Effects of Dietary Cerium Oxide Nanoparticles on Growth Performance, Plasma Biochemistry, and Histopathological Changes of Cyprinus Carpio Koi Fish Exposed to Florfenicol Antibiotic
Abstract
This study was conducted to investigate the effects of dietary cerium oxide nanoparticles (CeO2-NPs), on growth performance, plasma biochemistry and histopathological changes of koi fish (Cyprinus carpio koi) exposed to florfenicol (FF) antibiotic in a 60-day experiment. The fish with an average weight of 2.38 ± 0.44 g were completely randomly distributed into six different groups with three replications. The experimental groups included: Basal diet without additives (control), basal diet + oral antibiotic, basal diet + antibiotic bath, basal diet supplemented with CeO2-NPs, basal diet supplemnted with CeO2-NPs + antibiotic bath, and basal diet supplemented with CeO2-NPs + oral antibiotic. The experimental unit was a glass aquarium with a capacity of 110 L of water and 10 fish were stocked in it. At the end of the experimental period, growth performance of fish, blood plasma indices, and histopathology of liver, kidney, gill, and intestine were investigated. The results showed that the final body length of the fish in the basal diet + antibiotic bath group was significantly higher than the control group and the basal diet supplemented with CeO2-NP + oral antibiotic group (p < 0.05). Histological studies showed that the kidney, liver, and intestinal lesions of fish in different groups did not differ microscopically. In gill tissues, changes in secondary lamellae and their destruction occurred in different parts of the primary lamellae in different groups, but overall, no differences were observed among the different groups. The plasma aspartate transaminase (AST) and alanine transaminase (ALT) levels were significantly decreased in all groups compared to the control (p < 0.05), except the basal diet supplemented with CeO2-NPs + antibiotic bath group. The plasma blood urea nitrogen (BUN) level decreased significantly in the basal diet supplemented with CeO2-NPs + antibiotic bath group compared to the control, while this value increased in the basal diet + oral antibiotic and the basal diet supplemented with CeO2-NPs + oral antibiotic groups compared to the control (p < 0.05). The results of this study revealed that adding CeO2-NPs to the basal diet at level of 0.8 mg/kg decreased the alkaline phosphatase (ALP), AST, and ALP levels in plasma of koi fish compared to the control (p < 0.05). The simultaneous administration of dietary CeO2-NPs with the bath and oral FF antibiotic increased the AST and ALT levels and the BUN level in plasma, respectively (p < 0.05).
1. Introduction
Diet plays an pivital role in human health and welfare, and interest in healthy eating is growing. Nowadays, the total consumption of fish is increasing worldwide [1], because fish consumption is related to healthy nutrition [2]. Furthermore, aquaculture is recognized as the fastest growing business in the food production part [3]. The sharp decline in aquaculture production occurs for many reasons, including disease, which is stated to account for approximately 50% of the decline. This problem is more severe in developing countries because 90% of aquaculture companies are in developing countries [4]. Aquaculturists usually use antibiotics to control and treatment infectious diseases [5]. Disease outbreak has caused the misuse of antibiotics and decreased fish production [6, 7]. Research has shown that many antibiotics are genetically toxic to fish, and they also accumulate in fish tissues and aquatic environments. Their adverse effects include damage to the developmental, cardiovascular, and metabolic systems, as well as changes in antioxidant and immune responses in fish [8]. Several studies showed that various antibiotics compromised innate immunity response in different fish species [9, 10]. Elia et al. [11] reported that dietary administration of oxytetracycline at levels of 150 and 300 mg/kg for 10 days decreased superoxide dismutase (SOD) activity and increased catalase, glutathione (GSH) peroxidase, GSH reductase, and GSH activities in liver of common carp (Cyprinus carpio) fish. The administration of antibiotics also affect on growth performance and feed utilization of fish species. Most of studies indicate that the effects of antibiotics on fish growth are inconsistent depending on types of antibiotics, their doses and exposure methods and time [10]. The common route of antibiotic administration in fish is through feed, which appears to allow treatment of the entire population with minimal labor and drug expenditure. However, in some diseases, fish appetite can be significantly reduced, making it impossible to administer antibiotics in a suitable antimicrobial feed, and administering medicated feed during the early stages of fish growth is also problematic. Consequently, other routes of antibiotic administration should be considered and used as alternatives, including bath treatment, which offers the advantage of a convenient and uniform medication to the entire population [12]. In general, the administration of different antibiotics has raised serious concerns about their toxicity in fish, and more research and strategies are needed to prevent them in different regions of the world [8].
Florfenicol (FF) is a synthetic antibiotic that is exclusively used in veterinary industries and shows a powerful effect on gram-negative and gram-positive bacteria [13, 14]. On the other hand, the widely use of antibiotics has caused aquatic pollution and an alarming increase in drug-resistant bacteria, as well as this has led to many efforts to discover and develop environmentally friendly antibiotic alternatives [15]. Yun et al. [16] reported that an increase in the concentration of FF exposure results in the accumulation of oxidative products within tissues, inhibition of antioxidant enzyme activity, a substantial impairment of antioxidant capacity, and subsequently leads to oxidative damage in the liver and gill tissues of fish. The accumulation of oxidative products activates the toll-like receptor pathway, which in turn stimulates inflammation in the tissues, elevates the expression levels of inflammatory factors, and contributes to disorders within the immune system [16].
One of the measures that can be taken to reduce the side effects of antibiotics is the use of antioxidant, which prevent damage to important cellular components caused by free radicals, peroxides, and lipid peroxides caused by antibiotics [17]. Nanotechnology has developed greatly in recent years, leading to the introduction of nanoparticles, particularly inorganic ones, with intrinsic antioxidant activity [18]. Cerium oxide nanoparticles (CeO2-NPs) show antioxidant properties and mimic SOD, catalase, peroxidase, oxidase, and phosphatase, and inhibit hydroxyl radicals, nitric oxide radicals, and peroxynitrite in vitro and in vivo [19]. The antioxidant properties of CeO2-NPs are related to its large surface area to volume that creates a large number of structural defects, in particular, oxygen vacancies [20, 21]. These defects or oxygen vacancies on the surface of CeO2-NPs acts as active sites for binding and neutralizing active oxygen radicals [22]. Morever, CeO2-NPs create a safety effect against some destructive environmental agents by increasing the activity of natural killer cells and the expression of immune factors, as a result recovery the body’s immune ability and protecting hematopoietic tissue from damage in the body [23].
In recent years, research has shown that CeO2-NPs have reactive oxygen species (ROS)-scavenging capabilities [24]. Nelson et al. [24] also demonstrated the long-lasting antioxidant effect of CeO2-NPs in vitro and the ability of reductive antioxidant activity after a single dose of CeO2-NPs, which can essentially suppress intracellular ROS production. In another study, Ciofani et al. [25] showed that exposure to CeO2-NPs resulted in increased neuronal length, decreased ROS production in H2O2-stimulated cells, and increased dopamine production in a dose-dependent manner. CeO2-NPs significantly increase the production of GSH, which plays an important role in cellular defense against damage [26]. Nelson et al. [24] also reported that CeO2-NPs are ROS scavengers and can be used as alternative or co-treatments for diseases and disorders associated with oxidative stress and nitrosative stress.
To the best of our knowledge, there is no enough information on using CeO2-NPs as additive in fish feed and connection between CeO2-NPs and antibiotics. Therefore, this study was conducted to investigates the effects of dietary CeO2-NPs on growth performance, plasma biochemistry, and histopathological changes of Cyprinus carpio koi fish exposed to FF antibiotic.
2. Materials and Methods
2.1. Characterization of CeO2-NPs
In this study, the selected nanoparticle was cerium oxide (CeO2), sourced from a reliable supplier, the Iranian Pishgaman Nano Materials Company. According to information provided by the manufacturer, these nanoparticles were pure, the CeO2-NPs exhibited exceptional purity, reaching 99.97% (REO), with a particle size range of 10–30 nm. These nanoparticles possessed a light yellow color, a spherical morphology, and a specific surface area of 30–50 m2/g. The bulk density was approximately 0.8–1.31 g/cm, while the true density was measured at 37.132 g/cm.
2.2. Preparation of Experimental Diets
In the present study, a commercial common carp feed (EX-CG2; Raman Co., Aquatic Animals Feed Producer, Iran) with pellet size of 3.5 mm was supplemented with CeO2-NPs powder and FF antibiotic (10%) at a levels of 0.8 mg/kg [23] and 1 mg/kg [27] of dry feed, respectively. The proximate composition of commercial feed is shown in Table 1. To supplement the feeds with nanoparticles, 0.8 mg of CeO2-NPs powder was dissolved in 100 mL of distilled water and sprayed on surfaces of 1 kg of the pellets that were uniformly distributed on a layer of plastic sheets. The pellets were kept in a dark place overnight. In following, 10 g (1% w/w) of the gelatin powder from bovine skin was dissolved in boiling water and uniformly sprayed on surface of 1 kg of supplemented feed with CeO2-NPs to prevent the release of nanoparticles to water before intake of the pellets by fish [28]. The pellets were kept at room temperature for 24 h to dry and then, stored at 4°C unit use. To supplement the feeds with antibiotic, 1 mg of FF antibiotic solution (10%; Science Laboratories Co., Iran) was sprayed on surface of 1 kg of the pellets and coated with gelatin according to the method mentioned above. The pellets without adding CeO2-NPs and FF antibiotic as control were also coated with gelatin.
| Chemical composition | Content (%) |
|---|---|
| Crude protein | 35 |
| Crude lipid | 8 |
| Crude fiber | 5 |
| Total phosphorus | 4.1 |
| Ash | <10 |
| Moisture | <10 |
| Gross energy (kcal/kg) | 3500 |
2.3. Experimental Groups
In this study, six experimental groups were conducted to investigate the effects of dietary CeO2-NPs on Cyprinus carpio koi exposed to bath and oral FF antibiotic in triplicate as follows:
1-Basal diet (control). 2-Basal diet supplemented with FF antibiotic (oral administration; 1 mg/kg of dry feed). 3-Basal diet + bath FF antibiotic (5 mg/L). 4-Basal diet supplemnted with CeO2-NPs (0.8 mg/kg of dry feed). 5-Basal diet supplemnted with CeO2-NPs (0.8 mg/kg of dry feed) + bath FF antibiotic (5 mg/L). 6-Basal diet supplemnted with CeO2-NPs (0.8 mg/kg of dry feed) and FF antibiotic (1 mg/kg of dry feed).
2.4. Fish Culture and Feeding
This study was conducted at Abgineh Ornamental Fish farm (Mashhad City, Iran). A total of 180 cyprinus carpio koi fish with average weight of 32.38 ± 0.44 g were obtained from this farm and held in a 500-L polyethylene tank to acclimatize to their new culture conditions for 2 weeks. The fish fed with the commercial common carp feed two time daily (8:00 and 14:00) at a rate of 2.5% of body weight. In following, 18 glass-aquarium (80 × 40 × 40 cm) filled with 110 L of Tap water and aerated with two air stones connected to a central air blower pump were randomly allocated to the six experimental groups in three replications. Then, 10 fish were randomly allocated to each aquarium and fed with the experimental diets three time daily (8:00, 12:00, and 16:00) at a rate of 2.5% of body weight for 60 days. In bath administration of FF antibiotic groups, FF antibiotic solution (10%) was added to water of aquarium at dose of 5 mg/L [29], and the water of each aquarium was refreshed daily with a new antibiotic bath treatment for 60 days. The water of other aquariums was replaced with aerated freshwater at a rate of 20% and feces and unconsumed feed were siphoned from the bottom of each aquarium every day. During the experiment, the water quality parameters, including temperature, pH, and dissolved oxygen recorded using a portable multimeter (AZ-8603 model) as 26 ± 2°C, 7.3 ± 0.2, and 6.3 ± 0.4 mg/L, respectively. The fish were cultured under 14-h light/10-h dark regime.
2.5. Growth Performance Assessment
2.6. Blood Biochemical Assessment
Three fish were randomly sampled from each glass-aquarium, anaesthetized with clove oil (20 mg/L), and blood samples were obtained from caudal vein using a 2-mL heparinized syringe. The blood samples were centrifuged (Eppendorf 5417c model) at 10,000 × g for 10 min and obtained plasma was used to measure biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), bilirubin, albumin, total protein, blood urea nitrogen (BUN), and creatinine. These parameters were analyzed according to colorimetric method using commercial kits (Pars Azmoun Co., Iran) and a plasma autoanalyzer (Abbott Laboratories, Illinois, USA).
2.7. Histology Assessment
After 60 days, three fish were randomly sampled from each glass-aquarium for dissection. The internal organs, including the liver, kidney, gills, and intestine, were carefully removed, and transferred to vessels containing 10% neutral buffered formalin. After fixation for 72 h, the tissue samples were transferred to a autotechnicon tissue processor. After dehydration, clearing, paraffinization, and embedding, 4–5 µm thick sections were prepared from the samples using a microtome. The sections were mounted on glass slides and stained with haematoxylin and eosin. The slides were examined for the presence of lesions using a light microscope (Nikon Eclipse E200) at different magnifications.
2.8. Statistical Analysis
Data analysis was performed using SPSS software, version 19. Initially, the normality of the data distribution was assessed through the Kolmogorov–Smirnov test. Subsequently, one-way analysis of variance (ANOVA) was employed to compare means, followed by the Duncan’s New Multiple Range test to discern significant differences among the means at 95% confidence level. The data are presented as mean values with their corresponding standard deviations.
3. Results
3.1. Growth Performance
The effect of dietary CeO2-NPs on the growth performance of koi fish (C. carpio koi) exposed to bath and oral FF antibiotic is reported in Table 2. The results showed that the final weight, body weight gain, daily growth index, SGR, ADG, CF, and survival rate indices showed no significant differences between the experimental groups (p > 0.05). The final body length in the basal diet-oral antibiotic group was higher than the control and the basal diet + CeO2NPs-oral antibiotic groups (p < 0.05).
| Parameters | Experimental groups | ||||||
|---|---|---|---|---|---|---|---|
| Basal diet (control) | Basal diet-oral antibiotic | Basal diet-bath antibiotic | Basal diet + CeO2NPs | Basal diet + CeO2NPs-bath antibiotic |
Basal diet + CeO2NPs-oral antibiotic | p-Value | |
| Initial weight (g) | 32.01 ± 0.24 | 32.75 ± 0.63 | 32.87 ± 0.46 | 32.34 ± 2.45 | 32.16 ± 0.80 | 31.72 ± 0.51 | 0.64 |
| Initial length (cm) | 13.34 ± 0.06 | 13.70 ± 0.24 | 13.00 ± 0.61 | 13.48 ± 0.21 | 13.30 ± 0.63 | 13.65 ± 0.32 | 0.24 |
| Final weight (g) | 48.75 ± 1.50 | 50.32 ± 1.06 | 50.85 ± 1.84 | 47.01 ± 5.45 | 51.78 ± 1.56 | 50.91 ± 4.27 | 0.80 |
| Final length (cm) | 14.60 ± 0.35a | 15.21 ± 0.76b | 15.20 ± 0.56ab | 14.69 ± 0.30ab | 15.17 ± 0.17ab | 14.34 ± 0.12a | 0.03 |
| Weight gain (g) | 16.73 ± 1.73 | 17.57 ± 1.33 | 17.97 ± 2.29 | 14.67 ± 4.82 | 19.16 ± 0.76 | 19.19 ± 4.58 | 0.83 |
| SGR (%BW day−1) | 0.70 ± 0.06 | 0.71 ± 0.05 | 0.72 ± 0.08 | 0.62 ± 0.15 | 0.77 ± 0.01 | 0.78 ± 0.15 | 0.82 |
| ADG (g) | 0.27 ± 0.02 | 0.29 ± 0.02 | 0.29 ± 0.03 | 0.24 ± 0.08 | 0.31 ± 0.01 | 0.31 ± 0.07 | 0.83 |
| CF (g cm−3) | 1.56 ± 0.08 | 1.42 ± 0.19 | 1.44 ± 0.22 | 1.48 ± 0.08 | 1.48 ± 0.03 | 1.72 ± 0.17 | 0.15 |
| FCR | 3.03 ± 0.32 | 2.88 ± 0.19 | 2.70 ± 0.31 | 3.87 ± 1.02 | 2.65 ± 0.25 | 2.80 ± 0.76 | 0.65 |
| Survival rate (%) | 95 ± 5.77 | 95 ± 5.77 | 100 ± 5.77 | 95 ± 5.77 | 95 ± 5.77 | 95 ± 5.77 | 0.75 |
- Note: Values with dissimilar superscript letters in same rows are significantly different (ANOVA, p < 0.05).
- Abbreviations: ADG, average daily gain; CF, condition factor; FCR, food conversion ratio; SGR, specific growth rate.
3.2. Blood Biochemical Indices
Table 3 shows the variations of plasma biochemical indices of koi fish reared in different experimental groups. The plasma AST and ALT levels decreased in the all groups except the basal diet + CeO2NPs-bath antibiotic group compared to the control (p < 0.05). The lowest AST and ALT levels were observed in the basal diet-oral antibiotic and the basal diet + CeO2NPs-Oral antibiotic groups (p < 0.05). The plasms ALP level decreased significantly in the all experimental groups compared to the control (p < 0.05). The plasma LDH, bilirubin, albumin, total protein, and creatinine levels showed no significant differences between the experimental groups and control (p > 0.05). The plasma BUN level increased in the the basal diet-oral antibiotic and the basal diet + CeO2NPs-oral antibiotic groups compared to the control, while this value decreased in the basal diet + CeO2NPs-bath antibiotic group compared to the control (p < 0.05). The basal diet-bath antibiotic and basal diet + CeO2NPs groups had no significant effects on the plasma BUN level (p > 0.05).
| Indices | Experimental groups | ||||||
|---|---|---|---|---|---|---|---|
| Basal diet (control) | Basal diet-oral antibiotic | Basal diet-bath antibiotic | Basal diet + CeO2NPs | Basal diet + CeO2NPs-bath antibiotic |
Basal diet + CeO2NPs-oral antibiotic | p-Value | |
| AST (U/L) | 208.11 ± 72.25c | 51.83 ± 10.21a | 155.55 ± 69.21b | 156.44 ± 93.10b | 304.66 ± 85.11d | 47.44 ± 13.41a | <0.001 |
| ALT (U/L) | 15.73 ± 2.93c | 2.46 ± 1.30a | 10.68 ± 5.85b | 10.54 ± 5.01b | 28.68 ± 11.29d | 1.58 ± 1.23a | <0.001 |
| ALP (U/L) | 111.02 ± 39.65b | 68.5 ± 22.64a | 65.5 ± 28.01a | 52.27 ± 9.69a | 47.77 ± 15.80a | 47.22 ± 11.91a | <0.001 |
| LDH (U/L) | 1335.16 ± 786.70 | 1364.33 ± 570.05 | 1469.60 ± 589.80 | 1478.88 ± 773.08 | 1290.33 ± 687.72 | 1454.22 ± 267.69 | 0.98 |
| Bilirubin (mg/dL) | 1.31 ± 0.44 | 2.10 ± 1.39 | 1.26 ± 0.55 | 1.73 ± 0.59 | 2.04 ± 1.42 | 2.24 ± 1.43 | 0.26 |
| Albumin (mg/dL) | 0.51 ± 0.18 | 0.51 ± 0.19 | 0.49 ± 0.16 | 0.47 ± 0.09 | 0.58 ± 0.26 | 0.61 ± 0.20 | 0.59 |
| Total protein (mg/dL) | 2.61 ± 0.35 | 2.70 ± 0.31 | 2.66 ± 0.30 | 2.67 ± 0.14 | 2.89 ± 0.25 | 2.89 ± 0.37 | 0.22 |
| BUN (mg/dL) | 10.99 ± 1.0b | 17.74 ± 0.57c | 11.23 ± 1.60b | 12.11 ± 1.00b | 7.66 ± 1.48a | 17.77 ± 0.57c | <0.001 |
| Creatinine (µmol/L) | 0.55 ± 0.39 | 0.89 ± 0.63 | 0.71 ± 0.49 | 0.44 ± 0.33 | 0.30 ± 0.07 | 0.50 ± 0.33 | 0.062 |
- Note: Values with dissimilar superscript letters in same rows are significantly different (ANOVA, p < 0.05).
- Abrreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase.
3.3. Histology
In the histopathological examination of the kidney samples, mild-to-moderate hyperemia, infiltration of mononuclear inflammatory cells, especially lymphocytes and mild renal tubular epithelial cell degeneration and necrosis were seen (Figure 1). After examining the kidney tissue slides, no significant difference was observed between the control and the other groups in terms of histopathological lesions (Figure 1a–f).
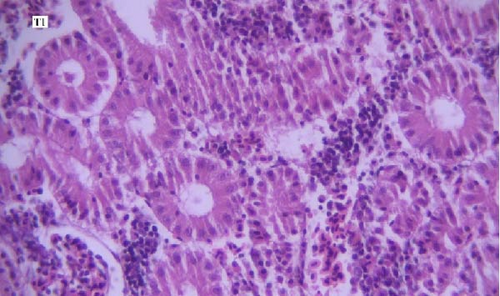
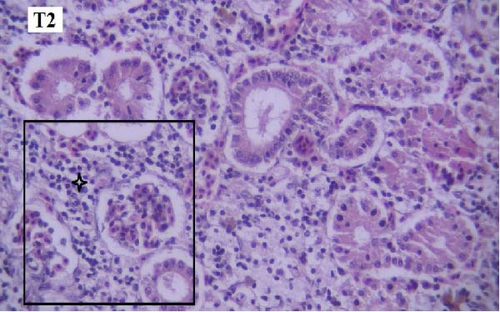
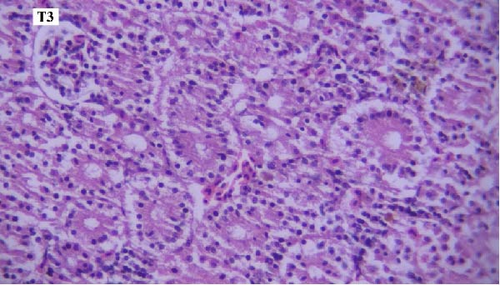
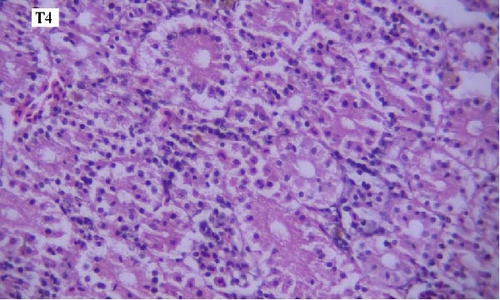
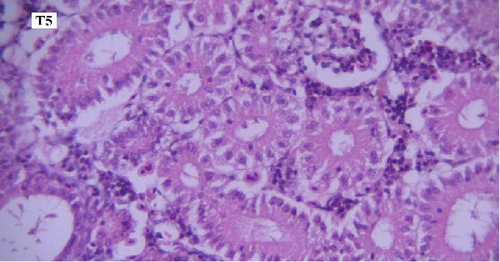
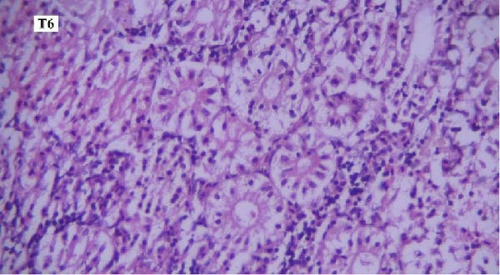
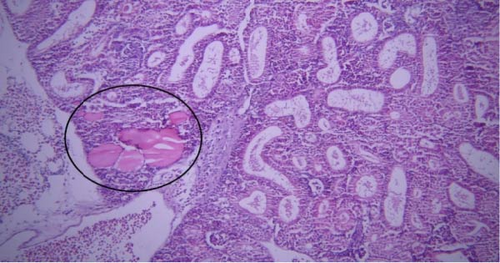
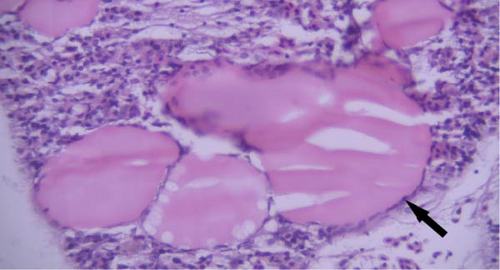
One of the findings that was seen in all fish in different groups was the thyroidization of the kidney tubules, which was in the form of dilation of the kidney tubules, the accumulation of hyaline casts within the lumens, and the change in the shape of the some tubular epithelial cells from columnar or cuboidal cells to squamous ones. Thyroidization of the kidney tubules is seen in cases of inflammation of the glomeruli, and when there are changes in the glomerular filtration barrier causing proteinuria (Figures 2 and 3).
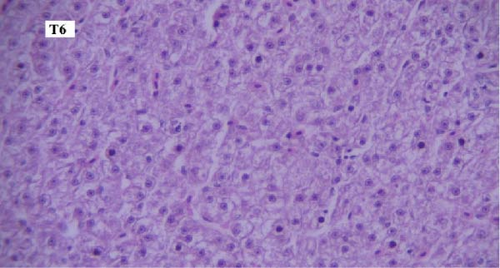
In the slides prepared from liver samples in different groups, microscopic lesions such as mild hyperemia, infiltration of mononuclear inflammatory cells, and hepatocellular degeneration and necrosis were observed, and no significant difference was seen between them. In all groups, some sections of pancreas were observed within liver, which is regarded as a completely normal finding considering the anatomy of the liver and pancreas inside the fish body (Figures 4a-f).

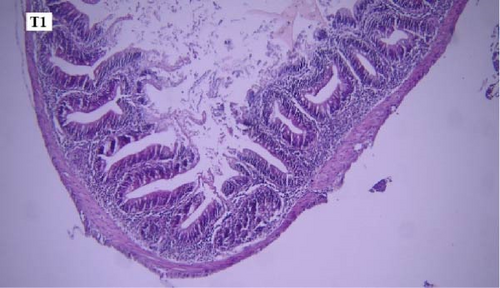
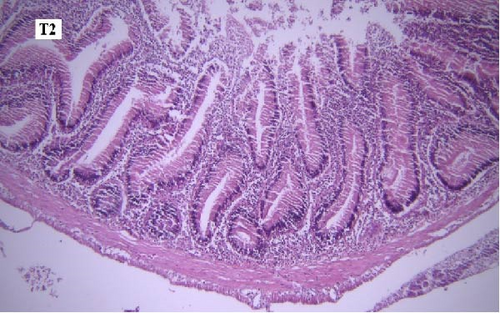
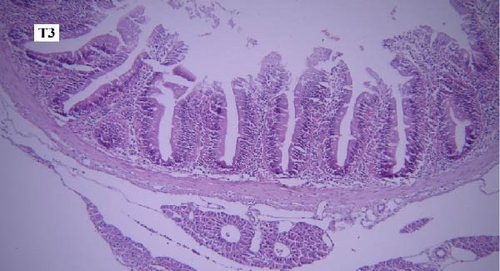
In the slides prepared from the intestines of fish reared in different groups, no significant difference was observed between them in terms of the presence of microscopic lesions such as hyperemia, infiltration of inflammatory cells, villar epithelial cells degeneration, and necrosis. Mild intestinal lesions were recorded in all experimental groups (Figure 5a-f).
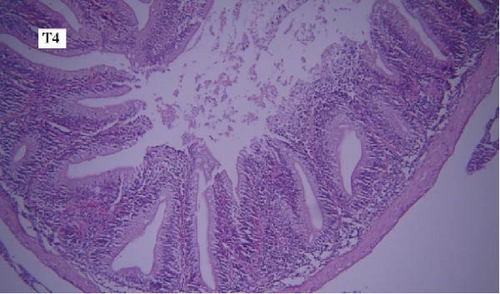
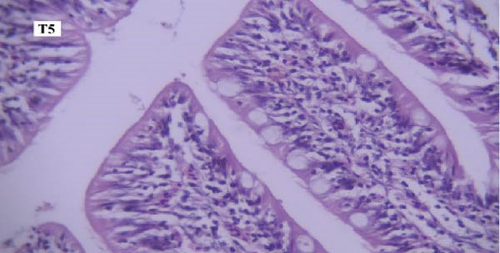
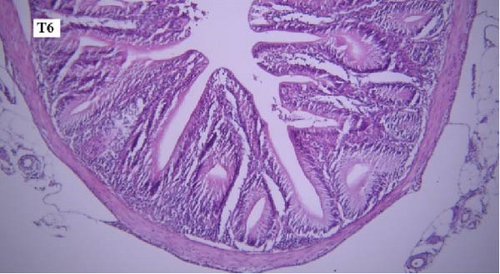
In the gill tissues, primary and secondary lamellae changes, including congestion, cellular hyperplasia, degeneration, necrosis, and sloughing, secondary lamellar fusion and their loss occurred in different groups, but overall, no significant difference was observed between different groups in terms of gill microscopic lesions (Figure 6a-f).
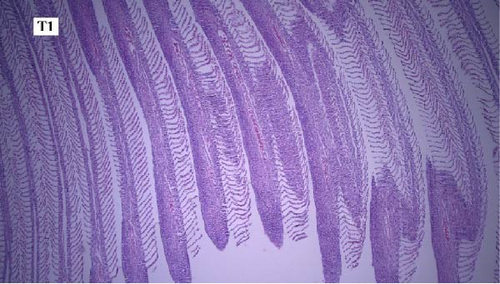
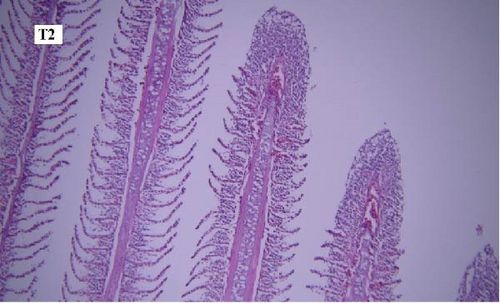
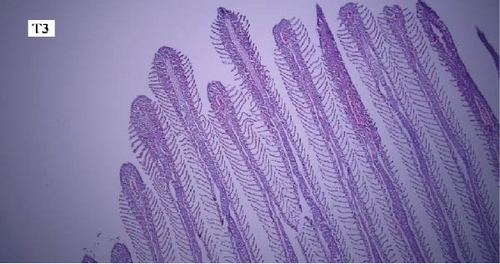
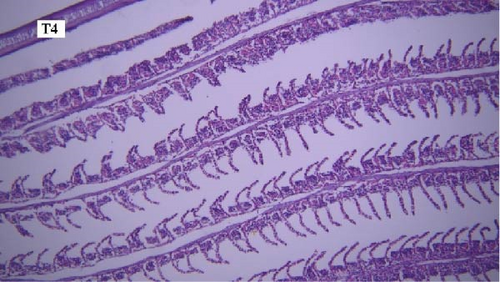
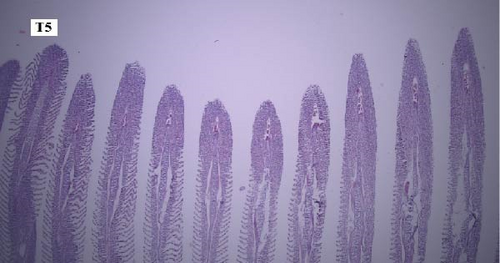
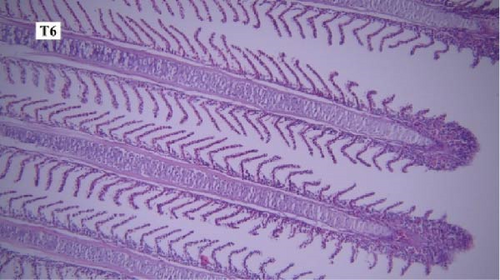
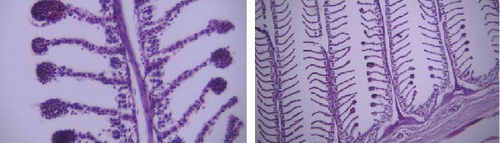
In this study, the mace-shaped or club-shaped tip of the secondary lamellae of the gills was seen randomly in the different groups (Figure 7) and this lesion is one of the first tissue damage in fishes. In this case, the circulatory disturbance of focal congestion in the tips of secondary lamellae was observed, and the presence of chemicals reduces the useful surface of the gills and, as a result, reduces the exchange.
4. Discussion
Cerium (Ce) is a rare earth element that has a wide range of biological activities, including anti-inflammatory, resistance to oxidative stress, immune regulation, and growth promotion [30, 31]. Qin et al. [23] reported that dietary CeO2-NPs increased weight gain rate and decreased feed coefficient at level of 0.8 mg/kg in Chinese mitten crab (Eriocheir sinensis). The results of the present study revealed that dietay CeO2-NPs had no significant effects on growth performance of koi fish. The primary use of antibiotics in aquaculture is for disease treatment and growth enhancement, which can lead to antimicrobial resistance in aquatic environments. Several studies have demonstrated the impact of FF on growth performance in various aquatic species. The results of present study showed that feeding the koi fish with FF-supplemented diet for 60 days led to increasing the final body length compared to the control, while bath exposure of FF had no significant effects on growth performance. Most of studies indicate that the effects of antibiotics on fish growth are inconsistent depending on types of antibiotics, their doses and exposure methods and time [10]. Improved growth performance of fish, after both oral and bath exposure to antibiotics, has been attributed to decreased gut microbiota, which increase nutrient utilization and reduced host maintenance costs [32] and decreased gut wall and villus lamina propria, which increase nutrient absorption [33]. Sanchez-Martinez et al. [32 ] found that dietary oxytetracycline and FF improved growth in channel catfish (Ictalurus punctatus). Decreased growth performance has been related to reduced appetite, voluntary feed intake, feed efficiency, and protein efficiency ratio [33, 34], and impaired digestive enzymes activities and nutrients digestibility [10]. Zhang et al. [35] found that exposure of European sea bass (Dicentrarchus labrax) to FF at dosage of 10 mg/kg body weight for 10 days decreased growth rates in both flow-through and recirculation aquaculture systems. This negative effect on growth was further reported by reduced weight gain and specific growth rates in sea cucumbers (Apostichopus japonicus) fed FF-supplemented diets for 60 days, as observed by Yang et al. [36]. The inhibitory action of FF on digestive enzyme activity, including amylase, lipase, and trypsin, was highlighted by Zhang et al. [35]. Additionally, Wang et al. [37] demonstrated that FF-enriched feed disturbed gut microbiota and impaired intestinal nutrition, ultimately contributing to reduced growth performance in fish. In the present study, both oral and bath exposue of FF at levels of 1 mg/kg of dry feed and 5 mg/L had no negative effects on growth performance of koi fish, respectively.
Enzymes such as ALT, AST, ALP, and LDH are crucial in fish physiology, significantly impacting metabolic functions and overall well-being. These enzymes, as highlighted by Nakano et al. [38], are valuable biomarkers in toxicological research, offering insights into the health status of fish. Research has shown that exposure to different toxic substances can lead to significant alterations in biochemical markers within fish physiology, particularly in blood and various organs. For instance, Abumourad et al. [39] observed a notable elevation in the activity of AST and ALT enzymes within the liver when exposed to various toxins. Similarly, Almeida et al. [40] conducted a study on Nile tilapia (O. niloticus) fish subjected to cadmium poisoning and found substantial changes in AST and ALT enzyme levels in both tissues and serum. These enzymes, prevalent in animal tissues, are crucial for carbohydrate and amino acid metabolism. They serve as vital indicators of tissue damage in fish, especially in vital organs such as the liver, gills, brain, and muscles. In the current investigation, it was noted that administration of CeO2-NPs at level of 0.8 mg/kg in koi fish feed did not result in an elevation of AST, ALT, and ALP enzyme levels in the blood plasma when compared to the control group. This finding suggests that there was no tissue damage to the liver, gills, brain, or muscle of the fish associated with the use of CeO2-NPs. Saleem Khan et al. [41] reported that pretreatment of Rohu (Labeo rohita) fish with dietary CeO2-NPs (50 µg kg−1) exposed to Ag-NPs (30 mg L−1) decreaed the AST and ALT levels in blood. They confirmed ameliorated role of CeO2-NPs in liver damage of Ag-NP. The administration of CeO2-NPs have gained attention in the field of aquaculture research due to their unique properties. Research by Duan et al. [42] revealed their potential as antioxidants, which is particularly intriguing considering the common association between nanoparticles and toxicity. Unlike many other nanoparticles, CeO2-NPs do not induce toxicity through the generation of ROS and subsequent oxidative stress [43]. Furthermore, Jemec et al. [44] found that these nanoparticles exhibit minimal toxicity towards various fish species, making them a promising candidate for further investigation in aquaculture. CeO2-NPs possess unique antioxidant capabilities, making them a promising candidate to combat toxicity, which often triggers oxidative stress in fish [45]. Research suggests that these nanoparticles can offer protective effects in biological systems, unlike many other nanoparticles that induce oxidative stress. Rundle et al. [46] demonstrated that exposure to 0.1 mg of CeO2-NPs for 25 h resulted in a mild physiological stress response without any signs of oxidative or osmotic damage. The levels of malondialdehyde, a lipid peroxidation marker, remained stable in the gill and heart tissue of fish treated with CeO2-NPs. In contrast, under identical conditions, 0.1 mg of Zn-NPs caused a significant increase in gill malondialdehyde, activated apoptosis pathways, and upregulated heat shock proteins [45]. Research indicates that CeO2-NPs maintain tissue malondialdehyde levels in fish, suggesting their unique ability to avoid elevating oxidative stress, unlike many other nanoparticles. This observation aligns with previous studies demonstrating the potential of these nanoparticles to regulate ROS species in the brain [47] and mitigate cardiac dysfunction by reducing oxidative stress [48]. The present study found that administration of FF as oral and bath exposure did not negatively impact AST, ALT, and ALP enzyme levels in blood plasma. Interestingly, it led to a reduction in these enzyme concentrations compared to the control group. The beneficial effects of the antibiotic might be attributed to its low concentration and nontoxic nature in koi fish. Previous research has shown conflicting findings, suggesting that significant alterations in AST and ALT enzyme activity in the blood can signify tissue damage caused by stress, toxicity, and drug-induced liver injuries. Duan et al. [42] explained that these changes disrupt the cell membrane, enabling enzymes to leak from the cells. However, the concurrent administration of CeO2-NPs along with FF bath exposue led to an increase in the concentrations of AST and ALT enzymes in the blood plasma. This rise in aminotransferase activity in the treatment may be attributed to their involvement in facilitating the necessary conditions for gluconeogenesis and supplying the energy required by the fish under stress, which could arise from the combined effects of CeO2-NPs and the antibiotic bath. Alternatively, this increase may indicate tissue damage resulting from toxicity. During stressful periods, fish are required to expend additional energy to mitigate the effects of toxins within their bodies. ALT and AST play crucial roles in protein and amino acid metabolism, as well as in transaminase activity. It is important to highlight that the elevation of transaminases serves as an immune response that occurs in the initial stages of stress [49]. The heightened activity of these two enzymes as a result of lead poisoning has been documented in carp [50] and tilapia [51], aligning with the findings of the current study. Additionally, an increase in the activity of these enzymes due to cadmium and copper poisoning has been observed in the brain, gills, and muscle tissues of African catfish [52]. Conversely, Gill et al. [53] reported a reduction in the activity of these enzymes in the liver, gills, and kidneys of rosy barb fish (Barbus conchonius) following poisoning. Furthermore,[54] indicated that while poisoning led to an increase in AST activity in the liver of tilapia, there was a corresponding decrease in ALT enzyme activity. Consequently, these findings suggest that varying outcomes may arise depending on the species, type of metal, and tissue examined [54].
ALP is an enzyme associated with membranes that facilitates the hydrolysis of a diverse range of phosphomonoester substrates and is also crucial for bone development [55]. This enzyme has been recognized as a reliable biomarker in toxicological research [54]. In the current investigation, the ALP index exhibited a decline due to the administration of CeO2-NPs nd the FF antibiotic in both oral and bath applications, in comparison to the control group. This suggests that the fish were not adversely affected by the antibiotics or CeO2-NPs. Han et al. [49] examined the levels of ALP enzyme in the liver of common carp (Cyprinus carpio) exposed to soluble heavy metal compounds over periods of one, eight, 16, and thirty-2 days. The findings indicated a progressive increase in the ALP enzyme from the first day to the thirty-second day relative to the control group [49]. The bioaccumulation of heavy metals induces oxidative stress in liver cells. The LDH enzyme serves as a key intermediary in the anaerobic glycolysis pathway in vertebrates and is utilized in fish to assess tissue damage. In this study, however, the activity of the LDH enzyme remained unaffected by the experimental treatments.
Blood urea and creatinine are reliable biomarkers for kidney functions [56], and glomerular filtration rate [57] in fish. The results revealed that BUN and creatinine levels in the plasma of koi fish treated with CeO2-NPs remained relatively unchanged in comparison to the control group. Oral administration of FF led to significantly elevated BUN concentrations in the plasma compared to controls. However, the bath exposure of FF did not show a similar effect. Reda et al. [58] showed that blood urea level increased in Nile tilapia (O. niloticus) fed diet supplemented with FF for 12 weeks compared to control, while the blood creatinine level decreased. The results of the current study showed that the administration of FF antibiotic as oral and bath had no significant impact on plasma creatinine level. FF, when administered to catfish at varying doses of 10, 30, or 50 mg/kg daily for a 20-day period, demonstrated a dose-related decrease in cellulose lesions within the hematopoietic and lymphatic tissues of the anterior and posterior kidneys, as well as the spleen [59]. In contrast, Atlantic salmon fed with FF at a higher dose of 100 mg/kg per day for 10 days exhibited no treatment-induced histopathological lesions in the liver or kidney [60]. A study conducted by Straus et al. [61] revealed that administering FF at a dosage of 75 mg/kg body weight to Sunshine Bass fish over a 20-day period did not result in any noticeable effects on liver or kidney tissue.
Histopathological changes have been widely used as indicators when evaluating the health of fish exposed to toxins and pollutants [62]. In our study, histopathological lesions in various organs, such as the kidney, liver, intestine, and gill, were examined, and no significant differences were observed between the different groups. It can be concluded that the treatments in this study do not result in significant microscopic lesions. Thyroidization of renal tubules in fish often occurs due to exposure to environmental pollutants or hormonal imbalances. This lesion in kidney were also reported by Naz et al. [62]. Considering that the histopathological lesions were observed in all groups, including the control group, it can be concluded that they were caused by other factors.
5. Conclusion
In the present study, the dietary CeO2-NPs and the administration of FF antibiotic as oral and bath exposure had no negative effect on growth performance, histology of liver, kidney, gill, and intestine and plasma parameters. Adding CeO2-NPs to the basal diet at level of 0.8 mg/kg decreased the ALP, AST, and ALP levels in plasma compared to the control. Nevertheless, the simultaneous use of dietary CeO2-NPs and FF antibiotic bath and dietary CeO2-NPs and oral FF antibiotic increased the AST and ALT levels and the BUN level in plasma of koi fish, respectively.
Ethics Statement
Fish care and handling were carried out based on the guidelines of Ferdowsi University of Mashhad (FUM) under code IR.UM.REC.1401.135.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Fatemeh Borjian Boroujeni performed the experiments and measurements and edited the manuscript. Davar Shahsavani conceptualized and designed the experiments and conducted biochemichal analysis. Mehrdad Sarkheil conceptualized and designed the experiments, analyzed the data, and wrote the original draft. Hossein Nourani performed histopathological examinations and edited the manuscript.
Funding
This study received financial support from Ferdowsi University of Mashhad (FUM) (Grant 3/58389).
Acknowledgments
The authors would like to express their gratitude to the staff of the Faculty of Veterinary Medicine of Ferdowsi University of Mashhad (FUM) and Abgineh Ornamental Fish farm (Mashhad City, Iran) for supporting and providing research facilities. This study received financial support from Ferdowsi University of Mashhad (FUM) (Grant 3/58389).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




