First Report of Calceostoma glandulosum (Monogenea) in Argyrosomus regius: Morphological and Molecular Characterization and Temperature Effects on Life Cycle
Abstract
The increase in fish production in aquaculture reflects the growth of the world population. However, this expansion is accompanied by challenges, such as the intensification of production systems through increased stocking density, which induces stress in fish, weakening their immune system and making them more susceptible to disease outbreaks, particularly those caused by parasites. Monogeneans are ectoparasites that attach to the external surfaces of fish and are characterised by their host-specificity. One of the emerging species in aquaculture, much appreciated in the Mediterranean region, is the meagre (Argyrosomus regius), whose production has been increasing due to its high-quality meat and excellent growth rates. However, the information available on the parasites that affect this species is limited, which makes it difficult to prevent and control disease outbreaks in aquaculture systems effectively. This study presents the first morphological and molecular characterisation of Calceostoma glandulosum, an ectoparasite of the Monopisthocotylea subclass, characterised by its lapel (L) in the cephalic area and an anchor-shaped haptor (Hp). The parasite’s life cycle, including eggs, larvae (oncomiracidium), and adults, has also been described. It was demonstrated that water temperature could influence the development of the eggs; at more favourable temperatures for the parasite, there was a higher percentage of developed and hatched eggs. The occurrence of the parasite in the farming tanks was higher when the temperature reached 20°C, and in vitro experiments have shown a hatching rate of 100% at 23°C. C. glandulosum reaches sexual maturity between 11 and 14 days and has a fertility period of 10 days. To assess the host-specificity of C. glandulosum for A. regius, eggs were investigated in other species (Sparus aurata, Diplodus sargus, and Seriola rivoliana). This work provides valuable insights into the behavior of monogeneans concerning environmental conditions and host interactions, offering critical information for implementing preventive measures in aquaculture.
1. Introduction
The growth of the human population over the years and the need to reduce the overexploitation of marine resources have led to the expansion of aquaculture worldwide. Apart from well-known species like gilthead seabream and sea bass, there is a need to diversify aquaculture production in the Mediterranean, and one promising species for this diversification is meagre, Argyrosomus regius, with a total production, of 13,670 tons in 2021 [1]. This species is an excellent candidate for aquaculture due to its high-quality meat, rapid growth rate, low feed conversion ratio, high fecundity rates, and rapid larval development [2]. One crucial requirement for introducing new species into aquaculture is understanding the pathologies that may affect them [3]. Although A. regius is less susceptible to disease occurrence compared to other species such as Dicentrarchus labrax, Solea senegalensis, and Sparus aurata [4], the intensification of production has led to higher densities and increased fish handling, resulting in elevated stress levels among farmed animals. This situation directly impacts the immunocompetence of individuals, leading to decreased disease resistance, with the occurrence of parasites, especially external parasites, frequently reported in intensive aquaculture systems [5].
Members of the class monogenea have been reported to parasitize marine vertebrates and some invertebrates, attaching themselves to the external surfaces of the host, primarily on gills, skin, and fins, classifying them as ectoparasites [6]. These organisms exhibit bilateral symmetry, with the body covered by a thin and colorless tegument [7] which can be divided into three zones: cephalic region (Cr), trunk, and haptor region. They are characterized by a haptor (Hp), an attachment organ, composed of hooks, anchors (hamuli (Ha)), or suction pads that can cause lesions to the organs where they attach [8]. With a direct life cycle, monogeneans can produce many eggs, and due to the use of various transmission strategies to ensure the survival of the next generations, particularly through mass hatching during the day [9], they can accumulate eggs in the aquaculture system and lead to new infections.
A precise understanding of the biology, ecology, and life cycle of these parasites, along with the influence of environmental parameters on parasite–host interactions, is crucial for comprehending infection mechanisms and developing mitigation measures. The capability to culture and maintain parasites in vitro [10] represents a significant resource for implementing measures to manage fish health and develop treatment strategies (e.g., disrupting parasite life cycles) for the aquaculture industry [11].
The identification of monogeneans is typically carried out by observing various anatomical structures. However, the similarity between different species can sometimes lead to incorrect taxonomic characterization [12]. Therefore, phylogeny can provide a more reliable, precise, and, faster identification method, enabling adequate and customized treatment or disease prevention strategies [13].
The class monogenea (phylum Platyhelminthes) is divided into two subclasses: the Monopisthocotylea and Polyopisthocotylea. These subclasses are distinguished by the morphology of their Hps and feeding methods [14]. Calceostoma glandulosum, belonging to the Monopisthocotylea subclass, has been found in the wild in Argyrosomus hololepidotus and cage farmed Argyrosomus japonicus in Australia [15, 16] and in Argyrosomus inodorus in Namibia [17]. This opportunistic parasite can cause gill hemorrhage when present in high numbers per branchial arch and may increase the risk of secondary infections.
This study aimed to characterize C. glandulosum morphologically and phylogenetically and to investigate its life cycle and the effect of temperature on its development. Additionally, the study aimed to determine the fecundity and lifespan of the oncomiracidium stage to estimate its infestation capacity. Furthermore, the study evaluated the species specificity of this parasite by examining its preference for different farming systems and assessing the lesions it provokes in the host.
2. Materials and Methods
During routine procedures at the Aquaculture Research Station of Olhão (EPPO/IPMA), monogenean parasites were observed on the gills of meagre (A. regius). The meagre used in this work comes from broodstock established at the EPPO several years ago, identified at the stock’s beginning [18].
2.1. Sample Collection
These parasites were subsequently collected for morphological and genetic characterization. Six meagre specimens, with a mean weight of 86.3 ± 16.6 g and a mean total length of 22.2 ± 1.5 cm, were collected from earthen ponds and transported immediately to the EPPO laboratory for gill observation. Animals were sacrificed by a spinal incision and the first two gill arches were examined under a light microscope (Nikon ECLIPSE Ci).
2.2. Morphological Analysis
Fourteen parasites were detached from the gills with a needle [19] and observed in fresh and photographed under a light microscope (Nikon ECLIPSE Ci). The parasite’s internal and external structures were dissected and measured using NIS-Elements imaging software.
For scanning electron microscope (SEM) observation, following the [20] protocol, five parasites and five eggs were collected randomly, obtained from meagre and cotton strips placed in the tanks, respectively, and stored in 70% ethanol [11]. The samples underwent gradual dehydration in ethanol concentrations ranging from 70% to 100%. Following this, two additional 10-min washes with 100% ethanol were carried out. After removing ethanol, the samples were embedded into HMDS for 10 min. Subsequently, C. glandulosum adults and eggs were mounted and dried at room temperature in aluminum tubes with carbon strips (Micro to Nano, The Netherlands) for observation under the electronic microscope (Hitachi TM4000Plus) [20]. The images were photographed at 15 kV accelerated voltage and the magnification ranged from 80x to 500x.
2.3. Adults’ Characterization
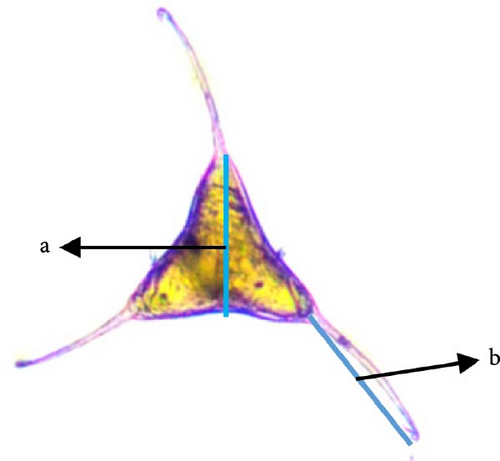
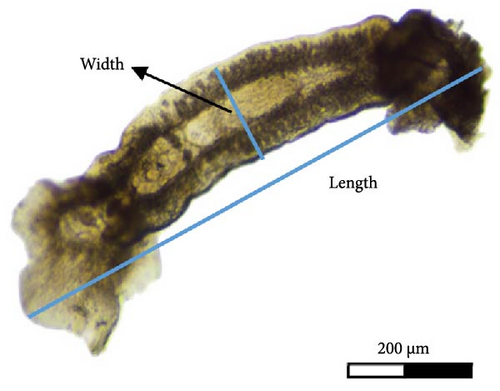
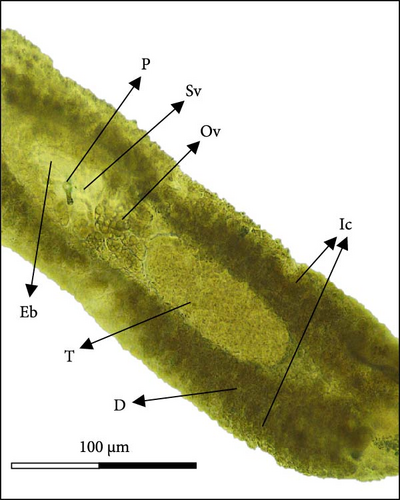
After collection (individuals were separated from fish gills using a needle), the length (from the top of the Cr to the Hp) and width (at the testis (T) area) of 14 parasites were measured (Figure 1B) and their external structures using NIS-Elements imaging software.
2.4. Primer Design
Based on the sequence comprising 5.8S, ITS, and 28S ribosomal RNA (rRNA) genes of C. glandulosum, with the accession number EF452640.1 (GenBank) and using Primer-BLAST, a forward (Fw; Cgla_Fw1 5′-GGTTGTGGATTGGACTGTAT-3′) and reverse (Rv; Cgla_Rv1 5′-GCACACAACAGAAACAATGA-3′) specific primers were designed.
2.5. DNA Extraction
The genomic DNA of 10 C. glandulosum was extracted using the NZY Tissue gDNA isolation kit (Nzytech, Portugal) following the manufacturer’s instructions. Subsequently, DNA concentration and purity were evaluated by spectrophotometry (DeNovix DS-11 FX).
2.6. PCR Amplification
A MasterMix solution (50 µl final volume), containing enzyme buffer at 1× concentration, 2.5 mM of MgCl2, 0.4 mM of each dNTP, 0.1 μM of Fw and Rv primers, and 2 U of Supreme NZYTaq II Polymerase (Nzytech, Portugal), was prepared according to the manufacturer’s protocol. For each reaction tube, 10 ng of the sample DNA was added, except for the negative control where the DNA was replaced by MilliQ water. PCR amplification was performed in a thermocycler (Biometra Personal Cycler) using the following program: initial denaturation at 94°C for 3 min (1×), followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 2 min, and a final extension step at 72°C for 10 min. The PCR products were then separated by electrophoresis for 1 h at 120 V using a BioRad electrophoresis system on a 1.5% agarose gel, stained with GreenSafe (Nzytech, Portugal). The gel was visualized using a transilluminator (BioRad, USA), expecting to see a band with a size of 129 bp, and the PCR products of interest were sent for Sanger sequencing at the Molecular Biology platform (CCMAR, UAlg) with both primers used in PCR amplification.
After the described procedure, Tedesco et al. [13] identified a species morphologically identical to the one described in our work. To evaluate the similarity between species, we tested the primers in their publication, designed for the sequence comprising 5.8S, ITS, and 28S rRNA genes of Ktariella polyorchis, on our sample using the conditions outlined in [13]. The PCR results were analyzed by electrophoresis on a 1.5% agarose gel run for 1 h at 120 V and visualized using a ChemiDoc system (BioRad, USA). Fragments with the expected size were amplified (129 bp) with Cgla Fw and Rv primer and 1600 bp of Tedesco et al. [13] and were excised using a scalpel, DNA purified using a kit (Nzytech, Portugal), and sent for sequencing at the Molecular Biology platform (CCMAR, Ualg). The sequence generated in this study was deposited in GenBank under the accession number PQ108666 with a 1211 bp final size.
2.7. Alignment and Phylogenetic Analyses
Sequences were initially aligned using the standard nucleotide BLAST+2.14.0 and the Vector NTI software. Subsequently, a multiple sequence alignment was performed using ClustalW Version 2.1 (https://www.genome.jp/tools-bin/clustalw). The sequences of the 5.8S, ITS, and 28S rRNA genes from different species, as we can see in Table 1, were aligned with the same fragment size and position. These genes were selected for primer design in this study primarily because they are the only ones available in the databases and are commonly used for the genetic identification of various organisms [30]. This is because rRNA genes have the advantage of being highly conserved [31]. All phylogenetic analyses and tree constructions were carried out using MegaX Version 0.1 software [32] using the maximum likelihood method and Kimura 2-parameter model with 60% bootstrap for 1000 replicas.
| Specie | Accession no. | Reference |
|---|---|---|
| Benedenia seriolae | AY033941.1 | Whittington et al. [21] |
| Calceostoma glandulosum | EF452640.1 | Hayward et al. [22] |
| Dactylogyrus extensus | AY553629.1 | Wu et al. [23] |
| Diplectanum aequans | MK208301.1 | Villar-Torres et al. [24] |
| Haliotrema magnihamus | MG593838.1 | Soo [25] |
| Ktariella polyorchis | OM985022.1 | Tedesco et al. [13] |
| Lamellodiscus japonicus | EF100561.1 | Simková et al. [26] |
| Neobenedenia girellae | MG193660.1 | Brazenor et al. [27] |
| Polylabris sillaginae | GU289509.1 | Catalano et al. [28] |
| Sparicotyle chrysophryii | AF311719.1 | Jovelin and Justine [29] |
| Calceostoma glandulosum | PQ108666 | This study |
2.8. Egg Collection and Characterization
To characterize monogenea eggs, they were collected from A. regius broodstock tanks (A, B, and C) maintained at EPPO at 19 ± 2°C and 35 ppm of salinity.
For egg collection, four cotton strips, 30 cm long, were placed in each tank, removed after 5 days, and transferred to a glass bottle with filtered seawater for sample maintenance. Each cotton strip was examined using a stereomicroscope (Nikon SMZ1000). The eggs were carefully separated with a needle for fresh observation on a slide, and photographs were taken. The external structure, color, and size of the eggs were characterized, and the height and total length of the filament were measured, as shown in Figure 1A, together with the adult stage (Figure 1B). Embryo development was also studied, and eggs were classified into four stages, according to Militz et al. [33].
2.9. Larvae (Oncomiracidium)
To observe and characterize the larval forms—oncomiracidium—all eggs presenting an oncomiracidium inside, with ocular operculum development, were collected with a needle and transferred to a petri dish, to be separated and cleaned from other debris, thus, avoiding contamination from different organisms. After collecting 100 eggs, they were assigned to a six-well plate, containing 3 ml of filtered and ultraviolet (UV) sterilized seawater, with one third of the water changed daily. The plate was then incubated at room temperature (23 ± 2°C) under a 12d:12n (day/night) photoperiod and observed daily simultaneously. The hatched larvae were transferred to a cavity slide, washed with 4% formalin (Produtos Sodacasa, Portugal) only to kill the larvae and stop their movement [34], observed under a light microscope (Nikon ECLIPSE Ci) for photographic documentation.
2.10. Lifespan
The methodology of Erazo-Pagador and Cruz-Lacierda [35] was followed with some modifications to determine the lifespan of the free-swimming larvae after hatching. New incubations were set up placing one individual larva in each well of a 24-well plate containing filtered and UV-sterilized seawater. Incubation was performed at room temperature (23°C ± 1) under a 12d:12n photoperiod. To maintain the water temperature at 23 ± 1°C, the plates containing eggs were kept refrigerated. The eggs were allowed to hatch in the morning and were observed after 24 h. Hatched larvae were transferred to a petri dish, with filtered seawater and observed every 2 h (from 9 am to 5 pm) for 3 days, recording the number of hours until their death. Larval death was determined when no movement was observed or when larvae showed signs of degradation.
2.11. Temperature Effect on Eggs and Parasites’ Development
To study the life cycle of the parasite C. glandulosum, eggs were collected, from the cotton strips introduced at the broodstock tank, as described above, over 7 months and counted.
The total number of eggs collected from strips of cotton was counted with a manual counter at water temperatures of 13, 14, 16, 17, 20, 22, and 24°C (temperature of the water in the tank, which varied over the 7 months).
The viability of the eggs was determined at the temperatures of 14, 16, and 17°C using the classification described by Militz et al. [33]: Phase I—unviable eggs exhibiting translucent coloration or damage; Phase II—opaque brown coloration due to the presence of oncomiracidium; Phase III—oncomiracidium with well-developed ocular points; and Phase IV—hatched eggs showing translucent coloration with the egg operculum open. To assess the optimal temperature for in vitro incubation, eggs were sorted and placed in 3 ml of UV-sterilized saltwater in a six-well plate (initial stock of 100 eggs). Eggs were then incubated for 48 h at temperatures of 17, 19, 21, 23, and 25°C, with a 12d:12n photoperiod and 1/3 of the water volume changed daily to maintain water quality. The eggs were examined after 24 h of incubation (considered sufficient time for all free-swimming larvae to hatch (time 0)) based on preliminary results from the larval morphological analysis. They were subsequently observed, 48 h after hatching. The hatching rate was determined using the formula: (No. of eggs hatched/total No. of eggs) × 100 [36].
2.12. Fecundity
To determine the fecundity of C. glandulosum, six meagres were transferred to a 20 l tank. Formalin at 300 ppm (Produtos Sodacasa, Portugal) was added for 30 min as mentioned by Sitjà-Bobadilla, de Felipe, and Alvarez-Pellitero [37], to eliminate any parasites present on the gills and skin. Each fish was then individually transferred to 20 l tanks with clean seawater at 23 ± 1°C.
To assess parasite fecundity, an infection experiment was conducted following the methodology described by Hoai [38] and Hoai, Hutson, and Orbán [39]. Briefly, the volume of water in each tank was reduced by 1/3, and one oncomiracidium was introduced into each tank. Aeration was stopped for 1 h to ensure successful infection. Due to the small size of the tank where the fish are kept, aeration results in increased water agitation, which may disrupt the swimming behavior of the oncomiracidium. This mechanical perturbation can impede its capacity to infect the host, compromising the effectiveness of the test [39]. After 1 h, the water volume was restored. The fecundity of C. glandulosum species was monitored by the daily egg production. Therefore, after transferring the fish to a new tank with UV-sterilized and filtered seawater at the same temperature, the water was filtered. Water filtration involves passing the water sequentially through a net with pore sizes of 125 and 55 µm. All the material caught by the second filter was observed under the stereomicroscope (Nikon SMZ1000) to detect the presence of eggs. This monitoring process was repeated every 24 h, following the same procedure. The experiment concluded after two consecutive days without the appearance of eggs.
2.13. Evaluation of C. glandulosum Species-Specificity
Six cotton threads, each measuring 30 cm, were placed in the meagre broodstock tanks labeled, A, B, and C; tanks D and E, housing gilthead seabream (S. aurata) and seabass (Diplodus sargus) broodstock, respectively; and tank F, with greater amberjack (Seriola dumerili) broodstock. After 5 days, the cotton threads were removed from each tank, and the number of eggs in each thread was counted.
Species from the semi-intensive earthen ponds system (including D. sargus, S. aurata, A. regius, D. cervinus, D. labrax, Epinephelus marginatus, and S. senegalensis) were monthly monitored for the presence of external parasites, during routine procedures. Therefore, five fish specimens were collected from each tank, weekly, and transferred to the laboratory for parasite observation. The fish were sacrificed and gills were removed for examination under a light microscope (Nikon ECLIPSE Ci). The presence of C. glandulosum and the intensity of the infection were recorded, with intensity determined by the number of parasites found in the first two gill arches of each host.
2.14. Statistical Analysis
For the C. glandulosum host specificity results, a one-way ANOVA was conducted to assess statistically significant differences in parasite fecundity among various fish species. Assumptions such as homogeneity of variances, using Levene’s test, and normality of data were thoroughly validated with Shapiro–Wilk test, with a significance level set at p < 0.05. In cases where violations of these assumptions were detected, a comparison of means was carried out using Games–Howell test. All statistical analyses were conducted using SPSS 20.0 software for Windows.
2.15. Ethical Statement
The present study was conducted by researchers certified in animal experimentation (EU functions A and B). All procedures related to animal handling and sample collection were in strict compliance with the ARRIVE (Animal Research: reporting of in vivo experiments) guidelines and followed ethical standards for the care and use of animals, always under the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA) and the Portuguese legislation for Laboratory Animal Science (EU Directive 2010/63; Decreto-Lei n° 113/2013). The research was approved by IPMA’s Animal Welfare and Ethics Body (ORBEA), overseen by the National Authority for the use of live animals, also known as the Directorate-General for Food and Veterinary (DGAV).
3. Results
During daily routines at the EPPO, the fish’s behavior was observed. If they exhibit abnormal symptoms, pathological analyses are performed to observe parasites’ occurrence. In this case, monogeneans were observed in the gills of A. regius. These parasites were collected for morphological and genetic identification, and for the first time, the monogenean C. glandulosum was identified in this species.
3.1. Characterization of Adult Organisms
C. glandulosum parasites (n = 14), collected on A. regius at temperatures between 19 and 21°C, were in the adult stage and had a mean length and width of 2230 ± 500 µm (1100–3900 µm) and 480 ± 100 µm (200–700 µm), respectively.
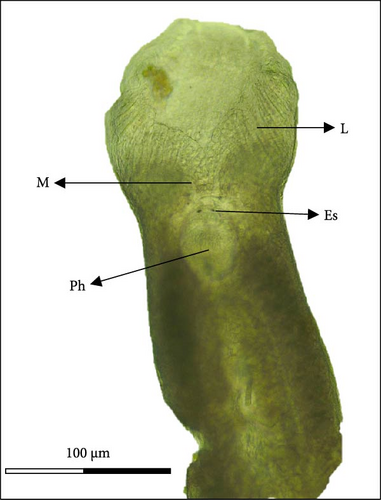
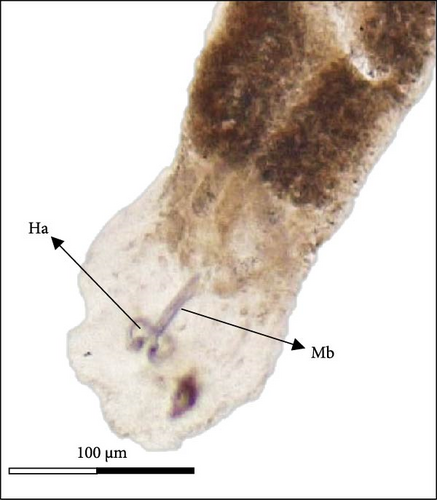
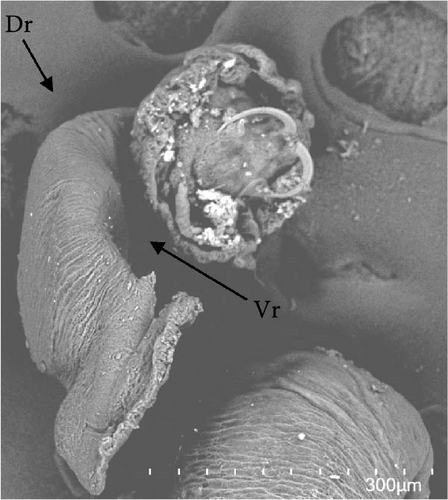
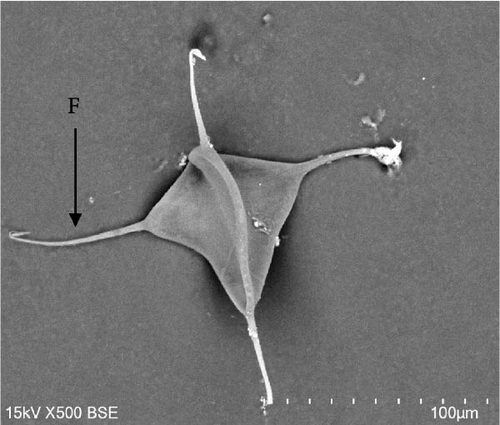
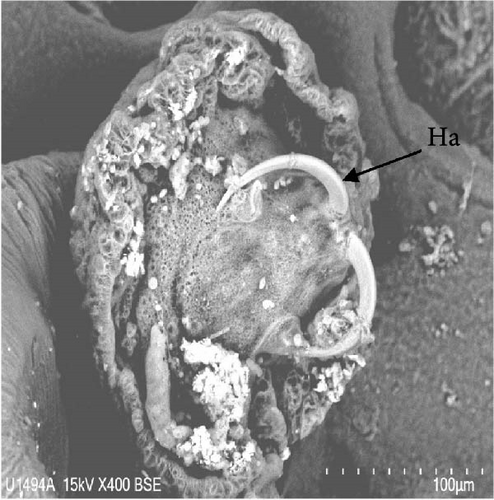
Morphologically, next to its mouth (M) cavity, there is a flap with two glandular projections. When expanded, its anterior end demonstrates a large and irregular lapel (L) with striated margins of 814 ± 144 µm width (613–940 µm). Additionally, in the Cr, two pairs of eye spots (Ess) are located anterior to the pharynx (Ph), which measures 197 ± 5 µm in length (191–203 µm) and 158 ± 7 µm in width (149–167 µm). The intestine ceca (Ic) bifurcates immediately after the Ph and extends to the anterior margin of the Hp, with well-developed lateral and medial diverticula (D) that extend laterally to the Ph. The reproductive system is composed of a prostatic reservoir, penis (P), ejaculatory bulb (Eb), seminal vesicle (Sv), ovary (Ov), and a large T, located between the Ov and the Hp. The P measures 106 ± 19 µm (85–145 µm) in length and 24 ± 4 µm (18–30 µm) in width. The Hp of C. glandulosum consists of two curved Ha with pointed ends, measuring 140 ± 25 µm in length (108–170 µm). These are associated with a T-shaped median bar (Mb) and a complex sclerotized structure, which measures 165 ± 27 µm in length (123–198 µm) and 27 ± 5 µm in width (21–34 µm). This Hp structure is protected by a suction cup-like structure that enables the parasite to attach firmly to the host (Figure 2). Detailed examination of external structures was achieved using electronic microscopy. The parasite’s tegument exhibits a concave shape on the ventral side and a convex shape on the dorsal side (Figure 3A, B). The Ha hooks on the Hp, originating from the central area and resembling anchor shapes, are evident (Figure 3D). Additionally, the eggs (n = 36) are pyramidal, with well-defined filament ends that function as hooks (Figure 3C).

3.2. Molecular and Phylogenetic Analysis
A fragment of 129 bp was successfully amplified using the Cgla primers, design specificity for C. glandulosum. In comparison, a larger fragment of 1600 bp was amplified using primers designed by Tedesco et al. [13] for the K. polyorchis. Both fragments were sequenced, revealing that amplification using the Cgla primers resulted in a 96.3% similarity between our sequence and C. glandulosum (EF452640), with no alignment to K. polyorchis (OM985021 and OM985022). Conversely, when using primers from the literature designed for another species within the same Calceostomatidae family, the obtained sequence had 1211 bp, exhibiting a higher similarity to C. glandulosum (96.1%) compared to K. polyorchis (92.5%).
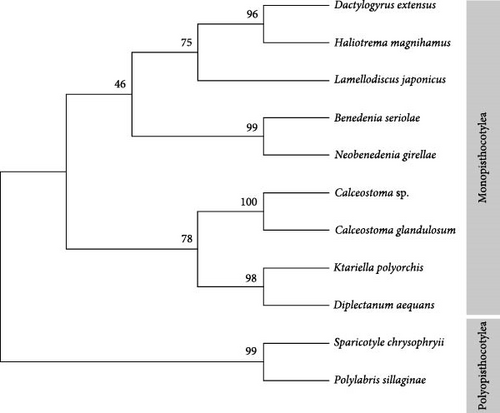
To further validate and position the species phylogenetically, an alignment of these sequences with nine additional sequences from different species of monogeneans was performed using ClustalW Version 2.1 (https://www.genome.jp/tools-bin/clustalw). A phylogenetic tree was constructed using the maximum likelihood method and the Kimura 2-parameter model (Figure 4). The phylogenetic tree illustrates the evolutionary relationships between various species of Monogenea, grouped into two primary clades: Monopisthocotylea and Polyopisthocotylea. Within the Monopisthocotylea clade, species such as C. glandulosum, K. polyorchis, and N. girellae are closely related, with high bootstrap support (100 and 99), indicating robust confidence in their evolutionary proximity. In contrast, the Polyopisthocotylea clade, including S. chrysophryi and P. sillaginae, is distinctly separated from the Monopisthocotylea, reflecting an early divergence between these two groups. The strong bootstrap values across the tree support the reliability of the inferred relationships, particularly within the Calceostoma genus. Calceostoma sp. and C. glandulosum are closely related, as indicated by the high bootstrap value of 100, representing strong statistical support for their evolutionary proximity. Ktariella polyorchis is placed as a sister taxon to C. glandulosum, with a moderately supported bootstrap value of 78 at their shared junction. This suggests a common ancestor between K. polyorchis and C. glandulosum species, although with lower confidence than the relationship between the Calceostoma species. Finally, D. aequans is more distantly related, but with strong support (98), indicating a clear evolutionary distinction from the Calceostoma–K. polyorchis grouping. The topology reflects robust evolutionary relationships within this monogenean subgroup, with Calceostoma species being more closely related to each other than to K. polyorchis or D. aequans.
3.3. Characterization of the Different Life Stages of C. glandulosum
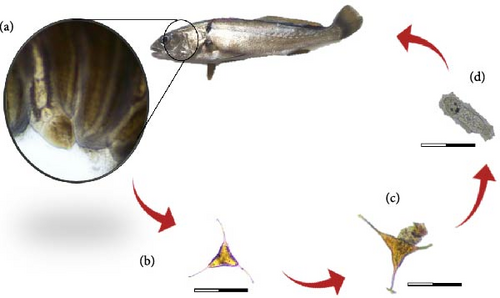
Calceostoma glandulosum is a parasite belonging to the class monogenea, subclass Monopisthocotylea. It follows a direct life cycle in which the adult organism attaches to the gills of the host, specifically in A. regius, and feeds on mucus and epithelial cells. Upon reaching sexual maturity, it produces eggs that are released into the water column. After hatching, the eggs give rise to an oncomiracidium (larval stage). These larvae exhibit rapid swimming capabilities and utilize the water flow generated by the host’s gills to attach to a host, thus, continuing the life cycle (Figure 5).
3.4. Eggs
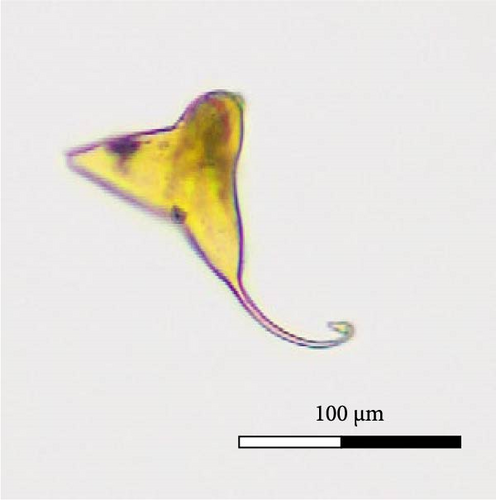
Calceostoma glandulosum eggs have a tetrahedral shape, with dimensions 79 ± 7 µm on each side. At each extremity, there is a filament measuring 54 ± 6 µm in length, rounded at its ends, except for one end which is “Y” shaped. Additionally, at one extremity, there is an opercular pole that facilitates the hatching of larvae. On correct embryo development, the egg takes on an opaque brownish coloration due to the presence of oncomiracidium. Conversely, it becomes translucent after hatching or if the egg is damaged (Figure 6).

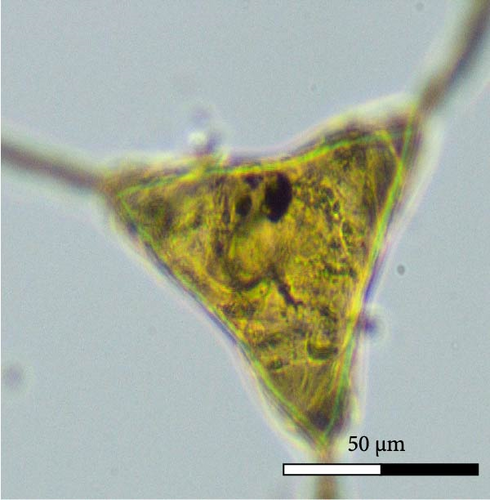

3.5. Oncomiracidium
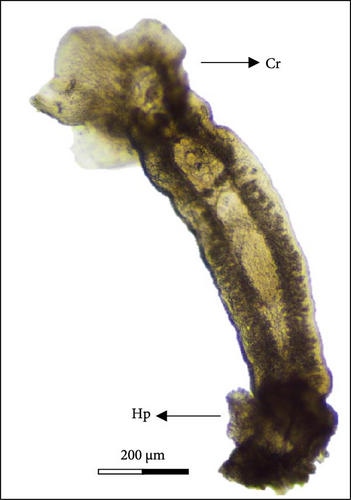
The oncomiracidium of C. glandulosum exhibits an oval shape with a length of 100 ± 15 µm and a width of 37 ± 9.3. Generally, it hatches when exposed to an optimal temperature of 23°C for 24 h and has a survival time of approximately 25.91 ± 2.7 h without a host. After hatching, these organisms display a fast-swimming behavior, aided by several sensilla (filaments around the body) arranged on the lateral and posterior areas of the body. In the Cr, the oncomiracidium possesses four ocular points and at the opposite pole, on the Hp, it presents two Ha hooks. No marginal hooks were observed under the light microscope (Figure 7).
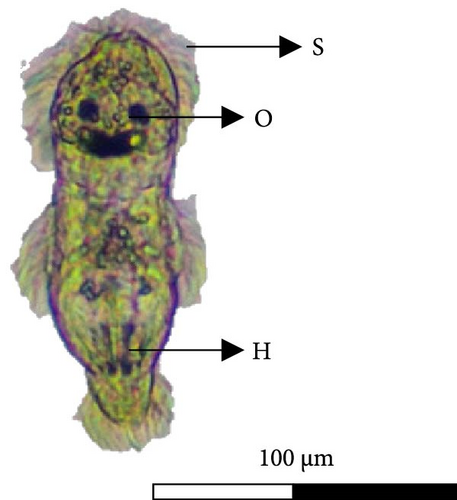
3.6. Temperature Effect
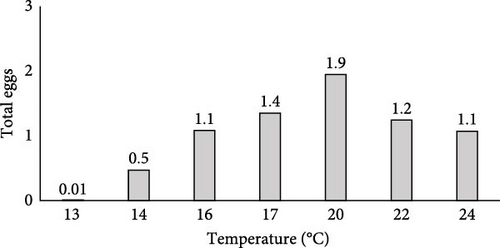
3.6.1. Egg Occurrence
In the broodstock tanks, the temperature fluctuated over the sampling months of this study. At 13°C, there was minimal egg occurrence (0.01 eggs/cm/day). There was a positive correlation between the temperature and the egg’s occurrence, with the peak being reached at 20°C (1.9 eggs/cm/day). At temperatures above 20°C (22 and 24°C), the egg occurrence tended to decrease (Figure 8).
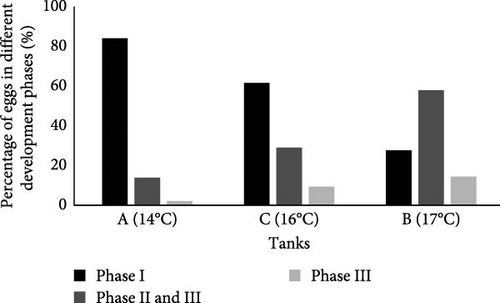
3.6.2. Egg Hatching
It was observed that water temperature not only influenced the reproduction of C. glandulosum, resulting in lower egg quantities but also impacted its embryo viability (Figure 9). A direct correlation between temperature and egg hatching was noted, with a significant decrease in egg production at lower temperatures. Eggs in Phase I (unviable) exhibit a consistent pattern: egg hatching increased as temperatures decreased. The lowest temperature tested (14°C) impacted egg hatching capacity, with almost nonexistent hatching rates (2%). This rate increased slightly (8%) at 16°C and further at 17°C (14%). The number of embryonated or developing eggs (Stage II and III) reached its peak at 17°C (58%), decreased at 16°C (29%), and decreased again at 14°C (14%).
3.6.3. Egg Incubation
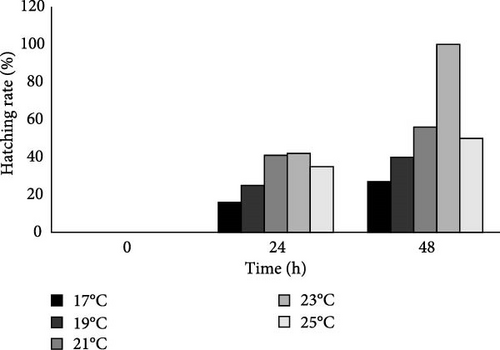
The moment 0 h corresponds to the time when the eggs were set to hatch under in vitro conditions. After 24 h, it was observed that eggs had hatched at all temperatures, although at a temperature of 17°C, the hatched eggs were lower, followed by 19°C. The temperature of 23°C had a maximum hatching rate similar to the eggs exposed to a temperature of 21°C. At 25°C, the hatching rate was lower than at 21°C and 23°C.
The highest in vitro hatching rate was observed at 23°C with 100% of eggs hatching after 48 h. As the temperature was reduced, the hatching rate decreased: hatching rates of 56%, 40%, and 27%, at 21, 19 and 17°C, respectively. As the temperature increased to 25°C, a decrease in hatching rate (50%) was also observed (Figure 10).
3.7. Fecundity
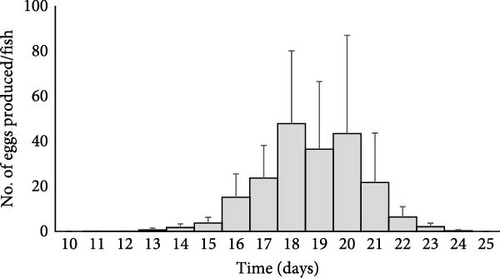
C. glandulosum reaches its sexual maturity between days 11 and 14, after infection on A. regius, corresponding to the initial appearance of eggs in the water column. The organism exhibited an increasing egg production, peaking on day 18 (287 ± 194 eggs per parasite). However, this fecundity capability then declined, and no additional eggs were produced 25 days postinfection. Therefore, the fecundity period lasted approximately 10 days at 23 °C, starting from the onset of egg production until production ceased (Figure 11).
3.8. Evaluation of C. glandulosum Species-Specificity
Counting the number of C. glandulosum eggs in the different broodstock tanks and earthen ponds with various fish species allowed the determination of C. glandulosum specificity to certain hosts. Calceostoma glandulosum eggs were not observed in broodstock tanks of S. aurata, D. sargus and S. dumerili, being found exclusively in A. regius (Table 2). In the earthen ponds, C. glandulosum was only found in A. regius with an average intensity of 3.83 ± 2.1 parasites per host. These parasites were primarily distributed in the first two gill arches on both sides of the fish.
| Broodstock tanks | Aa,b | Ba,b | Cb | Da | Ea | Fa |
|---|---|---|---|---|---|---|
| No. of eggs/cm/day | 0.33 ± 0.14 | 0.35 ± 0.11 | 0.98 ± 0.25 | 0 | 0 | 0 |
- Note:a,b,c represents the pairs that present statistically significant differences.
4. Discussion
This study represents the first report of C. glandulosum in A. regius, observed in intensive and semi-intensive aquaculture systems. In this study, C. glandulosum was exclusively found in fish gills. However, according to Hayward et al. [22], this parasite can also be found in the oral cavity and fins of the host. On the other hand, no parasites were observed in the skin and fins.
In Table 3, the measurements for the structures of various Calceostoma species and K. polyorchis from studies conducted by different authors can be found, for comparison with the species under study. Williams [15] mentions that C. glandulosum has two congeners, which differ in size, and both parasitize the wild A. hololepidotus species. The Calceostoma calceostoma parasites found in Tunis had an average length of 3295 µm (ranging from 2875 to 4002), while those found in the Bay of Biscay, referred to as Calceostoma herculana, had an average length of 6302 µm. Calceostoma herculana found in Morocco had an average total length of 2500–3500 µm. In contrast, C. glandulosum in Australia measured around 2724 µm (ranging from 1848 to 4176 µm). One of the distinctive features among species of this family is their size variability. In this study, we observed that the dimensions of C. glandulosum were smaller than those of C. calceostoma and C. herculana. Although Williams [15] reported C. glandulosum on A. hololepidotus in Western Australia, we found it on A. regius in southern Portugal, suggesting potential specificity to the same host genus in different geographical regions.
| Species | C. glandulosum | C. glandulosum | C. glandulosum | C. calceostoma | C. calceostoma | Calceostoma sp. | Ktariella polyorchis |
|---|---|---|---|---|---|---|---|
| Reference | This study | Williams [15] | Amakali et al. [17] | Williams [15] | Williams [15] | Amakali et al. [17] | Tedesco et al. [13] |
| Local | Algarve, Portugal | Wetern Australia | Northen Namibia | Tunis | Gulf of Gascoigne | Northen Namibia | Croatia |
| Host | Argyrosomus regius | Argyrosomus hololepidotus | Argyrosomus inodorus | Argyrosomus regius | Argyrosomus regius | Argyrosomus inodorus | Argyrosomus regius |
| Body length | 2230 (1100–3990) | 2724 (1848–4176) | 6250 (4200–8000) | 3295 (2875–4002) | 6302 | 4633 (2900–6900) | 3421 (2529–4806) |
| Body width | 477 (190–660) | 748 (592–960) | 1000 (900–1100) | 632 (598–644) | 759–1012 | 566 (400–800) | 687 (498–1035) |
| Lappet max width | 814 (613–940) | 794 (504–1296) | 1225 (200–1900) | 776 (644–897) | 943 | 2000 | — |
| Pharynx length | 197 (191–203) | 210 (157–272) | 289 (236–322) | 202 (17–216) | 273 (255–291) | 303 (293–312) | 262 (202–355) |
| Pharynx width | 158 (149–167) | 168 (132–208) | 270 (226–342) | 164 (156–179) | — | 276 (260–292) | 201 (141–255) |
| Penis length | 106 (85–145) | 182 | 188 (178–195) | 134 (124–152) | 189 (179–209) | 183 (172–202) | — |
| Penis width | 24 (18–30) | 23 | 24 (21–26) | 14 (12–14) | 19 (14–23) | 12 (11–13) | — |
| Hamulus length | 140 (108–170) | 129 (112–139) | 199 (188–213) | 94 (71–106) | 135 (124–147) | 143 (128–159) | — |
| Median bar length | 165 (123–198) | 238 (236–243) | 146 (132–172) | 178 (154–207) | 239 (230–251) | 146 (132–172 | — |
| Median bar width | 27 (21–34) | — | 36 (32–39) | — | — | — | — |
Amakali et al. [17] also reported the occurrence of C. glandulosum in northern Namibia, in A. inodorus, due to the structures of this population being consistently larger compared with the present study. This size difference is observed in several important morphological structures, such as body length and width, Ph length and width, P length, as well as the Ha and Mb. For example, while the C. glandulosum described in this study has an average body length of 2230 µm, the specimens from Namibia reach 6250 µm, a substantial difference that can be attributed to factors such as adaptation to different environmental conditions or host interaction. These variations suggest that the morphology of C. glandulosum may be highly influenced by geographical and ecological factors, raising questions about the phenotypic plasticity of the species or even the possibility of significant intraspecific differentiation among populations [40]. Tedesco et al. [13] described a species morphologically similar to monogeneans of the genus Calceostoma, called K. polyorchis, which differs in having multiple testes with evident structures, unlike C. glandulosum, which has only one T, as observed in this work. While morphological characteristics are crucial for monogenean identification, molecular techniques help clarify possible doubts in identification. In this case, we confirmed the species as C. glandulosum, supported by the high sequence similarity in the phylogenetic tree. Furthermore, another method of species’ identification in monogeneans is by examining the shape of their eggs. The C. glandulosum eggs in this study exhibited a tetrahedral shape, with an average size of 79 ± 7 µm, and filaments measuring 54 ± 6 µm at each extremity. These filaments had rounded tips at their ends, except for one that ended in a “Y” shape. The characteristics described, such as the tetrahedral shape of eggs with filaments, are shared by other species within the same family Calceostomatidae. For instance, Calceostomella inermis, typically found in Sciaena umbra and Umbrina cirrose [41, 42] and K. polyorchis in farmed A. regius in Croatia [13], exhibit similar egg morphology. Hayward et al. [22], also noted the tetrahedral egg shape of C. glandulosum in A. japonicus, but did not describe the positions of filaments or other characteristics in detail. It is important to recognize that many species within the monogenean group exhibit a diverse range of egg forms including spherical (e.g., Dactilogyrus vastator), ovoid (e.g., Paracaesicola nanshaensis n. gen), fusiform (e.g., Heterobothrium okamotoi), tetrahedral (e.g., Neobenedenia sp.), or urn-shaped (e.g., Neoentobdella natans) [43–46]. Monogenean species exhibit considerable variability in egg size and morphology, indicative of specific adaptations to their respective environments and the life cycles of their hosts. Kearn [47] suggests that these morphological variations predominantly arise from selective environmental pressures. Eggs from related species that develop in different habitats frequently exhibit adaptations aimed at optimizing their survival and developmental efficacy under fluctuating conditions. Factors such as egg shape and size are fundamental to reproductive success. Shape influences the relationship between surface area and volume, as well as the distance between the surface and the center of the egg, parameters that affect the diffusion of gases and nutrients. In hypoxic environments, such as specific sedimentary substrates where eggs may be deposited, natural selection may preferentially favor egg shapes that enhance gas exchange efficiency, particularly in larger eggs that encounter greater diffusion challenges. Furthermore, the mechanical integrity of the eggs is paramount for their protection against predation and in dynamic substrates such as sandy sediments. Egg morphology can also influence sedimentation rates, a factor of considerable relevance for monogenean parasites associated with pelagic hosts. These variables suggest that the morphological characteristics of monogenean eggs result from a complex interplay of evolutionary factors, adapting to diverse environmental exigencies.
The embryonic development process among monogeneans with oviparity as a reproductive strategy is similar across species. It typically involves four distinct stages, as described by Militz et al. [33] and Maciel, Muniz, and Alves [48], providing a common framework for understanding the development of these parasites.
To date, no comprehensive record has been detailing the morphological characterization, fecundity, and survival of the oncomiracidium stage of C. glandulosum. After completing its development within the egg, the ciliate oncomiracidium is released, embarking on its free-swimming phase to locate a suitable host before depleting its energy reserves or reaching the end of its lifespan [9]. According to our findings, the lifespan of C. glandulosum was approximately 26 h posthatching at 23°C, providing sufficient time for the parasite to locate a host at this temperature. Lifespan studies of oncomiracidium in other monogenean species have shown similar results. Repullés-Albelda et al. [49] reported that the lifespan of most oncomiracidium typically does not exceed 48 h. In contrast, Zeuxapta seriolae has a longer lifespan of approximately 4 days at 20°C [50]. Mladineo et al. [51] observed that S. chrysophrii oncomiracidium could survive in vitro for a minimum of 11.9 h and a maximum of 52 h, while Hirazawa, Kitagawa, and Hirata [50] found that survival at 20°C ranged from 2 to 52 h. These findings suggest that the larval development and hatching of S. chrysophrii are modulated by environmental factors, particularly temperature, which enhances parasite–host coordination and increases infection success. Despite the existing studies, the knowledge regarding the lifespan of oncomiracidium in various monogenean species remains incomplete. Therefore, the lifespan observed for C. glandulosum falls within the range of values published for other monogenean species, indicating consistency with the known characteristics of this life stage among related parasites.
Environmental factors, particularly temperature, play a crucial role in influencing egg production, larval hatching, and the overall life cycle of oncomiracidium in monogeneans. Monogeneans exhibit specific optimal temperature requirements, and maintaining this optimal temperature for an extended period can prolong the window of infection, leading to increased generations of parasites [52]. Transmission and the incidence of these parasites are generally seasonal, showing fluctuations throughout the year [53]. Understanding their behavior at different temperatures is essential for comprehending how monogeneans are impacted by temperature fluctuations and how to effectively manage outbreaks of diseases caused by these parasites [34]. In this study, it was observed that C. glandulosum was indeed influenced by fluctuations in water temperature. There was a notable decrease in egg production during the transition from autumn to winter, corresponding to minimum temperature values (13°C). Egg production significantly increased again in early spring (20°C), but decreased once more with very high temperatures in summer (22 and 24°C). Similar temperature effects were noted in other monogenean species. For instance, increasing temperature resulted in a higher occurrence of N. girellae, and the number of eggs laid by parasites from S. dumerili reared at 30°C was greater than from those reared at 20 and 25°C [34]. Likewise, Discocotyle sagittata exhibited increased egg production with rising temperatures, ranging from 1.5 eggs/day at 5°C to 7 eggs/day at 13°C and 12 eggs/day at 20°C [54]. The examples highlight the intricate relationship between temperature and the reproductive activity of monogenean parasites.
The development of monogenean eggs follows a similar process across species, typically occurring in four stages as described by Maciel, Muniz, and Alves [48]. However, temperature fluctuations can significantly impact this development process, leading to reduced egg viability. In our study, we observed that as temperatures decreased during colder seasons (13–14°C), the viability of C. glandulosum eggs tended to decrease, increasing translucent or damaged eggs indicating unsuccessful embryo development. This decrease in viability also correlated with a reduction in hatched eggs and eggs in embryonated or developing stages. Research by Zhang et al. [55] noted that eggs of D. vastor exposed to very low temperatures like 5°C, failed to hatch completely, highlighting the challenges posed by extreme temperatures on embryo development. However, in the case of C. glandulosum, when eggs are laid at low but tolerable temperatures, they can be preserved with important energy reserves and lipids that promote larval longevity and successful development, particularly when environmental conditions become favorable [10]. Similar observations were made in other monogenean species such as D. sagittata, Protopolystoma orientalis, and Protopolystoma xenopodis, where viability significantly decreased when temperatures dropped below 10°C [54, 56]. These findings emphasize the sensitivity of monogenean eggs to temperature variations and their reliance on suitable environmental conditions for successful development.
The results from in vitro tests assessing the hatching rate of C. glandulosum at different temperatures revealed that the optimal hatching temperature is 23°C achieving a 100% hatching rate within 48 h. However, at 25°C, the hatching rate decreased by 50%. Despite the higher occurrence of eggs in tanks at 20°C, the most successful hatching rates occurred at 23°C under controlled conditions. The increase in water temperature correlated with a higher number of hatched eggs also indicating accelerated life cycle completion for the parasite due to increased metabolism and enhanced embryo development, facilitating continued host infection [10]. Similar temperature preferences have been observed in other monogenean species. For example, B. seriolae shows optimal egg hatching between 14 and 28°C [57], Z. seriolae exhibits hatching success between 13 and 18°C [58], and D. anchoratus hatches eggs between 13 and 26°C [59]. Achieving successful in vitro hatching involves considering not only temperature but also other crucial factors such as daily water renewals to increase dissolved oxygen levels, appropriate light intensity and photoperiod, and ensuring the absence of mechanical disturbances [11]. In our study, we maintained constant water temperature with a 12d:12n photoperiod and changed 1/3 of the water volume daily to maintain optimal conditions for the experiment.
Parasites exhibit high fecundity to increase the likelihood of their offspring successfully finding and infecting new hosts, necessitating the production of numerous eggs [38]. In this study, we determined the sexual maturity of C. glandulosum for the first time, observing that when a host is infected with a larva at a temperature of 23°C, the parasite matures over a period of 11–14 days and becomes capable of producing a large number of eggs over 10 days. As time progresses, the ability of adult parasites to produce eggs diminishes until they cease reproduction altogether. Fecundity and sexual maturation can be influenced by temperature [58], but this varies significantly across species. For instance, Benedenia seriolae lays eggs between 16 and 20 days at 21°C, Cichlidogyrus spp. reach sexual maturity after 4–6 days at 20–28°C, Dactylogyrus vastor can achieve sexual maturity by day 9–10 at 22°C, Heterobothrium ecuadori after 33 days at 20−23°C, and Z. seriolae at day 25 at 20−23°C [35, 55, 60, 61]. Each species has specific requirements and timelines for fecundity and sexual maturation, which can be crucial factors in understanding these life cycles and infection dynamics.
Monogeneans are widely recognized for their high specificity, referring to the exclusivity or limited occurrence of a particular parasite species within a single host species [62]. Upon collecting cotton strips from various broodstock species such as D. sargus, S. aurata, and S. rivoliana, and not finding any eggs, it suggests that C. glandulosum may not infect species other than A. regius, a conclusion supported by statistical analysis confirming host specificity for C. glandulosum. Furthermore, observations of other species of monogeneans, evidenced by the presence of different types of eggs, in the earthen ponds where C. glandulosum was absent, such as S. chrysophrii in S. aurata and other species in D. sargus, reinforce the specificity of C. glandulosum to A. regius. Regular sampling of fish from these earthen ponds did not reveal C. glandulosum on any fish species except A. regius even when multiple fish species coexisted in the same production pond. This finding further substantiates the conclusion that C. glandulosum demonstrates a high degree of specificity toward its host. Previous studies by other researchers have also highlighted specificity within genera, such as Lamellodiscus in Sparidae, Thaparocleidus in Pangasiidae, Cichlidogyrus in Cichlidae, Dactylogyrus in Cyprinidae, and Gyrodactylus in Gobiidae [63]. Therefore, based on this study, it can be confidently stated that C. glandulosum shows specificity for meagre (A. regius).
5. Conclusion
This study provides a detailed morphological characterization and describes the fecundity and survival characteristics of the oncomiracidium of C. glandulosum for the first time. The study identified three distinct stages in the life cycle: eggs with a tetrahedral structure and unique filament patterns, oncomiracidium with rapid swimming behavior aided by sensilla, and adults characterize by specific anatomical features such as a Hp and sucker for attachment to gills. Calceostoma glandulosum demonstrated specificity towards A. regius, as evidenced by its absence in other fish species, such as S. aurata, D. labrax, D. sargus, D. cervinus, E. marginatus, S. senegalensis, and S. rivoliana both in adult and egg stages. The study highlights the significant influence of temperature on the life cycle of C. glandulosum. Egg occurrence showed seasonal patterns, peaking between 14 and 24°C, with decreased viability below 14°C and optimal hatching rates observed at 23°C in vitro. Understanding the life cycle of monogeneans like C. glandulosum is crucial for assessing infection risks during different seasons and under various environmental conditions, aiding in the development of effective preventive strategies in aquaculture. Monitoring parasite occurrence using methods such as cotton strips in tanks offers a noninvasive and efficient means of parasite control, particularly in broodstock where obtaining gill samples is challenging, while also reducing the need for sacrificial sampling to manage these parasites.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The present work was financed by the projects SAUDE&AQUA (MAR-02.05.01-FEAMP-0009), SAUDE&AQUA II (MAR-021.1.3-FEAMPA-00018), Be4AQUAHEALTH (MAR-02.05.01-FEAMP-0013), EMSO-PT (POCI-01-0145-FEDER-022157), Fundação para a Ciência e Tecnologia (FCT) (UIDB/04292/2020), and MARE—Marine and Environmental Sciences Centre (https://doi.org/10.54499/UIDB/04292/2020 and https://doi.org/10.54499/UIDP/04292/2020) and the project granted to the Associated Laboratory ARNET (https://doi.org/10.54499/LA/P/0069/2020).
Acknowledgments
The present work was financed by the projects SAUDE&AQUA (MAR-02.05.01-FEAMP-0009), SAUDE&AQUA II (MAR-021.1.3-FEAMPA-00018), Be4AQUAHEALTH (MAR-02.05.01-FEAMP-0013), EMSO-PT (POCI-01-0145-FEDER-022157), Fundação para a Ciência e Tecnologia (FCT) (UIDB/04292/2020), and MARE—Marine and Environmental Sciences Centre (https://doi.org/10.54499/UIDB/04292/2020 and https://doi.org/10.54499/UIDP/04292/2020) and the project granted to the Associated Laboratory ARNET (https://doi.org/10.54499/LA/P/0069/2020).
Open Research
Data Availability Statement
All data is stored at IPMA according to the internal guidelines.




