Assessing the Toxicity of Peracetic Acid to Parr, Smolt, and Post-Smolt Atlantic Salmon Salmo salar in RAS Water
Abstract
The risk associated with the presence of bacterial and fungal pathogens in recirculating aquaculture systems (RASs) has resulted in an industry-wide need for low-cost, safe, and effective disinfectants. The toxicity of peracetic acid (PAA) to Atlantic salmon (Salmo salar) eggs, fry, and fingerlings (~16.3 g) in freshwater RAS water has been previously assessed; however, its toxicity to later juvenile life-stages was yet to be investigated. The 24-h LC50 value of PAA was determined for parr ( = 47 g), smolt ( = 66.5 g), and post-smolt ( = 176.7 g) Atlantic salmon in RAS water static PAA treatments. The 24-h LC50 values were calculated using the trimmed Spearman–Karber (TSK) method and toxicity relationship analysis program (TRAP). TRAP LC50 values for parr, smolt, and post-smolt were 4.26, 4.27, and 4.78 mg/L PAA, respectively, while TSK LC50 values for parr, smolt, and post-smolt were 4.27, 3.94, and 4.65 mg/L PAA, respectively. These 24-h LC50 values provide novel guidance for developing safe PAA treatment protocols for Atlantic salmon parr, smolt, and post-smolt in freshwater RAS, although the influence of varying water quality scenarios needs to be considered.
1. Introduction
Land-based and closed-containment recirculating aquaculture systems (RAS) permit the production of aquatic species, including Atlantic salmon Salmo salar, while minimizing overall water consumption [1]. Water treatment technologies are employed to clean waste-water from culture tanks before it is cycled back into the culture tank [1, 2]. Due to low water exchange rates and typically high fish stocking densities in commercial RAS, there is potential for pathogenic microorganisms to proliferate [2–4]. Ubiquitous aquatic opportunistic pathogens, including Flavobacterium spp. and Saprolegnia spp., can be associated with clinical disease in RAS, ultimately leading to mortalities and decreased production [3, 5]. Some of the more common surface or water disinfectants used in the aquaculture industry to control microbial growth include chlorine and ozone; however, the use of both compounds carries significant risk [3, 6].
Peracetic acid (PAA) is sold commercially as an acidified, stabilized mixture of acetic acid, hydrogen peroxide, and water [7]. PAA serves as an antimicrobial disinfectant and has been used as a bactericide, viricide, and fungicide; however, to date the chemical has only been approved for use in aquaculture, in the presence of food fish, in the European Union [8]. PAA has been noted as being efficacious against bacteria and fungi at low concentrations due to its strong oxidizing capabilities and fat-soluble nature [3, 9, 10]. Because PAA rapidly degrades in water and does not produce any known toxic byproducts, it is considered to be environmentally benign [4, 10–12]. While there have been previous studies on numerous commercial fish species (e.g., channel catfish Ictalurus punctatus, goldfish Carassius auratus, largemouth bass Micropterus nigricans, rainbow trout Oncorhynchus mykiss, and walleye Sander vitreus) and their tolerance for PAA [13–15], there is still a multitude of farmed aquatic species, as well as their various life stages, that require investigation.
In the United States, PAA has only been registered by the U.S. Environmental Protection Agency (EPA) for aquaculture surface disinfection when fish are not present, and research investigating its applicability in RAS is limited. Redman et al. [15] investigated the toxicity of PAA to early Atlantic salmon (S. salar) life stages: eyed eggs in incubation systems, fry (~0.17 g), and fingerlings (~16.3 g) maintained in RAS. Briefly, the 24-h LC50 values of PAA—the concentration of PAA that results in approximately 50% mortality within 24-h posttreatment—were calculated after exposing eggs to PAA treatments for 5 and 10 min and after the fry and fingerlings were exposed to static PAA treatments for 24-h. The 24-h LC50 values for eyed eggs treated for 5 and 10 min were relatively high—781.5 and 485.0 mg/L, respectively—likely due to the thickness of the egg chorion providing protection against the strong oxidizer. The 24-h LC50 values for the fry and fingerlings, 4.0 and 5.3 mg/L PAA, respectively, were considerably less than egg LC50 values.
The degradation and efficacy of PAA in RAS can be impacted by water quality, and it is, therefore, imperative that RAS operators consider the specific water chemistry profiles of their systems prior to PAA application to ensure optimal disinfection [16]. Due to typically low water exchange rates, RAS can accumulate nutrients, dissolved metals, and nitrogen species over time [17, 18]. Limited research has been conducted regarding the effect of water chemistry on PAA degradation; however, it has been demonstrated that certain water quality characteristics impact PAA’s disinfection efficacy and degradation [16, 19]. Further experimentation is paramount to understand the ways in which water chemistry impacts PAA degradation, disinfection efficacy, and toxicity to aquatic species.
Due to its disinfection capacity for a variety of microorganisms, PAA could be a suitable water disinfectant for the United States aquaculture industry [3, 20–22]. This current research provides novel determination of 24-h LC50 values for exposure of parr, smolt, and post-smolt life-stages to PAA in RAS water. Redman et al. [15] provided guidance for developing safe PAA treatment protocols for early juvenile Atlantic salmon life stages (eggs, fry, and fingerling); the objective of the present study was to establish 24-h LC50 values for late juvenile Atlantic salmon life stages: parr ( = 47 g), smolt ( = 66.5 g), and post-smolt ( = 176.7 g). Establishing these toxicity values will assist industry stakeholders and facility operators in developing standard operating procedures for the application of PAA during various land-based RAS Atlantic salmon production phases.
2. Materials and Methods
2.1. General Procedures
The Conservation Fund Freshwater Institute (TCF/FI; Shepherdstown, WV, USA) received mixed-sex diploid Atlantic salmon eggs from a commercial supplier and were cultured on-site until specific life-stages were attained for the studies presented. All experiments were received Institutional Animal Care and Use Committee (IACUC) approval prior to commencement. At the end of each experiment, all remaining fish were humanely euthanized following TCF/FI’s IACUC-approved protocols.
A stock solution of PAA (15% PAA and 10% hydrogen peroxide; PeroxyChem LLC, Philadelphia, PA, USA) was verified using Hach method LIT2199 prior to individual treatments being administered (Hach 2014). Treatment concentrations were verified through CHEMetrics PAA Vacu-vials kits, as adapted from APHA Standard Methods (2017) Method 4500-Cl G-2000. All PAA treatments were administered as a single, static bath pulse dose in each trough compartment (see below), such that target concentrations were achieved at the start of the experiments and then allowed to decay over the course of the 24-h exposure periods.
Water quality analyses, aside from total and dissolved organic carbon (TOC and DOC, respectively), were carried out on-site. For TOC and DOC, samples were collected and shipped to the USDA-ARS Stuttgart National Aquaculture Research Center (Stuttgart, AR, USA) for analysis. Dissolved oxygen (DO), pH, and temperature (continuous, 24-h) were monitored using Hach HQ40d handheld probes and HOBO MX2203 TidbiT temperature loggers. Source water for each experiment was analyzed once prior to treatment initiation, including a 15-min potassium permanganate demand (PPD) for water comparability across toxicity trials [23, 24].
All salmon used in each experiment were obtained as eyed eggs from a commercial supplier (Benchmark Genetics Ltd., Hafnafjordur, Iceland).
2.2. Atlantic Salmon Parr
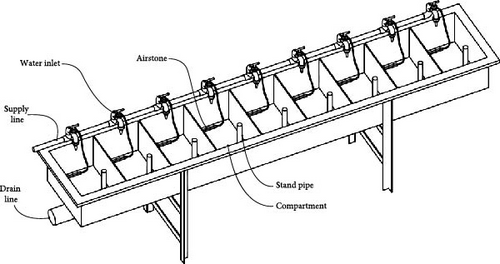
Ten Atlantic salmon parr ( = 47g, bulk weight estimate), previously fed to satiation, were stocked into challenge trough compartments and fasted for 24 h. Each trough was divided into nine individual 35 L compartments, each with water inlets and double standpipe drains (Figure 1). Source water for each trough was from a nearby 9.5 m3 freshwater RAS with a 5.3 m3 culture tank, fully stocked with Atlantic salmon. RAS water chemistry profiles are summarized in Table 1. Troughs were maintained as flow-through during 24-h acclimation, then switched to static baths with treatment initiation.
| Water quality parameter† | Study | ||
|---|---|---|---|
| Parr | Smolt | Post-smolt | |
| pH | 7.2 | 7.4 | 7.3 |
| Dissolved carbon dioxide | 11.1 | 8.5 | 9.9 |
| Dissolved oxygen (DO) | 10.5 | 11 | 10.3 |
| Temperature (°C) | 12.12 | 11.47 | 10.83 |
| Total alkalinity | 184 | 170 | 131 |
| Total hardness | 307 | 323 | 304 |
| Total suspended solids | 2.8 | 1.4 | 1.2 |
| Total organic carbon (TOC) | 7.62 | 7.69 | 4.46 |
| Dissolved organic carbon (DOC) | 6.92 | 7.58 | 4.25 |
| Nitrate-nitrogen | 56.4 | 69.9 | 45.9 |
| Nitrite-nitrogen | 0.011 | 0.012 | 0.025 |
| Total ammonia nitrogen | 0.34 | 0.27 | 0.22 |
| Potassium permanganate demand (PPD)‡ | 3 | 2.5 | 3.5 |
| Total residual chlorine | 0 | 0 | 0 |
Seven PAA treatments were selected following preliminary range-finding assessments (data not shown), with concentration ranges selected to include doses with expected 100% survival (low range) and 100% mortality (high range). The static bath treatments, in triplicate, were: 0, 2, 3, 3.5, 4, 4.5, and 5 mg/L PAA. For each treatment, the required volume of PAA was prepared from the stock solution of verified PAA concentration and based on trough compartment volume and target PAA concentration. PAA treatments were randomized among all compartments used. At 24 h following PAA administration, mortalities in each compartment were counted and LC50 values were calculated (see below).
2.3. Atlantic Salmon Smolt and Post-Smolt
LC50 assessment of PAA toxicity to Atlantic salmon smolt ( = 66.5g, bulk weight estimate; 10 fish per compartment) and post-smolt ( = 176.7g, bulk weight estimate; nine fish per compartment) was assessed utilizing the same approach as described above. Based on range-finding experiments, seven PAA treatment concentrations were selected for each life-stage: 0, 1, 2, 3, 5, 6, and 8 mg/L PAA (smolt), and 0, 1, 3, 4, 5, 6, and 7 mg/L PAA (post-smolt). Treatments were administered in triplicate, as 24-h static baths, after which mortalities were counted for LC50 calculations.
2.4. Statistical Analysis
In a similar approach to Redman et al. [15], LC50 values were calculated through (i) the toxicity relationship analysis program (TRAP) version 1.30a [26] and (ii) the trimmed Spearman–Karber (TSK) [27] method. Both of these approaches have been used frequently in previous studies [13, 14, 28–30]. The reason for using both TSK and TRAP was that, while TSK is a more commonplace in the past literature, it is now not widely available in statistical software packages, whereas the TRAP approach is becoming more popular and is freely available online [26].
3. Results
Experimental conditions were quite stable throughout the trial period, and the RAS from which study water was obtained performed normally and predictably. Water quality profiles for the RAS water used in the parr, smolt, and post-smolt toxicity trials remained relatively consistent across the three experiments (Table 1).
With the mortality data obtained following 24 h’s PAA exposure, the calculated LC50 toxicity values of PAA for each Atlantic salmon life-stage are summarized as follows:
3.1. PAA Toxicity to Parr
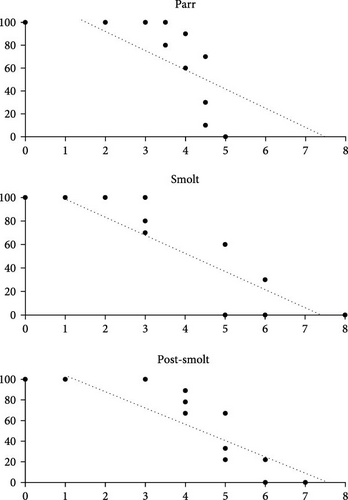
The calculated LC50 values for the TRAP and TSK analyses of parr were 4.26 ± 0.06 and 4.27 ± 0.13 mg/L PAA, respectively (Table 2). Twenty-four hours after PAA was administered, no mortality was observed in the 0, 2, and 3 mg/L PAA treatment groups; however, no surviving parr were observed in the 5 mg/L PAA treatment group (Figure 2).
| Life stage | TRAP LC50 | TSK LC50 |
|---|---|---|
| Parr | 4.26 ± 0.06 | 4.27 ± 0.13 |
| Smolt | 4.27 ± 0.16 | 3.94 ± 0.32 |
| Post-smolt | 4.78 ± 0.12 | 4.65 ± 0.27 |
3.2. PAA Toxicity to Smolt
The LC50 values for the TRAP and TSK analyses of smolt were 4.27 ± 0.16 and 3.94 ± 0.32 mg/L PAA, respectively (Table 2). Smolt mortalities were observed 24 h after treatment was administered in the 5 and 6 mg/L PAA treatment groups. Twenty-four hours after treatment, there were no observed surviving smolt in the 8 mg/L PAA treatment group. Smolt mortalities were not observed 24 h after treatment was administered to the 1 and 2 mg/L PAA treatment groups (Figure 2).
3.3. PAA Toxicity to Post-Smolt
The LC50 values for the TRAP and TSK analyses of post-smolt were 4.78 ± 0.12 and 4.65 ± 0.27 mg/L PAA, respectively (Table 2). No post-smolt mortalities were observed in the 1 and 3 mg/L PAA treatment groups 24 h after PAA treatments were administered; however, a notable number of mortalities were observed in the 5 and 6 mg/L PAA treatment groups. No surviving post-smolt were observed in the 7 mg/L PAA treatment group 24 h after treatment was administered (Figure 2).
4. Discussion
The 24-h LC50 values calculated for this study support the development of PAA as a disinfectant for the Atlantic salmon RAS industry. This study presents the first peracetic 24-h LC50 values for parr, smolt, and post-smolt Atlantic salmon, thereby, providing a basis for aquaculturists and regulators to develop safe protocols for applying PAA to the water for disinfection during these juvenile Atlantic salmon life-stages. Due to its uncommon usage, minimal research pertaining to PAA’s applicability in United States based RAS industry has been conducted [13, 31]. To date, PAA has only been registered for aquaculture surface disinfection by the EPA [14].
The LC50 values determined in the previous [15] and present study suggest that safe PAA application protocols can be developed to reduce bacteria in RAS without significant impact on the health of both early and late juvenile Atlantic salmon life stages [32]. It is important to note that surviving parr, smolt, and post-smolt were not monitored or assessed for long-term health implications following PAA treatments. Redman et al. [15] observed that surviving Atlantic salmon fingerling appeared lethargic 24 h after PAA treatments were administered, and it was unknown whether these fish would have recovered or died if they had been returned to normal culture conditions. Lethargic survivors were not observed during the present study’s toxicity trials; however, it is unknown if their health would have deteriorated over time as all exposed fish were humanely euthanized at the end of each trial. Future research should investigate whether PAA treatments similar to those administered in the present study are associated with long-term adverse health effects in juvenile Atlantic salmon.
Recent research has focused on the health and physiological responses of salmonids to acute and longer-term PAA exposure. Liu et al. [33] assessed antioxidant responses in rainbow trout O. mykiss to biweekly single-dose 1 mg/L PAA applications or continuous 0.2 mg/L PAA exposure over a 6-week period, and determined that, while antioxidant defenses instigated (skin, gill, and systemic) were mild, periodic PAA applications would likely be a better option to reduce the risk of oxidative damage. Similarly minimal antioxidant defense triggering was noted by Carletto et al. [34] following Atlantic salmon parr being exposed to 1 mg/L PAA pulse (every 3 days) or continuous exposure over 4 weeks duration. Alterations in mucosal morphometrics in Atlantic salmon in response to a range of therapeutic concentrations of PAA have been observable but, in general, mild and transient, providing further evidence that low-dose PAA exposure is relatively benign for this species [35, 36]. Good et al. [5] investigated the use of low-dose PAA treatments to prevent postvaccination saprolegniasis in juvenile Atlantic salmon; PAA treatment in general was significantly associated with a reduction in saprolegniasis-affected fish postvaccination, and no treatment-associated histopathologic lesions in gill, spleen, or kidney tissues were noted. Finally, Mota, Eggen, and Lazado [37] exposed Atlantic salmon parr to a range of PAA doses (maximum 6.4 mg/L) for 1 h, and the no-observed-effect concentration for PAA was determined to be below 1.6 mg/L, which is in agreement with the data presented in the present study.
As mentioned previously, water chemistry has been demonstrated to affect the degradation and disinfection efficacy of PAA [12]. PAA degrades as it oxidizes organic matter, which is supported by Liu et al.’s [16] observations of high DOC increasing PAA degradation rates. One of the primary limitations of the current study was that RAS water quality profiles slightly differed between each life-stage trial. Specifically, DOC for the post-smolt trial (4.3 mg/L) was less than that of the smolt trial (7.6 mg/L). Higher DOC content in the RAS water during the smolt toxicity trial could have resulted in faster PAA degradation, thereby, reducing the amount of disinfectant to which the smolt were directly exposed. RAS water nitrate-nitrogen concentrations also differed between the toxicity trials for the three life stages. The nitrate-nitrogen concentration was highest during the smolt toxicity trial (69.9 mg/L) and lowest during the subsequent post-smolt toxicity trial (45.9 mg/L). Additionally, the RAS water temperature was highest for the parr toxicity trial (12.1°C) and lowest for the post-smolt toxicity trial (10.8°C). It is possible that these water quality differences could have influenced the 24-h LC50 values for each life stage, and therefore, additional research is necessary to investigate how variation in water chemistry affects the degradation and toxicity of PAA in RAS water.
In summary, the PAA 24-h LC50 values calculated for parr, smolt, and post-smolt Atlantic salmon in RAS water can support the development of PAA as a disinfectant in the United States RAS industry. Future research should address the limitations of this study by including consistent RAS water chemistry profiles among individual experiments. As each RAS will have different water chemistry and quality, baseline toxicity tests of Atlantic salmon in well water would also be helpful. Because PAA toxicity and efficacy will differ between RAS water chemistry profiles, farmers should consider their unique system characteristics when developing PAA treatment protocols. While the LC50 values presented in this study answer important questions regarding the chemical’s applicability in RAS, it is unknown how these low dose treatments would holistically affect the operation of a RAS, including biofilter function. Research pertaining to the effect of low dose PAA treatments on nitrifying bacteria that thrive in RAS biofilters should also be investigated to assess the disinfectant’s compatibility with RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Study design and protocols were developed by Natalie Redman, David L. Straus, and Christopher Good. Study execution and water assessments were carried out by Natalie Redman and Megan Murray. Manuscript writing and data analyses were performed by Natalie Redman, David L. Straus, and Christopher Good. Manuscript review and editing were performed by all listed authors.
Funding
This research was carried out by The Conservation Fund Freshwater Institute as an independent third party, with funding support from PeroxyChem LLC (Philadelphia, PA, USA).
Acknowledgments
Special thanks are extended to Travis May, Curtis Crouse, Anna Knight, Scott Tsukuda, Shanen Cogan, J.C. Stanley, Cindy Ledbetter, and Kata Sharrer for their assistance. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by The Conservation Fund Freshwater Institute or the U.S. Department of Agriculture. The Conservation Fund and the U.S. Department of Agriculture are equal opportunity employers and providers.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




