Effects of Dietary Indian Sphagnum junghuhnianum Peat Moss Pectin on Growth, Digestive Enzymes, Antioxidant Capacity, Immune Responses, and Disease Resistance in Catla catla Fingerlings
Abstract
In this study, the effects of dietary supplementation of Indian Sphagnum junghuhnianum peat moss pectin were evaluated in Catla catla fingerlings. A total of 375 fish (10.47 ± 0.2 g) were randomly divided (n = 25/tank) into basal diet (Sphagnum peat moss pectin [SPMP] at 0% control) (crude protein: 37%, crude fat: 8%) or four treatment diets, 2% (SPMP2), 4% (SPMP4), 8% (SPMP8), and 16% (SPMP16) for 60 days. The water temperature was 27.5 ± 0.6°C, and fish were fed with the experimental diets at 4% of their live body mass. The results of this research revealed that the SPMP8 diet substantially enhanced growth indices in catla fingerlings (p < 0.05). The highest and lowest whole-body lipid levels were in SPMP8 (5.03%) and control (4.06%), respectively. The gut amylase activity was significantly higher in the SPMP8 group, while lipase and protease remained unchanged. Additionally, fish given 8% and 16% SPMP had a significant decrease in the liver’s malondialdehyde concentration. Superoxide dismutase (SOD) activity was increased in SPMP-supplemented groups, with the highest value in group SPMP8 while catalase (CAT) activity was higher in SPMP4, SPMP8, and SPMP16 compared to the other groups. Supplementing the diet with 4%–16% SPMP increased the fish’s red blood cell count and hemoglobin level. An 8% SPMP diet increased white blood cell count, phagocytic activity, lysozyme, acid, and alkaline phosphatase (AKP) activities compared to the other groups. The cumulative mortality of fish after challenge with virulent Aeromonas hydrophila significantly decreased in SPMP8 and SPMP16 groups, compared to the other treatments. The results of this study indicated enhancements in immunological markers, specifically an increase in levels of AKP, SOD, and CAT. This research proposes that providing catla fingerlings with 8% SPMP effectively boosts their growth, immune response, and disease resistance.
1. Introduction
Aquaculture is the fastest-growing sector of food production system due to its recent increase in global expansion [1]. In response to the increasing demand for fish products, aquaculture is expanding its share of total production to ensure a steady supply. According to the State of World Fisheries and Aquaculture (SOFIA), global production of fisheries and aquaculture rose to 223.2 million tonnes in 2022, marking a 4.4% increase compared to 2020. This total included 185.4 million tonnes of aquatic animals and 37.8 million tonnes of algae. Production of aquatic animals will increase by 10% by 2032, reaching an estimated 205 million tonnes [2]. This heightened demand is driven by population growth and a decline in capture fisheries [3]. However, despite efforts to meet this demand, the aquaculture industry has also caused significant environmental harm, leading to reduced land availability, microbial infections, water pollution, eutrophication, the use of harmful chemicals, and disruptions to the food chain [4]. Disease outbreaks continue to be a significant obstacle to sustainable production, even with a high level of intensification [5]. The prevalence of disease greatly impacts aquaculture productivity, and fish can be affected by a variety of diseases and stressors that reduce fish resistance [6]. Many would concur that adopting a preventative strategy for disease management is significantly more effective than having to respond to an outbreak after it has already taken place. The use of antibiotics or chemicals to eliminate external pathogens usually constitutes a reactive strategy. In contrast, prevention can be enhanced through vaccines or the use of high-quality functional feeds, such as various herbs (or their derivatives), probiotics, and seaweeds. An effective vaccine that targets a specific disease of concern is often the most preventing or reducing the impacts of disease; however, the route of administration, the use of effective adjuvants, and, most importantly, the lack of effective results are the main challenges of vaccination in fish health management [7]. According to a variety of recent studies, medical herbs are safe and environmentally friendly dietary additives that enhance growth and immune function [5, 8].
A widespread freshwater carp in South Asian nations, Catla catla, is highly profitable [9]. With more than 4 million metric tons harvested in 2022, catla ranks as the seventh most produced aquatic species worldwide [10]. In Bangladesh’s and India’s freshwater aquaculture sectors, C. catla is the second most popular native carp species because of its high market value as well as good flavor [11]. Around the world, carp are the most significant group of freshwater fish used as study models and as food sources. According to Sanyal et al. [11], the two most common bacteria found in carp cultures are Aeromonas and Pseudomonas. A. hydrophila is a bacterium that has been extensively studied since it can be found in water [12], food [13], and estuaries [14], is resistant to antibiotics, and can infect humans and animals [15]. According to recent studies, the primary cause of many infections is motile Aeromonas species, particularly A. hydrophila [6]. Numerous virulence factors, including hemolysins, proteases, and aerolysin-related enterotoxins, are responsible for the pathogenic manifestation of A. hydrophila. These factors can cause apoptosis, which can result in death, hemorrhagic septicemia, dropsy, exophthalmia, and tail and fin rot [6, 16, 17]. A. hydrophila enters the fish body through the gills and damaged skin [18], causes macrophage apoptosis [19], and destroys the intestinal mucus layer [20], according to several studies on other fish species. These infections reduce the effectiveness of the intestinal mucosa in defending against this pathogen.
Sphagnum is a quite distinct kind of plant that belongs to the Bryophyta group of mosses. They are commonly found in moist and wetland environments. Their ability to retain water allows them to keep the ground in their habitats high in humidity and at an ideal acidity, which speeds up the decomposition process and leads to the accumulation of peat [21]. Sphagnum species are estimated to range between 350 and 500 around the world [22]. According to studies, Sphagnum junghuhnianum, an Indian peat moss, can lower blood sugar and plasma cholesterol, prevent colon cancer, and increase wound healing, among additional positive health effects [23]. Consequently, the use of peat moss can be beneficial for both industrial and agricultural applications [24, 25], and it can also be used for the creation of products with value-added and significant advantages for health [26, 27]. The antimicrobial abilities of S. papillosum [28], S. fallax [29], and S. junghuhnianum [30] have all been investigated.
Pectin is a polysaccharide high in galacturonic acid that can be globally derived from fruits and other plants [31, 32]. Due to its low toxicity and therapeutic properties, pectin and pectin-based composites have been the subject of substantial research in the culinary, medical, and cosmetic industries. Consuming plants containing antioxidants has been shown to prevent different diseases [33]. Pectin has been shown to have anticancer, antisenescence, and plasma cholesterol-lowering therapeutic effects in humans, according to pharmacological research [34, 35]. Immune reactivity affects the use of pectin in drug delivery, tissue engineering, and biological applications [36–38]. Pectin and other immunomodulators are used to control reactivity and boost the efficacy of applications requiring changes to the immune system, not to inhibit the immune response. Previous studies have shown that pectin can decrease inflammatory reactivity by increasing anti-inflammatory cytokines and decreasing the production of proinflammatory cytokines [39]. Dietary pectin has also been shown to have positive effects on disease resistance, innate immune responses, and improved growth performance in a change of aquaculture hosts, including juvenile rainbow trout (Oncorhynchus mykiss) [40], pearl gentian grouper (Epinephelus fuscoguttatus × E. lanceolatus) [31], common carp (Cyprinus carpio) [41], and white-leg prawn (Litopenaeus vannamei) [42]. In particular, in the current study, pectin was extracted from S. junghuhnianum Indian peat moss for the first time as a feed additive for the Indian major carp, C. catla. This can improve the nutritional value of the fish diet, which may improve growth performance and overall health while also supporting sustainable aquaculture strategies.
2. Materials and Methods
2.1. Collection and Extraction of Sphagnum Peat Moss Pectins (SPMPs)
S. junghuhnianum peat moss was collected from Darjeeling, West Bengal, India. The gathered peat moss was rinsed several times with tap water to eliminate soil and dirt particles after being cleaned with deionized water. An electronic blender was used to grind the peat moss after it had been dried for 24 h at 70 °C. With some slight modifications, the pectin extraction technique was based on the Kratchanova et al. [43] method. 1 L of distilled water was mixed with 100 g of dried Sphagnum peat moss to extract it. Using 0.5 M HCl, the pH was reduced to 1.5. The mixture was boiled at 80°C for an hour with frequent stirring. Following cooling, the extract was centrifuged (8000 rpm) in a 50 mL centrifuge tube for 10 min at 4°C. After gathering the pellet and mixing it with the same amount of 96% (v/v) ethanol, it was rinsed three times with 96% ethanol to get rid of the mono- and disaccharides, and then it was dried in an oven at 60°C until it was needed.
2.2. Experimental Diet
Five isolipidic (8% crude lipid) and isoproteic (37% of crude protein) diets were formulated to contain graded levels of SPMP (0%, 2%, 4%, 8%, and 16%) (Table 1). Previous studies found that 30%–40% dietary protein content was an appropriate protein content for catla fingerlings [44–46]. Ingredients and proximate composition of the four experimental diets are given in detail (Table 1). All the ingredients were thoroughly mixed until they were homogenous in the mixer. After mixing SPMP into the basal food slowly and evenly in a drum mixer, and allowed to air dry for 24 h in a clean, dry space. The prepared diets were kept at −20°C until the experiment was conducted.
| Sphagnum peat moss pectin levels (%) | |||||
|---|---|---|---|---|---|
| Ingredients | SPMP0 | SPMP2 | SPMP4 | SPMP8 | SPMP16 |
| Soybean meal | 35 | 35 | 35 | 35 | 35 |
| Fish meal | 10 | 10 | 10 | 10 | 10 |
| Rice polish | 10 | 10 | 10 | 10 | 10 |
| Wheat starch | 15.5 | 14 | 13.5 | 11.5 | 7.5 |
| Wheat bran | 8 | 8 | 8 | 8 | 8 |
| Soybean oil | 2 | 2 | 2 | 2 | 2 |
| Binder | 15 | 14.5 | 13 | 11 | 7 |
| Vitamin and mineral mixa | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Chromic oxide | 1 | 1 | 1 | 1 | 1 |
| Ascorbic acid | 1 | 1 | 1 | 1 | 1 |
| SPMP (%) | 0 | 2 | 4 | 8 | 16 |
| Chemical composition (%) | |||||
| Crude protein | 37.11 | 37.24 | 37.62 | 37.75 | 37.12 |
| Total lipid | 8.23 | 8.31 | 8.34 | 8.22 | 8.09 |
| Moisture | 7.12 | 7.23 | 7.35 | 7.43 | 7.65 |
| Ash | 11.13 | 11.20 | 11.32 | 11.41 | 11.49 |
| NFEb | 36.41 | 36.02 | 35.37 | 35.19 | 35.65 |
| Gross energy (MJ kg−1) | 12.40 | 11.51 | 12.27 | 10.34 | 13.19 |
- aComposition of the mixture per kg dry weight (Suplevite M obtained from Sarbahi chemicals, Wadi, Baroda-390007, India); Vit A (700,000 IU), Vit D3 (70,000 IU), Vit E (250 mg), Nicotinamide (1000 mg), Co (150 mg), Cu (1200 mg), Io (325 mg), Fe (1500 mg), Mn (1500 mg), K (100 mg), Se (10 mg), Na (5.9 mg), S (0.72%), Zn (9600 mg), Ca (25.5%), P (12.75%).
- bNitrogen-free extract = 100 – (protein + lipid + moisture + ash).
2.3. Experimental Fish and Design
A commercial fish farm in Dimapur, Nagaland, India, provided the C. catla fingerlings (10.47 ± 0.2 g), and their health was assessed immediately as they arrived. For 14 days before the experiment, the fish were acclimated in 500 L aerated cement tanks. Totally 375 fish were split into five groups (25 fish/tank) in triplicate 180-L reinforced plastic containers. For 60 days, the groups were fed diets supplemented with 0%, 2%, 4%, 8%, and 16% SPMP twice daily (09.00 and 17.00) at a rate of 4% of their body weight. The fish were acclimated to the tank conditions (temperature 27.54 ± 0.6 °C, dissolved oxygen 5.33 ± 0.31 mg/L, pH 7.5 ± 0.4, salinity 0 ppt, and ammonia less than 0.01 mg/L) for 2 weeks. About 20% of the water from each tank was replaced with fresh dechlorinated water every day in the process of siphoning out the feces and uneaten feed.
2.4. Sampling Procedure
All fish were fasted for 24 h before weighing and sampling, at the beginning and end of the trial, and all the fish were individually weighed [47]. After biometry, a 1.0 mL hypodermal syringe was used to draw blood samples (n = 9/tank) from the caudal vein. For hematological analysis, a part of the blood sample was placed into a tube containing an anticoagulant (2.7% EDTA) and kept at 4°C. To collect the serum, the remaining blood was placed in a tube, allowed to stand at room temperature for 1 h, then centrifuged at 5000 g, and then kept in a freezer at −20°C. The liver, gut, and whole body of the three fish from each replicate were removed and stored at −80°C.
2.5. Composition Analysis
The proximate chemical composition of fish samples and experimental diets was examined using the procedures of the Association of Official Analytical Chemists (AOAC) [48]. In brief, samples were dried in an oven (model: LHAON-95; Labindia Instruments, Thane, Maharashtra, India) at 105°C until they obtained an equivalent weight that allowed them to quantify the moisture content. The total amount of ash was determined by burning it in a muffle furnace (Model: TC303, Hasthas Scientific Instruments India (P) Ltd, Chennai, Tamil Nadu, India) at 550°C for 8 h. The standard Kjeldahl method (model: STXKJD3; Stericox India Pvt. Ltd, New Delhi, India) was used to assess crude protein. To determine the crude lipid, the Soxhlet extractor (model: ACM-54097-W; ACMAS Technologies Pvt. Ltd, Haryana, India) method was used [48].
2.6. Digestive Enzyme Activity
For each container, three gut samples were sliced and homogenized in 0.9% saline at 1:9 (m/v) ratio. The supernatant was then collected by centrifuging the samples at 3500 g for 15 min at 4°C, and it was stored at −80°C for further analysis. 50 µL of the tissue homogenate and 250 µL of a 1% soluble starch solution were combined and incubated for 30 min at 37°C to measure amylase activity. Before measuring the absorbency at 540 nm, an aliquot of 0.5 ml of 1% dinitro-salicylic acid was added, heated for 5 min, cooled, and then 5 ml of distilled water was added [49]. In accordance with Rúa et al. [50], lipase activity was measured. In short, 20 µL of the sample, 60 µL of p-nitrophenyl butyrate (50 mM), and 1 mL of buffer (50 mM Tris-HCl, pH 8.0, with 4% ethanol) were combined to create the mixture. For 5 minutes, at 30-s intervals, the rate of p-nitrophenyl butyrate (p-NPB) hydrolysis was recorded at 405 nm. The measurement of protease activity was based on Folin and Ciocalteau [51], which used casein (Sigma–Aldrich) as the substrate at pH 8.0 and the Folin reagent to quantify the reaction product. Activities of the enzymes amylase, lipase, and protease were expressed as U/mg protein.
2.7. Antioxidant Capacity
A 0.4 M Tris buffer solution with a pH of 7.0 was used to homogenize 100 mg of liver samples, which were afterward centrifuged for 12 min at 6,000 g. The supernatant was stored at −20°C. We used commercial kits provided by Nanjing Jiancheng Company (Nanjing, China) to measure antioxidant levels. By measuring the samples’ capacity to prevent superoxide radical-dependent reactions at 550 nm, the activity of superoxide dismutase (SOD) was determined [52]. By detecting hydrogen peroxide reduction at a wavelength of 405 nm, catalase (CAT) activity was determined [53]. Malondialdehyde (lipid peroxidation) was determined using the technique described by Yu et al. [47].
2.8. Hemato–Immunological Parameters
About 20 mg/L of tricaine methanesulfonate (Sigma) was used to anesthetize 12 fish per tank [54]. Hematological parameters, including white blood cells (WBCs), red blood cells (RBCs), and hemoglobin (Hb) content, were determined according to standard methods described by Blaxhall and Daisley [55]. To determine respiratory burst activity, 100 µL of blood containing an anticoagulant was transferred into a 96-well plate, and then a similar amount of 0.2% NBT solution was added to the plate to determine the nitro blue tetrazolium chloride (NBT) activity. A glass tube containing 1 mL of N, N-dimethylformamide was filled with 0.05 mL of NBT blood suspension, which was then centrifuged at 800 g for 3 min at 4°C after 30 min of room temperature incubation. At 540 nm, the OD was determined using spectrophotometry [56]. Serum lysozyme activity, a turbidometric test, was carried out slightly differently from Demers and Bayne’s [57] report. The initial step was to fill a 96-well plate with 200 μL of lyophilized Micrococcus lysodeikticus (MTCC 4598, IMTECH, Chandigarh, India) suspension in PBS (0.75 mg/mL, pH 6.4, 0.1 M) and then thoroughly mix the suspension with 20 μL of serum. At room temperature, absorbance was measured at 570 nm at 0 and 30 min using a microplate reader. About 0.1 mL of blood samples was combined with 1 × 107 cells of formalin-killed A. hydrophila in a sterile microplate for the phagocytosis assay. The well was thoroughly mixed, and the mixture was then incubated for 30 min at 25°C. Fifty μL of the blood-bacteria suspension was applied to three glass slides after it had been gently mixed after incubation. The smears were allowed to air dry before being fixed in 95% ethanol, redried, and stained using May-Grunwald’s Giemsa. Both the phagocytosed bacteria and the phagocytic cells were counted [58]. The commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to determine the total alkaline phosphatase (AKP) and acid phosphatase (ACP) levels using colorimetric methods following the manufacturer’s instructions [59].
2.9. Challenge Test
The source of A. hydrophila (MTCC 1799) was IMTECH in Chandigarh, India. Tryptone Soya Agar (TSA) was used as the medium for the bacterial culture. A. hydrophila (1 × 107 cells/mL) was injected intraperitoneally into 12 fish from each group following the feeding trial’s 60-day duration. For 14 days, fish mortality was monitored. According to Sattanathan et al. [60], the RPS was calculated as follows: RPS = [1 – (% mortality in dietary treatment/% mortality in control)] × 100.
2.10. Statistics
The dataset’s homogeneity of variance and normality were evaluated using the Levene and Shapiro–Wilk tests, respectively. Following that, using IBM SPSS software (SPSS Inc., version 21, Armonk, NY: IBM USA), all the data were analyzed into a one-way ANOVA test with Duncan’s multiple range test. For statistical significance, a p-value of less than 0.05 was used, and the means are shown as mean ± standard error (SE).
3. Results
3.1. Growth Performance
After 60 days, the growth and feeding performance of C. catla fed various doses of SPMP-enriched diet at 2%, 4%, 8%, and 16% were analyzed (Table 2). Feeding fish with various doses of dietary SPMP up to an 8% diet showed a significant increase in fish growth performance, but at 16%, the growth parameters decreased. Among the treatments, fish fed above 8% dietary SPMP exhibited a significant increase in weight gain (p < 0.05). There was no significant difference in FCR (p > 0.05). No mortality occurred throughout the experiment in SPMP4, SPMP8, and SPMP16 dietary groups. The highest survival rate (94.5%) was found in the control compared to SPMP dietary groups (p < 0.05).
| Sphagnum peat moss pectin levels (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | SPMP0 | SPMP2 | SPMP4 | SPMP8 | SPMP16 | p-Value |
| IBW (g)a | 10.76 ± 0.33a | 10.29 ± 0.11a | 10.41 ± 0.07a | 10.46 ± 0.06a | 10.42 ± 0.05a | 0.374 |
| FBW (g)b | 20.91 ± 1.23a | 23.69 ± 0.59b | 26.71 ± 0.64c | 29.43 ± 0.42d | 27.66 ± 0.40cd | 0.001 |
| RGR (%)c | 94.58 ± 12.7a | 130.22 ± 7.09b | 156.57 ± 5.52c | 181.39 ± 4.84c | 165.32 ± 4.06c | 0.001 |
| SGR (%)d | 1.10 ± 0.10a | 1.38 ± 0.05b | 1.56 ± 0.03bc | 1.72 ± 0.02c | 1.62 ± 0.02c | 0.001 |
| FCRe | 1.83 ± 0.23a | 1.65 ± 0.10a | 1.52 ± 0.06a | 1.46 ± 0.04a | 1.62 ± 0.01a | 0.854 |
| SR (%)f | 94.6 ± 1.33a | 98.6 ± 1.3b | 100 ± 0.0b | 100 ± 0.0b | 100 ± 0.0b | 0.005 |
- Note: Values are means ± SE of three replicates. Mean values with different letters in the same row are significantly different (p < 0.05).
- aInitial body weight.
- bFinal body weight.
- cRelative growth rate (RGR; %) = 100 × (final weight − initial weight/initial weight).
- dSpecific growth rate (SGR; %) = 100 × (ln [mean final body weight] − ln [mean initial body weight])/days.
- eFeed conversion ratio (FCR) = dry feed intake/wet weight gain.
- fSurvival rate (SR; %) = 100 × (final no. of fishes − initial no. of fishes)/initial no. of fishes.
3.2. Body Proximate Composition
There were no significant differences in the five diet groups’ muscle moisture, ash, and crude protein contents. Whole-body lipid levels increased with SPMP level up to 8%, then decreased to 16% (Table 3; p > 0.05).
| Sphagnum peat moss pectin levels (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | SPMP0 | SPMP2 | SPMP4 | SPMP8 | SPMP16 | p-Value |
| Crude protein | 15.02 ± 0.24a | 15.24 ± 0.58a | 15.59 ± 0.28a | 16.01 ± 0.62a | 15.67 ± 0.70a | 0.710 |
| Crude lipid | 4.06 ± 0.20a | 4.61 ± 0.15bc | 4.82 ± 0.09bc | 5.03 ± 0.05c | 4.44 ± 0.09ab | 0.004 |
| Moisture | 76.23 ± 0.03a | 76.41 ± 0.16a | 76.55 ± 0.17a | 76.53 ± 0.15a | 76.33 ± 0.18a | 0.538 |
| Ash | 2.79 ± 0.02a | 2.68 ± 0.08a | 2.74 ± 0.06a | 2.86 ± 0.13a | 2.80 ± 0.09a | 0.698 |
- Note: Values are means ± SE of three replicates. Mean values with different letters in the same row are significantly different (p < 0.05).
- Abbreviation: SPMP, Sphagnum peat moss pectins.
3.3. Digestive Enzyme Levels
The amylase activity was significantly enhanced with SPMP8 and SPMP16 dietary groups compared to the control (p < 0.05). However, lipase and protease concentrations did not differ significantly in any groups (Table 4).
| Sphagnum peat moss pectin levels (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | SPMP0 | SPMP2 | SPMP4 | SPMP8 | SPMP16 | p-Value |
| Digestive enzymes | ||||||
| Lipase (U/mg prot) | 0.84 ± 0.17a | 0.96 ± 0.03a | 1.03 ± 0.16a | 1.36 ± 0.40a | 0.99 ± 0.03a | 0.177 |
| Amylase (U/mg prot) | 3.77 ± 0.24a | 4.87 ± 0.18b | 5.56 ± 0.28bc | 5.79 ± 0.33c | 5.16 ± 0.12bc | 0.001 |
| Protease (U/mg prot) | 2.21 ± 0.05a | 2.31 ± 0.06a | 2.38 ± 0.06a | 2.45 ± 0.20a | 2.04 ± 0.05a | 0.085 |
| Antioxidant enzymes | ||||||
| SOD (U/mg prot) | 6.07 ± 0.15a | 7.43 ± 0.20b | 8.50 ± 0.09c | 9.40 ± 0.14d | 8.73 ± 0.15c | 0.001 |
| CAT (U/mg prot) | 73.57 ± 0.58a | 75.61 ± 0.44a | 81.27 ± 1.20b | 83.84 ± 0.79b | 82.87 ± 1.81b | 0.001 |
| MDA (nmol/g tissue) | 2.43 ± 0.15a | 2.41 ± 0.04a | 2.28 ± 0.06a | 2.13 ± 0.01b | 2.24 ± 0.09b | 0.296 |
- Note: Values are means ± SE of three replicates. Mean values with different letters in the same row are significantly different (p < 0.05).
- Abbreviations: CAT, catalase; MDA, malondyaldehyde; SOD, superoxide dismutase.
3.4. Antioxidant Capacity
The SOD was increased in SPMP-supplemented groups compared to the control, with the highest activities in SPMP8 (Table 4). CAT activity increased in SPMP4, SPMP8, and SPMP16 groups compared to the other treatments. Regarding lipid peroxidation, MDA contents were noticeably reduced in groups SPSP8 and SPSMP16 as compared to the other treatments (p > 0.05).
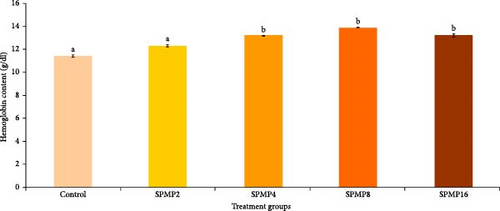
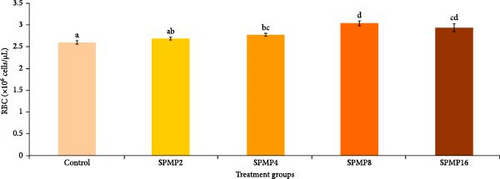
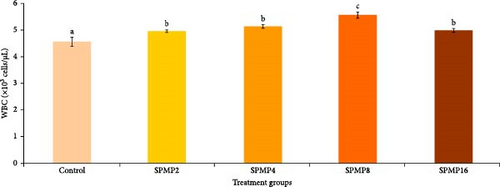
3.5. Hemato–Immunological Parameters
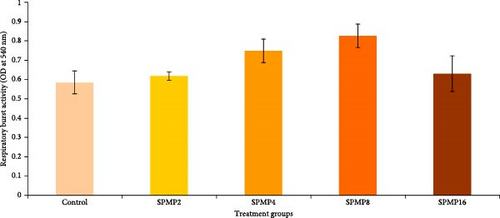
Blood hemoglobin (Figure 1A) and RBC counts (Figure 1B) increased markedly in the SPMP4, SPMP8, and SPMP16 groups compared to the other treatments (p < 0.05). WBCs significantly increased in SPMP-supplemented groups, and SPMP8 had the highest WBC count (Figure 1C). When comparing the dietary groups to the control, the respiratory burst activity findings revealed no statistically significant differences (p > 0.05; Figure 2A). Lysozyme activity (Figure 2B) in fish fed SPMP-supplemented diets was higher than in the control. Phagocytosis activity (Figure 2C) in SPMP8 was higher than in the other experimental groups (p < 0.05). The ACP and AKP activities in fish-fed SPMP4, SPMP8, and SPMP16 diets were higher than in the other treatments (Figure 2D,E).
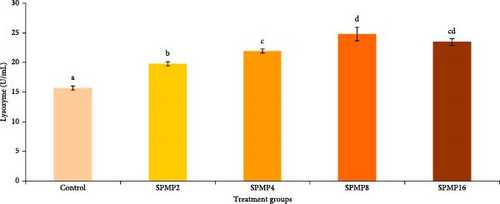
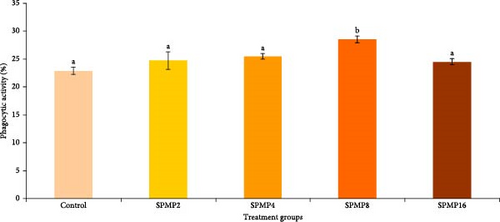
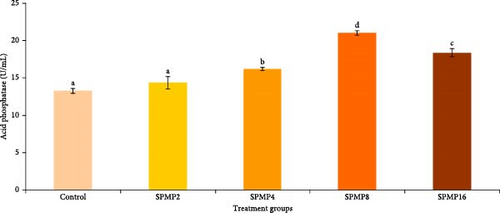
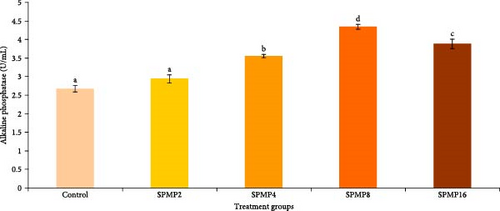
3.6. Challenge Study
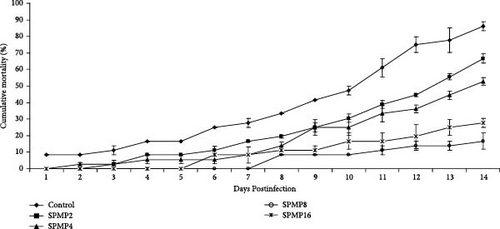
The results of the challenge study showed that the cumulative mortality rate in fish-fed SPMP-supplemented diets was higher than the control (Figure 3). The lowest mortality was recorded in SPMP8 and SPMP16, followed by SPMP4 and SPMP2 during the 14 days of the challenge test against acute A. hydrophila.
4. Discussion
Phytochemicals are increasingly being used for environmentally friendly and efficient disease control. In addition, these natural products’ combined advantages, such as their affordability, stability, and dependability, and their absence of negative impacts, have become increasingly popular [5]. Using phytochemicals in aquafeeds is a promising method to handle the challenges of intensive farming and improve profitability in aquaculture. Pectin, a configurational fiber located in the primary cell wall and middle lamella of plant cells, is prevalent in the peels of fruits such as apples, oranges, and lemons [61, 62]. Research indicates that pectin and its breakdown product, pectin oligosaccharides, can enhance digestion, absorption, and growth in terrestrial vertebrates, including rats, chickens, and pigs [63–66]. According to the findings of the current study, adding 8% SPMP to the diet considerably increased growth performance and improved feed utilization in C. catla fingerlings, mainly associated with increased digestive enzyme activity, better antioxidant capacity, and promoted health conditions in this species. In this context, it has been reported that growth performance and feed utilization improved 2% dietary apple peel-derived pectin fed common carp [41]. Furthermore, growth performance in rainbow trout, is considerably enhanced by dietary supplementation of 8%–16% commercial pectin [40]. Moreover, supplementing the diet with 1% pectin derived from apple pomace increased the weight gain and specific growth rate in rainbow trout [67]. In addition, dietary 8% low methyl-esterified pectin improved the growth performance of largemouth bass (Micropterus salmonids) [68]. According to Zhu et al. [31], 8% amidated low-ester pectin in the diet appears to improve feed efficiency in pearl gentian grouper. In Nile tilapia, a combination of 1% pectin from passion fruit peel and 1% red yeast enhanced growth and feed utilization [69]. However, it has been demonstrated that adding 30% pectin to the diet reduces the growth performance of yellow catfish (Pelteobagrus fulvidraco) [70, 71]. In considering this, it is appropriate to assume that the physiological effects of dietary pectin are species-specific and depend on pectin dosage, culture conditions, and dietary feed composition, among other factors.
Fotschki et al. [72] found that a combination of pectin–oligosaccharides and raspberry polyphenol extracts reduced the production of short-chain fatty acids in the cecum, which reduced liver fat, cholesterol, triglyceride content, and liver steatosis, benefiting liver lipid metabolism. We speculate that fish may be able to use stored energy if supplemented diets enhance their metabolism. Considering the exception of crude lipid concentration, the whole chemical composition of fish given SPMP revealed no discernible differences in the other biochemical components. The findings of Hoseinifar et al. [67] are comparable to this study. They reported that adding 1% pectin obtained from apple pomace to the diet induced nonsignificant changes in the rainbow trout’s body composition. According to Zhou et al. [40], rainbow trout fed a diet containing 4%–16% commercial pectin did not significantly differ in moisture, crude protein, or ash content; however, the crude lipid content significantly decreased between the control group and the pectin-treated groups. While it has no adverse effects on fish meat, 8% pectin has been recognized as a safe feed addition.
Pectin is a soluble prebiotic that promotes the growth of beneficial microorganisms in the gastrointestinal tract and boasts a range of biological properties, including antioxidant, antiglycosylation, anticoagulant, and anti-inflammatory effects [73, 74]. Additionally, it helps reduce serum cholesterol levels and stimulates the immune response [75]. The activity of digestive enzymes produced by the intestines serves as a key indicator of the gut chemical barrier function [76], with these enzyme activities being influenced by dietary components [77]. While Deng et al. [78] suggested that nonstarch polysaccharides (NSPs) in diets can hinder digestion and absorption processes in fish, other researchers have argued that dietary pectin actually enhances digestive enzyme activities, thereby improving digestion and nutrient absorption [79, 80]. Based on earlier research, pectin and the breakdown product of pectin oligosaccharides can enhance the growth, digestion, and absorption of terrestrial vertebrates, such as pigs, rats, and chickens [81–83]. In contrast to the control diet, we observed that SPMP8-supplemented diet enhanced the amylase activity, compared to control. In this regard, supplementing the diet with 8% amidated low-ester pectin in pearl gentian grouper increased lipase activity [31]. Moreover, rainbow trout fed a diet supplemented with 1% pectin extracted from apple pomace showed improvements in the activity of the digestive enzymes amylase and protease [67]. Comparable to this, feeding narrow-clawed crayfish (Postantacus leptodactylus) with a combination of Lactobacillus salivarius (1 × 107 CFU/g) and 0.5% pectin significantly enhanced the activities of amylase and protease [32]. Supplementing the diet with 8% to 16% of commercial pectin enhanced lipase, amylase, and protease activities in rainbow trout [40]. Further research is necessary to fully comprehend the mechanism of pectin at the level of digestive enzymes produced by fish.
Aquatic animals manage oxidative stress with a various of antioxidant enzymes, including SOD, CAT, and glutathione peroxidase (GPx) [84]. These enzymes are crucial for preserving cellular integrity by neutralizing reactive oxygen species (ROS) [85]. However, under challenging conditions, the host may not have sufficient antioxidant capacity to effectively eliminate ROS, necessitating the addition of external resources to enhance this capacity [86, 87]. One of the most common methods to enhance the antioxidant defense in fish is by incorporating stimulant compounds into their feed. In this study, the inclusion of SPMP in the diet demonstrated a high antioxidant capacity, with notable increases in SOD and CAT activity. Additionally, levels of MDA, a reliable indicator of oxidative stress (the imbalance between antioxidant defenses and ROS levels), were significantly lowered in fish fed synbiotic diets [88]. These findings may be attributed to pectin’s ability to enhance antioxidant capacity or neutralize ROS, as previously reported in carp by Harikrishnan et al. [89]. Furthermore, the beneficial effects of prebiotics on antioxidant capacity are likely linked to their ability to counteract ROS, chelate ions, and produce antioxidant compounds such as folate, butyrate, and glutathione [90].
In this investigation, we found that MDA concentration decreased in the liver of SPMP8 group that associated with the increment of SOD and CAT activities. These results imply that increasing the antioxidant capacity of C. catla can be achieved by including an appropriate amount of SPMP in the diet. This is in line with past studies showing that dietary apple peel-derived pectin supplementation increases the antioxidant capacity in common carp [41], and rainbow trout [67], or orange peel-derived pectins in common carp [91].
The degree of methyl esterification significantly influences the inflammatory properties of pectin. Differences in pectin’s methyl esterification levels contribute to the polysaccharide’s ability to inhibit the functional activity of WBCs and leukocytes [92]. The results of the present study showed that supplementing diet with 4%–16% SPMP enhanced Hb level and RBC count, suggesting SPMP can induce hematopoiesis in C. catla juveniles. In this context, Hosseini et al. [91] attributed the increase in common carp Hb content, leucocytes, and RBC counts by supplementing a diet with 1%–2% pectin derived from orange peels. Moreover, considering garlic peels contain 27% pectin, Chitsaz et al. [93] found that 1% dietary garlic peel provided to beluga sturgeon (Huso huso) increased the total Hb content, RBC, and WBC counts. A combination of pectic polysaccharides (0.1% β-glucan and 0.4% mannan oligosaccharide) that increased the levels of total RBC and WBC in silver pompano (Trachinotus ovatus) showed similar trends [94]. In wister male rats fed a diet with or without pectin, where ferric iron was the only Fe source, pectin increased the final Hb concentration [95]. In another study, pectin complexation with Na, Fe, and Ca increased white rat Hb and RBC levels while inhibiting erythropoiesis [34]. Additionally, the increased WBC counts in fish fed with SPMP-supplemented diet in the current study were linked to induced humoral (lysozyme, acid, and AKPs) and cellular (phagocytosis activity) immune responses, indicating that this supplement can enhance catla immunocompetence.
The structural characteristics of pectin contribute to its biological activities, including immunomodulation [96]. Immunomodulation encompasses a range of therapeutic strategies aimed at regulating the immune system. Immunomodulators interact with the immune system through two primary mechanisms: immunostimulation and immunosuppression. The immunosuppressive effects are linked to the backbone of pectin polysaccharides. Modifications in the galacturonic chain of pectin influence the macromolecule’s ability to diminish immune reactivity [96]. A higher concentration of galacturonic acid residues is associated with enhanced immunosuppressive activity. Consequently, the quantity of galacturonic acid residue fragments present in pectin plays a crucial role in determining its immunomodulatory effect [97].
Phagocytic and lysozyme activities are important markers of the innate defensive systems [81]. An inducible humoral component with bacteriolytic action, fish lysozyme aids in the activation of phagocytes, such as neutrophils and macrophages and the complement system [82]. AKP is a lysosomal enzyme that plays a vital role in several essential functions, including hydrolytic processes, antibacterial actions, and wound healing across all animal species [98]. ACP, another lysosomal enzyme, is involved in the breakdown of microbial pathogens and serves as a marker for evaluating the ability of macrophages to eliminate invading agents [99]. According to the current study, fish given 8% SPMP had increased phagocytic activity, serum lysozyme, ACP, and AKP activities, suggesting this supplement can trigger both cellular and humoral nonspecific immune responses in C. catla juveniles. These findings were in agreement with earlier studies [91], which discovered that diets administered to common carp that contained 1% pectin derived from orange peels increased skin mucus lysozyme activity and total immunoglobulin levels. In pearl gentian grouper, the 8% amidated low-ester pectin diet did not affect the levels of intestinal mucosal chemical barrier-related indicators (lysozyme, histone deacetylase, and mucroprotein), according to a study by Zhu et al. [31]. According to Zhang et al. [83], ACP is another lysosomal enzyme that is known to be involved in the digestion of microbial pathogens and is used as a gauge to determine the extent to which macrophages can break down invading agents. In addition, AKP is a lysosomal enzyme that has been associated to many significant functions, including wound healing in all species, hydrolytic and antibacterial activities [100]. The present study found that serum ACP and AKP activity in C. catla was notably elevated in fish fed diets with 8% SPMP compared to the control group, indicating enhanced humoral immune responses. Also, in narrow-clawed crayfish, lysozyme, phenoloxidase, nitroxide synthetase, ACP, and AKP increased when fed with L. salivarius (1 × 107 CFU/g) and pectin (0.05%) [32]. These findings are consistent with the immunology findings of rainbow trout, which showed increased levels of lysozyme, total alternative complement activity, and total immunoglobulin following feeding with 1% of pectin obtained from apple pomace [67]. According to a similar pattern, Hoseinifar et al. [41] found that common carp that were given diets, including 1% apple peels derived pectin exhibited increased levels of mucus, serum lysozyme, and total immunoglobulin. Furthermore, rats administered (1 mg) citrus pectin had improved immunological characteristics by improving antigen presentation and immunostimulating and activating macrophages, according to Khramova et al. [101].
Pectin has demonstrated antibacterial properties against various pathogenic bacteria. Its antibacterial effects are believed to stem from its ability to bind to bacterial cell walls, which disrupts their structure and inhibits growth and replication [102]. Notable pathogens, such as Escherichia coli, Aeromonas hydrophila, Salmonella enteritidis, and Staphylococcus aureus, are significant contributors to food and waterborne illnesses and infections [103]. Studies indicate that pectin can inhibit the growth of these and other bacteria. Additionally, when combined with other compounds like chitosan and essential oils, the antibacterial efficacy of pectin appears to be enhanced, highlighting its potential as a natural alternative to traditional antibacterial agents [104]. The antibacterial properties of pectin represent a promising research area with potential applications in aquaculture industry.
When researching pectin as an “alternative antibiotic feed additive,” one of the most important indicators is the improvement in animal disease resistance [105, 106]. An opportunistic conditional pathogen called A. hydrophila caused widespread aquatic diseases and caused major losses for the aquaculture sector [6]. In the present investigation, after feeding 8% SPMP, the relative percent survival rate of catla challenged by A. hydrophila was above 94.4%. The SPMP effectively improved Catla’s nonspecific immunity by preventing A. hydrophila from growing and increasing its survivability. The symbiotic (pectin + probiotic) feeding of narrow-clawed crayfish has been shown to improve disease resistance in A. hydrophila and change the way their innate immune systems function [32]. The survival rate of tilapia against Streptococcus agalactiae infection is boosted up by pectin produced from orange peel, according to a study by Doan et al. [107]. The findings of the current study confirmed that SPMP can promote disease resistance in C. catla that could be related to triggering nonspecific immune responses in this species.
5. Conclusions
The results of this study confirmed that the administration of SPMP at 8% SPMP can improve growth that may be associated with higher amylase activity and better antioxidant capacity in C. catla juveniles. Furthermore, disease resistance against A. hydrophila in fish fed 8% SPMP-supplemented diet may be attributed to immunostimulating effects of this phytochemical, such as increased WBC, lysozyme, phagocytosis, ACP, and AKP activities in this species. Further studies at a molecular level are required to clarify the SPMP mode of action on growth and disease resistance in C. catla.
Ethics Statement
All procedures involving animals in this study were conducted strictly with ethical guidelines and approved by the Ethics Committee on Animal Experimentation at St. Joseph University, Nagaland, India (SJU/ZOO/40/2021).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Govindharajan Sattanathan and Hairui Yu contributed equally to this work.
Funding
Financial supports for this study were provided by the Shandong Provincial Key Research and Development Programs (Major Scientific and Technological Innovation Projects, MSTIP, Grant/Award Numbers: 2018CXGC0102 and 2019JZZY020710).
Acknowledgments
The authors gratefully thank St. Joseph University, Chumoukedima, Nagaland, India for carrying out the research.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding authors upon reasonable request.




