regrowth up to 24 h of incubation (Figure 2f).
3.4.2. Time-Kill Kinetics of CAE
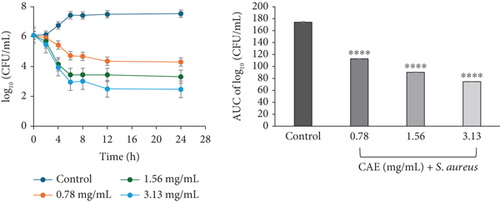
The time-kill kinetics profiles of CAE also showed different characteristics depending on the type of bacteria, similar to what was observed in CAA. S. aureus exposed to CAE displayed a rapid decline in bacterial cell count for the first 12 h at 0.78 (×1 MIC) and 3.13 mg/mL (×4 MIC) and 6 h at 1.56 mg/mL (×2 MIC). Each of these was followed by a slight decline up to the 24th hour. The kill rate indicated a concentration-dependent course of anti-S. aureus activity of CAE, which was bacteriostatic at ×1 MIC and ×2 MIC but bactericidal at ×4 MIC (Figure 3a).
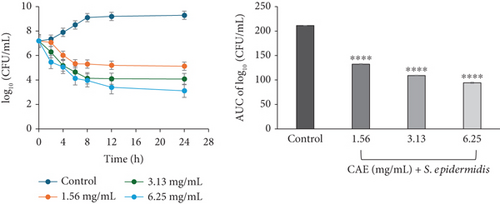
For CAE against S. epidermidis, a rapid decline in the number of bacterial cell count was observed for the first 6 h at 1.56 mg/mL (×1 MIC) and 8 h at 3.13 mg/mL (×2 MIC). Each of these was followed by a gradual decline up to 24 h of incubation. At 6.25 mg/mL (×4 MIC) CAE, a rapid continuous reduction in bacterial cell count was observed over the entire 24 h of incubation. The rate-kill curve suggested a concentration-dependent antibacterial activity, which was bacteriostatic ×1 MIC but bactericidal at ×2 and ×4 MICs CAE (Figure 3b).
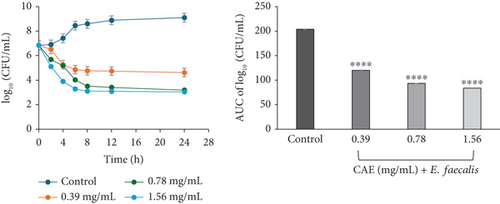
The kill rate curve of CAE against E. faecalis revealed a concentration-dependent decline in the bacterial cell count for the entire 24 h of exposure at all multiples of MICs studied. The profile suggested the course of the kill kinetics of anti-E. faecalis activity as bacteriostatic at 0.39 mg/mL (×1 MIC) CAE but bactericidal at 0.78 (×2 MIC) and 1.56 mg/mL (×4 MIC) CAE (Figure 3c).
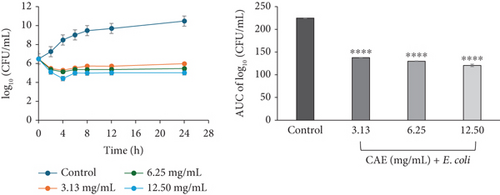
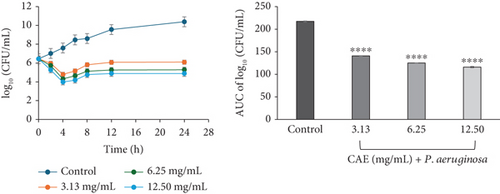
For CAE against E. coli (Figure 3d) and P. aeruginosa (Figure 3f), the kill rate curves revealed a concentration-dependent reduction in the bacterial cell count for the first 4 h at all multiples of MICs studied, followed by slight regrowth up to 24 h of incubation. The course of antibacterial action at all concentrations was observed to be bacteriostatic.
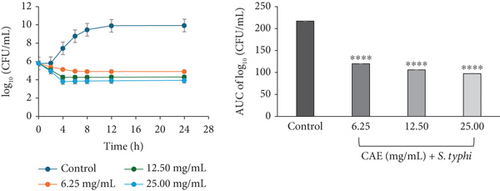
The profile of CAE against S. typhi exhibited concentration-dependent and bacteriostatic activity at all multiples of MICs studied. This was characterized by a steady decline in bacterial cell count at 6.25 mg/mL (×1 MIC) CAE over the 24-h incubation, whereas a rapid decline in the bacterial cell count in the first 4 h, followed by a slight reduction over the 24-h incubation period, was observed at 12.5 (×2 MIC) and 25.00 mg/mL (×4 MIC) CAE (Figure 3e).
The area under the curves (AUCs) of CAA (Figures 2a, 2b, 2c, 2d, 2e, and 2f) and CAE (Figures 3a, 3b, 3c, 3d, 3e, and 3f) against all test bacteria at all the multiples of MICs studied revealed that the number of bacterial cell count was significantly (p ≤ 0.0001) reduced when compared to the bacterial growth control.
3.5. Interaction Between Extracts and Standard Antibiotics in Combination
The MICs of some commonly used antibiotics alone ranged from 0.25 to 64 μg/mL (Table 4). The interaction effect of combining CAA or CAE with the antibiotics was determined using the checkerboard assay. It was observed that the effects varied depending on the type of antibiotic and the bacterial species. The FICI values ranged from 0.31 to 1.45 for CAA and 0.28 to 2.00 for CAE in combination with the antibiotics. The interactive effects observed were synergy, additive, or indifferent; none of the combinations resulted in an antagonistic effect (Table 4). CAA produced a synergistic effect in combination with all antibiotics against at least 16.67% (1/6) of the test bacteria. The highest CAA–antibiotic synergism combination was observed with tetracycline in 50.00% (3/6) of the test bacteria. CAA in combination with all antibiotics displayed a synergy effect against E. coli. On the contrary, none of the CAA–antibiotic combinations resulted in a synergy against S. epidermidis and E. faecalis.
| Bacteria | MIC of antibiotic alone (μg/mL) | Antibiotics in combination with CAA | Antibiotics in combination with CAE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FICI/effect | FICI/effect | |||||||||||
| AMP | CIP | CHL | TET | AMP | CIP | CHL | TET | AMP | CIP | CHL | TET | |
| S. aureus | 0.31 | 0.50 | 2 | 16 | 1.00/A | 0.75/A | 0.75/A | 0.37/S | 0.75/A | 1.50/I | 1.00/A | 0.50/S |
| S. epidermidis | 2.50 | 0.50 | 4 | 64 | 1.25/I | 1.45/I | 1.00/A | 0.75/A | 1.25/I | 0.75/A | 0.75/A | 0.38/S |
| E. faecalis | 1.25 | 1.00 | 32 | 8 | 0.75/A | 1.00/A | 0.75/A | 1.25/I | 0.75/A | 1.50/I | 0.38/S | 1.25/I |
| E. coli | 2.50 | 0.25 | 64 | 4 | 0.38/S | 0.31/S | 0.31/S | 0.37/S | 0.28/S | 1.50/I | 0.28/S | 0.56/A |
| S. typhi | 5.00 | 0.25 | 2 | 2 | 1.00/A | 0.56/A | 0.50/S | 0.50/S | 1.50/I | 1.50/I | 0.50/S | 0.28/S |
| P. aeruginosa | 10.00 | 0.25 | 8 | 32 | 0.50/S | 0.75/A | 0.56/A | 0.75/A | 1.00/A | 2.00/I | 0.62/A | 0.37/S |
- Abbreviations: A = additive; AMP = ampicillin; CAA = C. adenogonium leaf aqueous extract; CAE = C. adenogonium leaf ethanol extract; CHL = chloramphenicol; CIP = ciprofloxacin; FICI = fractional inhibitory concentration index; I = indifferent; MIC = minimum inhibitory concentration; S = synergy; TET = tetracycline.
CAE exhibited a synergy effect in combination with 75% (3/4) of the antibiotics (ampicillin, tetracycline, and chloramphenicol) against 16.67% (1/6) to 66.67% (4/6) of the test bacteria. In contrast, CAE in combination with ciprofloxacin displayed an indifferent effect against 83.33% (5/6) of the test bacteria, with no synergy effect observed.
4. Discussion
Phytochemical screening in the current work revealed the presence of saponins, phenolic compounds and tannins, reducing sugars, flavonoids, phytosterols, and terpenes in the extracts of C. adenogonium leaf (Table 1). This finding is consistent with previous reports [16, 24]. These phytochemicals are known for their biological activities such as antimicrobial, antioxidant, antidiabetic, anti-inflammatory, antiplatelet aggregation, hepatoprotective, and anticarcinogenic activities. The observation that alkaloids were absent in both extracts agrees with earlier reports [16, 24, 41]. The absence of alkaloids and cyanogenic glycosides could suggest a lower risk of acute toxicity of the plant, though there is an overlap between toxicity and therapy. These two phytochemicals are noted to cause mild toxicity including rapid respiration, drop in blood pressure, itching, nausea, vomiting, mild gastrointestinal discomfort, and mental confusion [42, 43].
The antibacterial activities of CAA and CAE were assessed against medically important bacterial. The agar well diffusion assay revealed that both extracts inhibited both the Gram-positive and Gram-negative bacteria in a concentration-dependent manner (Table 2). This suggests that phytochemicals that were more favorable to inhibit bacterial growth were present in the extracts as the concentration increases. Moreover, it suggests the presence of broad-spectrum antibacterial compounds in the extracts [44]. Similar observations were made by previous studies that extracts from the leaves of C. adenogonium had inhibitory activities against Proteus vulgaris, Streptococcus oralis, E. faecalis, P. aeruginosa, Proteus mirabilis, Micrococcus luteus, S. epidermidis, S. aureus, Enterobacter aerogenes, Bascillus subtilis, and Sarcina spp. [24, 45]. The broad-spectrum activities of CAA and CAE observed in this study support the traditional usage of the leaf and other organs of the plant for the treatment of various ailments including diarrhea, new and chronic wounds including diabetic wounds, skin infections, syphilis, and ulcers [20, 46]. The observation that the Gram-negative bacteria were less susceptible compared with the Gram-positive is consistent with the proposition that Gram-positive bacteria are usually more susceptible to plant extracts than Gram-negative bacteria [47]. This is attributed to Gram-positive bacteria having only an outer peptidoglycan layer that is not an effective permeability barrier [48]. In contrast, Gram-negative bacteria are distinguished by the presence of an outer phospholipidic membrane containing structural lipopolysaccharide components, resulting in impermeability of the cell wall to chemotherapeutic agents. This feature allows the microorganisms to effectively regulate and impede the passage of antibacterial agents to the target region [35].
According to Balouiri and colleagues [33], the broth dilution assay is useful to evaluate the efficacy of antimicrobial agents and assess their bactericidal or bacteriostatic effects. The MICs of the extracts were between 0.39 and 12.50 mg/mL in the current study (Table 3). It was observed that the MICs of CAA were higher than CAE, suggesting that ethanol was able to extract more active compounds compared with aqueous. In addition, the MICs of the Gram-positive bacteria were observed to be less than the Gram-negative bacteria, a confirmation of the earlier proposition that the Gram-positive bacteria were more susceptible to both extracts than the Gram-negative bacteria. Previous studies have reported contrasting MIC values of different extracts of the plant against several bacterial species that include those that have been tested in the current work [16, 24, 25]. The disparity in the MICs obtained in this work and previous studies could be attributed to factors including the inoculum size, the type of growth medium, incubation time, and the inoculum preparation method [33]. It is proposed that extracts with MICs between 1 and 8 mg/mL are deemed to exhibit moderate antimicrobial activity, while those with MICs ≤ 1 mg/mL exhibit significant antimicrobial activity [49, 50]. Furthermore, Boakye et al. noted that plant extracts exhibiting MIC values ranging from 2.5 to 8 mg/mL have resulted in the isolation of efficacious antimicrobial agents [51]. Based on these propositions, it could be suggested from this study that CAA and CAE exhibited moderate to significant antibacterial activities against the test bacteria, except against E. coli and S. typhi.
Antimicrobial agents are characterized as bacteriostatic if MBC/MIC ratio is > 4 and bactericidal if MBC/MIC ratio is ≤ 4 [35]. In this study, the ratios obtained for the extracts varied depending on the bacterial species. Both extracts showed bactericidal activity against all Gram-positive bacteria, except CAA against E. faecalis which presented as bacteriostatic. On the other hand, the Gram-negative bacterial strains were characterized as either static or uncertain (Table 3). We therefore performed time-kill kinetics study to confirm their bacteriostatic or bactericidal status over the period and to afford quantitative information about the rate of kill of the extracts. Generally, an activity is considered bacteriostatic when there is a < 3-log10 reduction in CFU/mL relative to the growth control (initial inoculum with no drug) and bactericidal when there is ≥ 3-log10 reduction in CFU/mL relative to the growth control [37, 38]. Time-kill kinetics also affirms whether a particular bacterial agent is concentration-dependent or time-dependent (concentration-independent) [52]. According to Pankey and Sabath [53], concentration-dependent activity occurs when the rate and extent of killing increase with progressively higher antibacterial concentrations, while time-dependent activity occurs when increasing antibacterial concentrations to more than the MIC does not result in proportional increase in killing.
In the current study, both extracts exhibited bacteriostatic activity against all test bacteria at 1× MIC (Figures 2a, 2b, 2c, 2d, 2e, and 2f and Figures 3a, 3b, 3c, 3d, 3e, and 3f). This confirms the observation that none of the MBC/MIC ratios was equal to 1 (Table 3), which would have corresponded to a bactericidal activity. It is worth noting that the Gram-negative bacteria that were characterized as “uncertain” per the MBC/MIC ratio (Table 3) have now been established as bacteriostatic at all the multiples of MICs studied using the time-kill kinetics studies. This makes sense since those extracts recorded MBC values > 25 mg/mL. It is interesting to note that per the MBC/MIC ratios, the activities of CAA and CAE against S. epidermidis were classified as bactericidal (Table 3), which is in variance with the bacteriostatic activity shown by the kill rate analysis. Moreover, in the current study, time-dependent activity was observed in CAA against S. aureus (Figure 2a) and S. epidermidis (Figure 2b). This contrasts with the earlier observation that noted that the activity of CAA against all test bacteria was concentration-dependent using the agar well diffusion assay (Table 2). This is not unexpected as the literature shows that time-kill analysis is the most appropriate assay for determining bactericidal or bacteriostatic activities, because it provides a more dynamic assessment of the interaction between an antimicrobial agent and a given organism than the static MBC or agar well determinations [33, 36]. Bacteriostatic antimicrobial agents work by impeding the growth and proliferation of microbes, which enables the immune system to eradicate them effectively, ensuring that the host is not overrun by the presence of microbes and eventually healing from the infection [53]. The rate-kill curves also demonstrated that each test bacteria responded to the extracts differently, reinforcing the suggestion that the extracts are selective in their activities against the test bacteria. This could be attributed to the presence of different compounds in the extracts inhibiting bacterial growth or killing bacteria via different mechanisms, resulting from differences in the cell wall composition and/or genetic differences between the test bacteria. In this study, the AUCs of the time-kill kinetics (Figures 2a, 2b, 2c, 2d, 2e, and 2f and Figures 3a, 3b, 3c, 3d, 3e, and 3f) for the extracts were all significantly (p ≤ 0.0001) less than the bacterial growth control. This confirms the suggestion that the extracts significantly inhibited the bacterial growth and were broad-spectrum. Literature search indicated no documentation of employing kill kinetics in assessing the antibacterial activity of C. adenogonium leaves. However, other authors have previously documented the time-kill kinetics of mushrooms [54], plant extracts [55], and standard antibiotics [56] against different pathogenic microorganisms.
The antimicrobial effects of CAA and CAE may be ascribed to the presence of phytochemicals, as indicated in Table 1. Anand et al. [14] have demonstrated that these active compounds function by disrupting cellular membrane functions and structure, interfering with intermediary metabolism, disturbing DNA/RNA synthesis and function, inhibiting normal cell communication (quorum sensing), and inducing cytoplasmic constituent coagulation. Phenolic compounds are documented to attach to bacterial enzymes or cell envelopes through hydrogen bonds, leading to a significant loss in the integrity of bacterial cells. This could lead to the release of cellular contents, destruction of membrane function or structure, reduction in intercellular pH, coagulation of cytoplasmic constituents, inhibition of biofilm formation, enzyme inhibition or inactivation, hindrance of nucleic acid synthesis, ATP synthesis and electron transport chain inhibition, and chelation of metal enzymes [57, 58]. Tannins could potentially affect microorganisms by deactivating microbial adhesion, enzymes, cell membrane transport proteins, and mineral uptake or polymerization through oxidation reactions [47, 59]. Saponins are glycosides and possess detergent-like properties, which destabilize the bacterial membrane by altering the membrane permeability. They can also interfere with the energy metabolism through interaction with catabolic enzymes and the electron transport chain [50]. Phytosterols and terpenes possess the ability to impede bacterial cell surface proteins and bring about changes in bacterial membrane composition at the same time [60]. In comparison, CAE was found to be more effective than CAA. This could be ascribed to the presence of phytosterols and terpenes in CAE, which were not present in CAA.
In the face of the spread of bacterial AMR, diverse alternative approaches have been employed to overcome, impede, or bypass resistance mechanisms in pathogens. These prospects include traditional plant-based medicines, combinational therapies, bacteriophage therapy, antimicrobial peptides, antibacterial antibodies, and nanoparticles [9]. In this work, aqueous and ethanol extracts of leaves of C. adenogonium were combined with different classes of conventional antibiotics to evaluate their synergistic outcome. All test bacteria were susceptible to all antibiotics with MICs between 0.25 and 64 μg/mL (Table 4). The difference in the MICs of antibiotics used in this study is consistent with the fact that different classes of antibiotics act by different mechanisms [61]. It was observed from the current work that the extract–antibiotic combinations resulted in synergy, additive, and indifferent outcomes depending on the antibiotic and bacterial strain, with none resulting in an antagonistic effect (Table 4). To the best of our knowledge, this is the first report involving combination studies of C. adenogonium extracts with antibiotics for their antibacterial activities. However, Silén et al. [62] reported that other species of the genus Combretum have been investigated for their synergistic and/or modifying effect when combined with antibiotics. According to the authors, none resulted in an antagonistic effect, which is consistent with the current work.
Anand and colleagues [14] elaborated on the rationale for combining antibiotics with medicinal plant extracts, highlighting the importance of achieving bactericidal synergy in order to broaden the antimicrobial spectrum, inhibit the emergence of drug-resistant mutants, or reduce toxicity levels. The authors also proposed that plant extracts can enhance the activity of antibiotics through a range of mechanisms, including enhancing drug pharmacodynamics, modulating biotransformation in the liver and intestines, regulating active transport, reducing elimination, and strengthening the immune system. The potentiating effects could be due to the fixation of bioactive compounds present in the extracts and antibiotics at different sites of the bacterial cell. For instance, Khan and colleagues [63] posited that saponins possess properties akin to that of detergents; hence, they could contribute to the increase in the permeability of bacterial cell membranes, thereby facilitating the influx of antibiotics through the bacterial cell wall membrane.
5. Conclusion
Taken together, this study investigated the effect of ethanol and aqueous leaf extracts of C. adenogonium alone and in combination with four conventional antibiotics against six clinically important bacteria, S. aureus, S. epidermidis, E. faecalis, P. aeruginosa, E. coli, and S. typhi. Additionally, the killing kinetics of the extracts was also investigated. The results showed that both extracts alone have varied activities against the bacterial strains, with the Gram-positive bacteria relatively more susceptible than the Gram-negative. It was further revealed that the ethanol extract was more effective than the aqueous extract, which could be due to the presence of phytosterols and terpenes in the former but absent in the latter. Time-kill kinetics analysis revealed that the antibacterial activities of the extracts were either concentration-dependent or time-dependent depending on the extract and bacterial strain. For the first time, the interaction between the extracts of C. adenogonium in combination with some commonly used antibiotics has been successfully established, and there was no antagonistic outcome observed. The focus of the authors is to extend the current work to explore the mechanism of antibiotic activity of the extracts alone and in combination with antibiotics at the molecular level.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
M.N.D., R.M., J.A., and A.-M.D. conceived and designed the experiments; M.N.D. and R.M. performed the experiments; M.N.D., R.M., J.A., and A.-M.D. contributed reagents and analysis tools; M.N.D. and A.-M.D. performed statistical/data analysis; M.N.D., J.A., and A.-M.D. drafted the manuscript; M.N.D., R.M., J.A., and A.-M.D. edited and revised the final version of the manuscript.
Funding
The authors did not receive any funding for this research.
Acknowledgments
We are grateful to the staff of the School of Chemical and Biochemical Sciences, C. K. Tedam University of Technology and Applied Sciences, Navrongo, Ghana, for support.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.




