Pathogen Screening and Genetic Analysis of Brazilian Black Tiger Shrimp (Penaeus monodon) Populations for Enhancing Aquaculture Stocks
Abstract
Black tiger prawn (shrimp) (Penaeus monodon) populations that have established in the wild across the north of Brazil provide an alternative for shrimp aquaculture systems that are primarily based on pacific white-leg shrimp (Litopenaeus vannamei). A population of P. monodon that was sourced from the state of Ceará in Brazil, used to evaluate husbandry and breeding systems, and was assessed for pathogen incidence and genotyped to evaluate inbreeding, genetic diversity, and population structure after 10 generations of domestication. Ninety-six P. monodon were screened for 19 pathogens listed by WOAH, MAPA, and other significant pathogens. All 1133 qPCR/RT-PCR tests were negative, and the histopathology of 42 samples showed no significant morphological changes when inspected for general health status. Genotype data from other populations of P. monodon collected and genotyped with the same DNA marker panel were used to estimate inbreeding coefficients and population diversity estimates. The Brazilian population’s inbreeding coefficient estimate was low (0.03) compared to most comparison populations. Genetic distance estimates (Fst) showed Brazilian broodstock were genetically similar to both domesticated populations from Vietnam and wild populations from Australia, while being genetically different to populations originating from the Mozambique Channel region. The absence of pathogens and lack of inbreeding indicate that the population is well adapted to this region, making it suitable for the development of a structured breeding program to provide a reliable domestic source of genetically improved P. monodon. Such a program would contribute significantly to the long-term sustainability, resilience, and productivity of the shrimp industry in north/northeast of Brazil, reducing dependence on imported stocks and enhance local aquaculture development.
1. Introduction
Black tiger prawn (shrimp) (Penaeus monodon) is widely cultivated in aquaculture and has been introduced into many regions of the world for intensive, semi-intensive, and extensive culture. This species is also believed to have been inadvertently introduced within the ballast of cargo ships to result in the establishment of the species in the north of Brazil. The species has been reported in wild populations across northern Brazil on multiple occasions since the late 1980s [1–5]. Once the wild population of P. monodon is established in Brazil, its domestication can promote positive implications for the sustainability by reducing reliance on wild stocks, diversifying production systems, providing higher market prices for producers, and reducing losses from disease.
Marine shrimp farming in Brazil has developed into a significant industry with more than 3300 producers in more than 300 municipalities providing 130,000 jobs [6]. The industry has been primarily based in the commercial culture of Penaeus japonicus, P. monodon, Penaeus stylirostris, Penaeus shimitti, Penaeus subtilis, Penaeus paulensis, and Penaeus brasiliensis and started in the 1970s replacing the white-leg shrimp (Litopenaeus vannamei) that has grown significantly since the species was introduced for aquaculture in 1983. Despite the success that Brazilian producers have experienced using L. vannamei, problems with pathogens such as Infectious Myonecrosis virus (IMNV) and White Spot Syndrome virus (WSSV) have led to financial losses in the region [6–10]. P. monodon provides an alternative species of shrimp with different levels of susceptibility to the major pathogens that limit productivity. For example, P. monodon has been found to be somewhat more resistant to infection with IMNV than L. vannamei [11] and has the capacity to develop a level of resistance against white spot syndrome virus [12, 13]. In the market, P. monodon will complement L. vannamei production by offering a species with distinct advantages on prices that is very well suited for small-scale farm production [14].
Developing a domesticated population of specific pathogen-free P. monodon from the stock that has become naturalized across the north of Brazil would provide local aquaculture operations another option for domestic shrimp production systems. Ensuring these breeding populations are free from specific known pathogens will be critical for increasing the proportion of P. monodon that is produced in the region. While managing the P. monodon population in a production environment for 10 generations provides evidence that the population that was introduced to the region is suitable for aquaculture systems, determining if the population is pathogen free will ensure investments into genetic selection may be applied for long-term improvements. The advent of a specific pathogen free L. vannamei breeding population was a critical point in moving global shrimp production from P. monodon to the white-leg shrimp. Reliance on wild P. monodon broodstock for aquaculture has resulted in financial losses from disease. These losses have been mitigated by moving production to less intensive systems and maintaining lower stocking densities, where P. monodon is managed extensively with little additional feed with production targeting organic and other high-margin, low volume markets. Knowledge of pathogen incidence and the genetic diversity of Brazilian P. monodon will help to guide the development of locally adapted breeding populations from the genetics that have become established in the region. Genetic diversity plays a key role in enhancing resilience by ensuring populations can adapt to change, which is essential for overcoming challenges like disease outbreaks and improving productivity. These factors were considered in evaluating the suitability of a population of P. monodon that has been domesticated over 10 generations in northern Brazil.
2. Materials and Methods
2.1. Sampling
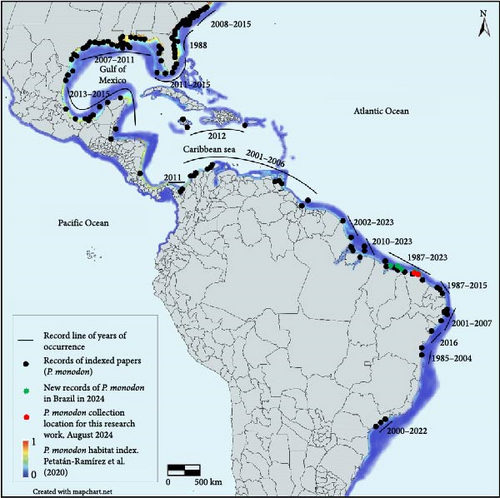
In 2019, a population of several 100 P. monodon collected from Aranaú (2°48′15"S 40°13′36"W “) and Acaraú (2°48′22″S 40°08′56″W) in the state of Ceará in Brazil to initiate a domestication program (Figure 1). The collection sites were chosen based on reports of naturally occurring P. monodon caught by shrimp fishermen and their proximity to the genetic breeding unit. These animals were used to develop breeding and husbandry systems as part of a collaboration between Crustaceans Diseases Diagnostic Laboratory in the State University of Maranhão (LAQUA-UEMA) and prawn producers in the region in 2024. After 10 generations in culture, samples from 138 animals were collected for both pathogen screening and genotyping. For pathogen screening, tissue from 21 broodstock and 75 progeny (40 juveniles and 35 small juveniles) derived from four of these broodstock were collected for genotyping to evaluate genetic diversity and similarity with other populations. For pathogen screening, pleopod samples were taken from all animals along with 10 fecal samples from broodstook that were sampled for enteric pathogens and one pool of spermatophore sample that was also sampled. For the 75 progeny, pleopod and hepatopancreas samples were also included for systemic and enteric pathogen analysis. For histological analysis, 42 of the shrimp (21 juvenile and 21 small juvenile) that were progeny of 4 broodstock that were sacrificed and fixed with Davidson AFA (1 L: 330 mL of 95% ethyl alcohol, 220 mL of 100% formalin; 115 mL of glacial acetic acid and 335 mL of distilled water, pH ~ 3–4). After fixation, Davison’s fixative was removed and replaced by 70% ethanol. For Histological processing, samples were washed in a series of alcohol/xylene solutions, embedded in paraffin, section at 5 µm and stained with H&E and examined using a bright field light microscope following standard procedures [16]. The severity of the pathogen infection was graded based on a semi-quantitative scale that ranges from Grade 0 to Grade 4 following a previous publication [17]. The samples were transported to be analyzed at the Crustaceans Diseases Diagnostic Laboratory (LAQUA-UEMA) that meets the requirements of accreditation by the National Institute of Metrology, Quality and Technology – INMETRO for the execution of biological animal health testing services in the scope of crustacean diseases in attention to the international standard ABNT NBR ISO/IEC 17025 : 2017. Accreditation number CRL 1799, also through the mutual recognition of the International Laboratory Accreditation Cooperation (ILAC) and the Interamerican Accreditation Cooperation (IAAC).
Genotype data from the 21 broodstock were merged with data from 98 other animals representing 12 other populations to allow for comparisons of genetic diversity and similarity. Marker data from these 12 other populations were used for comparisons: AU = various Australian populations, FJ = Fiji, ID = Indonesian, MC = Mozambique Channel, VN = Vietnamese, AUSNT = Australia, Northern Territory, AUSWA = Australia, Western Australian, GL1 = Global breeding program 1, GL2 = Global breeding program 2, GL3 = Global breeding program 3, GL4 = Global breeding program 4, GL5 = Global breeding program 5. As few individuals represented some of these populations (GL4 = 4; ID = 3; GL1, GL2 & FJ = 2), they were excluded from analyses of within population genetic diversity.
2.2. Pathogen Screening—Molecular and Histopathology
In total, 96 P. monodon were screened for a total of 19 etiological agents using protocols recognized by World Organization for Animal Health [18] WOAH-Technical diseases card to new emerging significant pathogens, the Brazilian Ministry of Agriculture Livestock and Supply (MAPA), and other pathogens of significance were also screened using protocols recommended by WOAH-reference laboratories. Pathogens included in this study are 1) White Spot Syndrome virus (WSSV); 2) Penaeus stylirostris pestyldensovirus 1 (PstDNV1) [Infectious hypodermal and hematopoietic necrosis virus (IHHNV)]; 3) Hepatobacter Penaei Bacterium (NHP-B); 4) Vibrio parahaemolyticus [ (PirA and PirB) Acute Hepatopancreatic Necrosis Disease (AHPND “EMS”)]; 5) Enterocytozoon hepatopenaei (EHP); 6) Decapod Iridescent Virus – type 1 (DIV1 [SHIV]); 7) V. parahaemolyticus [ (VHVP-2) Transparent Postlarval Disease (TPD)]; 8) Baculovirus penaei (PvSPNV); 9) Monodon baculovirus (MBV); 10) Infectious Myonecrosis Virus (IMNV); 11) Taura Syndrome Virus (TSV); 12) Yellow Head Virus type 1 (YHV-1); 13) Macrobrachium rosenbergii nodavirus (MrNV); 14) Laem Singh virus [Monodon Slow Growth Syndrome (LSNV)]; 15) Covert mortality Nodavirus (CMNV); 16) Penaeus vannamei Nodavirus (PvNV); 17) Penaeus vannamei Solinvivirus (PvSV); 18) Mourilyan Virus (MoV); and 19) Decapod hepanhamaparvovirus (Hepatopancreatic Parvovirus (HPV).
For molecular diagnostics of these 19 pathogens, both real-time PCR and standard for molecular diagnostics of these 19 pathogens, both real-time PCR and standard PCR/RT-PCR were used on samples taken from 21 broodstock and 75 progeny (40 juveniles and 35 small juveniles). Real-time PCR/RT-PCR with TaqMan hydrolysis probes was used to determine the presence of WSSV [19], IHHNV [20], IMNV [21] and unpublished method developed in LAQUA-UEMA], PvSV [8, 9], NHP-B [22], AHPND (EMS) [23], EHP [24], DIV1 [25], MrNV [26], LSNV [27], CMNV [28], PvNV [29], TSV [30], and YHV [31]. Standard PCR was used to determine the presence of MoV (unpublished method developed in Aquaculture Pathology lab University of Arizona), MBV [32], HPV [33], PvSPNV (BP) [34], and TPD (unpublished method developed in Aquaculture Pathology lab University of Arizona). All analysis was carried out in parallel with negative control (SPF shrimp tissues used since sample preparation) and positive reference controls, all certified within ISO 17043 standards. All the intermediary precision (accuracy) on the procedures of references molecular diagnostics methods were also supported by accredited international standards (ABNT NBRISO/IEC 17025:2017) established in LAQUA-UEMA. The primer, sequence, and references are provided in Table S1.
2.2.1. Nucleic Acid Extraction
For nucleic acid extraction, the Maxwell 16 Viral (TNA) Purification Kit method was used in the Maxwell 16 MDX equipment for the automated purification of genomic total viral nucleic acid (DNA and RNA). A 50 mg piece of tissue was macerated for every 300 uL of lysis solution until no tissue fragment was visible to the human eye, homogenizing in the vortex for 10 s and proceeding to incubate the tube in a thermoblock for 20 min at 80°C. The samples are then used in the Maxwell MDx 16 device, according to the manufacturer’s instructions (insert manufacturer name). TNA was eluted in 50 μL of elution buffer. Then the optical density (OD), purity, and concentration were recorded, by adding 1 μL of the extracted TNA (DNA and RNA) in a Nanodrop Lite equipment.
2.2.2. Realtime RT-PCR/PCR
For the detection of DNA, the GoTaq Probe qPCR Master Mix kit (Promega, A6101) was used, and for the detection of RNA, the GoTaq reProbe 1-step RT-qPCR system (Promega, A6120) was used. A total of 1 μL of sample template was added to 9 μL of the kit master mixture, including 1 μL of the respective target Forward/Reverse primers (5 μM final concentration) and 0.5 μL of the TaqMan probe (2 μM final concentration) for the qPCR/RT-qPCR reaction. The primer and probe sequences are listed in Table S1.
The analysis was conducted using a QuantStudio 3 real-time qPCR machine (Thermo Fisher Scientific), and the raw data were processed using the Quant-Studio Design & Analysis software to produce cycle threshold (Ct) values of positive detections (v. 1.5.2; Thermo Fisher Scientific). The cycle conditions for DNA targeting assays included an initial denaturation step of 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle conditions for the RNA targeting assays included an initial denaturation step of 45°C for 15 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A positive target control and a negative control were included in each analysis.
2.2.3. Standard PCR/RT-PCR
The PCR/RT-PCR reaction was performed in a 25 µL reaction mix containing 2 µL of template (100–900 ng/µL DNA/RNA), with the AcessQuick RT-PCR System protocol, 12.5 µL Master Mix (2x), 1 µL of AMV transcriptase (5u), and 0.5 µL of MoV-254F/R primer. With the GoTaq Hot Start Polymerase protocol, 5 µL of 5x Green Buffer, 2 µL MgCl2. (1.0 mM), 0.5 µL of dNTP (0.2 mM of each dATP, dGTP, dCTP, and dTTP), 0.25 µL GoTaq Hot Start Polymerase and 1 µL of each primer (MBV 261F/R, BP 6581F/6582R, HPV-2F/R, and VpTPD-vhvp-2-F1/R1).
PCR/RT-PCR amplification was performed in a thermocycler (Applied Biosystems, Veriti 96-well) amplification at 60°C for 30 min for the RT step, followed by denaturation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 45 s, annealing at 62°C for 45 s and final extension at 60°C for 7 min. For the other primers, sequences were performed with a denaturation profile at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s, followed by final extension at 72°C for 5 min. Subsequently, 6 µL of the PCR products were analyzed by electrophoresis in 1.5% agarose gels. DNA ladders of 100 bp and 1 kb (Promega) were used as markers. The agarose gel was examined under UV transillumination and photographed using an imaging capture system L-PIX EX (Loccus do Brasil).
2.2.4. Histopathology
The 42 shrimp were prepared using Davidson’s fixative (330 mL 95% ethanol, 220 mL 100% formalin, 115 mL glacial acetic acid, 335 mL distilled water, and pH ~ 3.0–4.0) following standard procedures [16]. Davidson’s fixative was then removed and replaced with an equal volume of 70% ethanol. For histological processing, samples were washed in a series of alcohol/xylene solutions while embedded in paraffin for sectioning at 5 µm and staining with an hematoxylin and eosin (H&E) microtome following [16]. Histological slides were examined using a bright field light microscope. The severity of the pathogen infection was graded based on a semi-quantitative scale that ranges from Grade 0 to Grade 4 following a previous publication [17].
2.3. Genotyping and Genetic Analysis
A total of 96 animals were genotyped using an Illumina array of 8000 single nucleotide polymorphism (SNP) markers [35] under review). The panel was created using sequence from many wild and domesticated populations of P. monondon so that it is informative in a broad range of populations. Brazilian P. monodon was not included in the reference set that was used for panel design. Samples were shipped to Genics (Brisbane, Australia) for commercial array genotyping. Markers were used to identify the broodstock that were the parents of the 75 juvenile samples prior to removing marker data for all progenies. Genotype data from the 21 broodstock were merged with marker data from the 12 other populations identified above. Quality control (QC) of markers was performed using the AGHmatrix in R [36] to remove SNP with call rates (CR) less than 75%, and minor allele frequency (MAF) less than 5%. Over 96% of the SNP were retained after QC, which removed 275 SNP for CR and 43 SNP for MAF to provide a final marker matrix of 7,567 markers for the evaluation of genetic diversity. Levels of genetic diversity within each population were calculated as expected heterozygosity (HE), average pairwise genetic distance (DST), and inbreeding coefficients (F) were estimated using the hierfstat package [37]. Populations with more than 10 individuals were used to provide more reliable estimates of genetic variation among and within populations.
To provide broader comparisons, additional genotype data for individuals from other populations with less than 10 individuals represented were used to estimate inbreeding and relatedness coefficients following [37, 38]. A genetic distance matrix was estimated using Pearson’s correlation estimates of standardized data and Ward’s method (D2) was used for clustering individuals into a fixed number of panmictic populations identified using the snapclust [39] function in the adgenet package to minimize the Akaike and Bayesian Information Criteria for group number. Among population genetic distances (Fst) were estimated using the Cavalli-Sforza and Edwards Chord’s method [40–42]. The matrix of Fst estimates was used to produce a phylogenetic tree with neighbor-joining tree estimation [43] in the ape package [44]. Principal component analysis of marker data with the Base R function prcomp was used to display similarities of marker profiles among individuals labeled by population of origin [45].
3. Results
3.1. Pathogen Screening of Breeding Population
A total of 1133 analyses were performed to assess the incidence of 19 pathogens in 138 P. monodon, whereas 96 by molecular pathology and 42 shrimps were analyzed by histopathology (Tables 1 and 2). A range of tissue types and methods were used to account for variation in the development of pathogens from post-larvae to mature broodstock. There were no pathogens detected in any of the analyses undertaken, indicating that the population that has been managed for 10 generations in a closed system is free of the examined pathogens. Light micrographs of paraffin sections stained by hematoxylin & eosin taken from P. monodon analyzed in this study are provided in Figures S1 and S2.
| Diagnostic molecular method | Sample ID#a | Total analysis by each pathogen | |||
|---|---|---|---|---|---|
BOX GENICS (FK00640204) |
From A1 (FR42887682) to E3 (FR42887702) | From F3 (FR42887703) to E8 (FR42887742) | From F8 (FR42887743) to H12 (FR42887777) | ||
| LAQUA-UEMA | OS – 012–24 From 1 to 21 broodstook (Pleopods and feces) |
OS – 013–24 From 1 to 8 pools of 5 Juveniles (pleopods and dissected Cephalothorax) |
OS – 014–24 From 1 to 7 pools of 5 Small juveniles (Cephalothorax) |
||
| Realtime (qPCR/RT-qPCR) using TaqMan probe | WSSVb | ND ∗ | ND | ND | 74 |
| IHHNVc | ND | ND | ND | 74 | |
| DIV1d | ND | ND | ND | 52 | |
| NHP-Be | ND | ND | ND | 52 | |
| EHPf | ND | ND | ND | 52 | |
| AHPND (EMS)g | ND | ND | ND | 74 | |
| IMNVh | ND | ND | ND | 74 | |
| TSVi | ND | ND | ND | 74 | |
| YHVj | ND | ND | ND | 74 | |
| CMNVk | ND | ND | ND | 74 | |
| PvSVl | ND | ND | ND | 74 | |
| PvNVm | ND | ND | ND | 74 | |
| MrNVn | ND | ND | ND | 74 | |
| LSNVo | ND | ND | ND | 37 | |
| PCR/RT-PCR | MBVp | ND | ND | ND | 37 |
| PvSPNV (BP)q | ND | ND | ND | 37 | |
| HPVr | ND | ND | ND | 37 | |
| MoVs | ND | ND | ND | 37 | |
| TPDt | ND | ND | ND | 37 | |
| Total of analysis | — | — | — | — | 1.133 |
| Number of shrimps analyzed | — | 21 | 40 | 35 | 96 |
- aID# = Sample ID.
- bWhite spot virus (WSSV).
- cPenaeus stylirostris pestyldensovirus 1 (PstDNV1) [infectious hypordemal and hematopoietic necrosis virus (IHHNV)].
- dDecapod iridescent virus – type 1 (DIV1 [SHIV]).
- eHepatobacter penaei bacterium (NHP-B).
- fE. hepatopenaei (EHP).
- gV. parahaemolyticus [(PirA and PirB) acute hepatopancreatic necrosis disease (AHPND “EMS”)].
- hInfectious myonecrosis virus (IMNV).
- iTaura syndrome virus (TSV).
- jYellow Head Virus type 1 (YHV-1).
- kCovert mortality nodavirus (CMNV).
- lPenaeus vannamei solinvivirus (PvSV).
- mPenaeus vannamei Nodavirus (PvNV).
- nM. rosenbergii nodavirus (MrNV).
- oLaem Singh Virus [Monodon Retarded Growth Syndrome - (LSNV)].
- pMonodon Baculovirus (MBV).
- qBaculovirus penaei (PvSPNV).
- rMourilyan Virus (MoV)’.
- sDecapod hepanhamaparvovirus (Hepatopancreatic Parvovirus [HPV]).
- tV. parahaemolyticus (Transparent Postlarval Disease [TPD]).
- ∗ND = Not detected.
| Pathogen | ID#a Sample | |
|---|---|---|
| OS-013–24 | OS-014–24 | |
Slides 1–11 22 shrimp |
Slides 1–9 18 shrimp |
|
| HP VACb | L0 a L4 | L2 a L3 |
| IHHNVc | G-v | ND |
| WSSVd | ND ∗ | ND |
| IMNVe | ND | ND |
| LSNVf | ND | ND |
| PvSVg | G- | ND |
| AHPNDh | ND | ND |
| EHPi | ND | ND |
| DIV1j | ND | ND |
| NHP-Bk | ND | ND |
| TSVl | ND | ND |
| YHVm | ND | ND |
| TPDn | ND | ND |
| BPo | ND | ND |
| MBVp | ND | ND |
| MrNVq | ND | ND |
| PvNVr | ND | ND |
| CMNVs | ND | ND |
| MoVt | ND | ND |
| HPVu | ND | ND |
- aID# = Sample identity.
- bHP VAC = Hepatopancreas vacuolation levels, an indicator of nutrient absorption, digestion and storage (L0 = lowest; L4 = highest).
- cIHHNV = Decapod pestylhamaparvovirus 1 (PstDNV1), Infectious hypodermal and hematopoietic necrosis virus.
- dWSSV = White Spot Virus.
- eIMNV = Infectious Myonecrosis Virus.
- fLSNV = Virus Laem Singh (Monodon Slow Growth Syndrome).
- gPvSV = Penaeus vannamei solinvivirus.
- hAHPND “EMS” = V. parahaemolyticus (PirA and PirB), Acute Hepatopancreatic Necrosis Disease.
- iEHP = Infection with E. hepatopenaei.
- jDIV1/SHIV = Decapod Iridescent Virus – type 1.
- kNHP-B = Hepatobacter Penaei Bacterium.
- lTSV = Taura Syndrome Virus.
- mYHV = Yellow Head Virus type 1.
- nTPD = V. parahaemolyticus (VHVP-2), Transparent Postlarval Disease.
- oPvSPNV “BP” = Baculovirus penaei.
- pMBV = Monodon baculovírus.
- qMrNV = M. rosenbergii nodavirus.
- rPvNV = Penaeus vannamei Nodavirus.
- sCMNV = Cover mortality Nodavirus.
- tMoV = Virus Mourilyan.
- uHPV = Decapod hepanhamaparvovirus (Hepatopancreatic Parvovirus).
- vG- to G4 = Degree of severity of infection/injury according to the severity grade table.
- ∗ND = Not detected.
IHHNV was detected in 2 of 42 juvenile shrimp and PvSV was detected in 5 of 42 juvenile shrimp located outside area of maturation located in the nursery area. All signs found were with severity grade G-Trace, which can be classified as suspected infections and below level of detection. Both IHHNV and PvSV were not detected by molecular pathology in those groups of juveniles, so, we cannot conclude that those juveniles were infected by both IHHNV and PvSV because it does not meets the WOAH’s Aquatic manual [18] criteria of a “confirmed” case, but meets the criteria as “suspected” case (detect by only one criteria/diagnostic method). No other lesions associated with WSSV, IMNV, LSNV, NHP-B, EHP, AHPND, YHV, TSV, MrNV, MoV, DIV1, TPD, BP, HPV, MBV, PvNV and CMNV were observed in the shrimp assessed with histopathology.
3.2. Within-Population Genetic Diversity
To begin evaluating relative levels of genetic diversity, the expected heterozygosity HE and average pairwise genetic distance (DST) were estimated for the 21 broodstock of the Brazilian population to compare with other populations that had been genotyped previously. Within-population genetic diversity estimates are provided for populations with more than 10 individuals. When compared to other populations, the Brazilian broodstock were relatively outcrossed with low inbreeding coefficient (F = 0.03). The inbreeding coefficient for the global breeding program animals (F = 0.34) and the Mozambique Channel (MC) animals (F = 0.34) included in the study were more than 10 times greater than the estimate from the Brazilian animals. The samples from Australian (AU) wild animals and the MC wild population had the lowest and highest levels of inbreeding, respectively. The other populations were compiled from genotypes of individuals in domesticated populations that were sourced from various regions to provide examples of how different inbreeding management strategies have impacted changes in genetic diversity (Table 3).
| Origin | Name | Type | N | HE | DST | F |
|---|---|---|---|---|---|---|
| Mozambique channel | MC | Wild | 11 | 0.049 | 0.197 | 0.344 |
| Vietnam | VN | Dom | 10 | 0.540 | 0.187 | 0.004 |
| Australian wild | AUS | Wild | 15 | 0.554 | 0.167 | 0.008 |
| West AUS | AUSWA | Dom | 19 | 0.476 | 0.120 | 0.064 |
| Global breeding pop 3 | GL3 | Dom | 10 | 0.052 | 0.127 | 0.341 |
| Global breeding pop 5 | GL5 | Dom | 10 | 0.395 | 0.133 | 0.104 |
| Northern Terr. AUS | AUSNT | Dom | 10 | 0.364 | 0.139 | 0.125 |
| Brazil | Brazil | Dom | 21 | 0.526 | 0.111 | 0.033 |
- Note: The number of animals (N), proportion of SNP with polymorphic 280 (PN), number of monomorphic SNP (M), expected heterozygosity (HE), average pairwise genetic distance (DST), and inbreeding coefficient estimates (F) are provided.
3.3. Divergence Among Animals and Populations
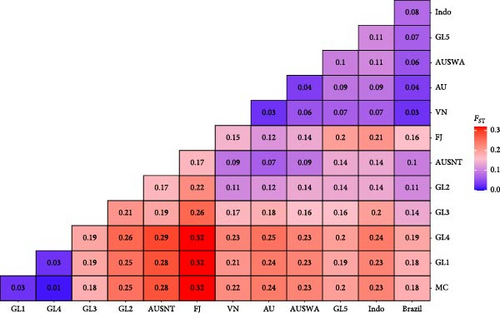
Comparisons among individuals within populations incorporated samples from an additional five populations that were represented by less than four genotyped animals each. Estimates of the genetic distance (Fst) among populations are provided in Figure 2, which identifies animals from the Mozambique Channel (MC) region as genetically distinct from most other breeding populations, and very similar to two of the global breeding programs (1 and 4) that were sampled. The MC population was used to root the phylogenetic tree created with the neighbor joining approach used with population Fst estimates (Figure 3). The GL3 population appears to be intermediate between the African and the Asian or Oceania populations, perhaps indicating the population is derived from mating among these distinct sub-populations. The wild and domesticated Australian populations formed a separate cluster with the genetically distinct population from Fiji. Considering among population Fst estimates of genetic distance, the Brazilian population was placed between the Indonesian and Vietnamese populations and was not significantly different to most of the Asian and Australian populations.
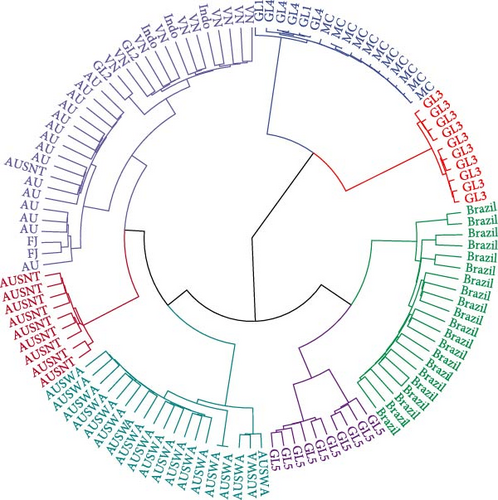
Maximum likelihood inference of the number of genetic groups that were present in the data suggested seven groups were present using Aikaike’s information Criteria and three groups were present using a Bayesian Information Criteria (BIC). The seven clusters are presented in Figure 3 and provide similar classification results to what is provided by the estimates of population specific Fst estimates in Figure 2. There were strong similarities among populations the GL2, Indonesian and Vietnamese populations as well as in the GL1, GL4 and Mozambique Channel (MC) populations. There was little genetic variation within the cluster of GL1, GL4 and MC animals or among the set of GL3 animals, The inbreeding estimates of all these populations were high. All animals in the domesticated population from Western Australia clustered separately from other Australian populations, supporting the long-standing hypothesis that the Western Australian population is genetically distinct from other Australian P. monodon populations [46–49]. When three clusters are assumed to represent the underlying population structure, the MC and GL3 clusters are joined, the Brazilian and GL5 clusters are joined and the rest of the populations from Asia and Oceania form a third cluster.
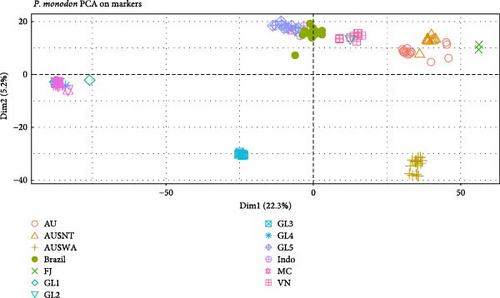
Principal components (PC) analysis identified strong differentiation among many populations as well as similarities among others that was evidenced with individuals from different populations overlapping one another in the first two dimensions (Figure 4). A total of 28.3% of the allelic variation among individuals in the population was accounted for by the first two latent variables identified with PC analysis. Individuals from the Mozambique Channel and the three global breeding populations with high inbreeding coefficients were tightly clustered compared to the other populations with animals that were more distributed along the PC axes. The Australian and Fiji populations positioned to the right of the plot had the greatest genetic distances when compared to the populations clustering with the Mozambique Channel animals. The Western Australian population was positioned closely to the other Australian populations along the first PC but was differentiated in a similar manner as the GL3 population using the second PC.
4. Discussion
A genetically diverse breeding population of P. monodon may be used to provide the Brazilian industry with an alternative to L. vannamei for domestic production. Initiating a breeding program with a pathogen free and genetically diverse population will ensure production constraints imposed by disease and inbreeding depression are avoided [50].
4.1. Pathogens
In global shrimp farming, pathogens like EHP, WSSV, IMNV, CMNV, HPV-like, PvSNPV, VpAHPND, EHP, and DIV1 have been associated with widespread production losses. In Brazil, PvSV, IMNV, WSSV, IHHNV, and PvSNPV are common pathogens in shrimp-producing areas, and the development of disease has been caused by pathogen presence and multiple associated factors. Over reliance on molecular diagnostics and concurrent neglect of histopathology has created challenges for the shrimp farming community. Implementing the combination of histopathology and molecular diagnostics will provide fundamental safeguards for protecting the shrimp farming industry by reducing detection failures of new or rapidly evolving pathogens, improving disease detection and identification, and improving animal health management practices [51].
Prevention of pathogen spread via monitoring of broodstock and post-larvae for emerging and established pathogens (PvSV, IMNV, microsporidian, etc.) is essential to identify and prevent future outbreaks of disease. It is important to note that molecular diagnostics (i.e., PCR-based assay) is used to test for a specific pathogen and not the general health status of shrimp that is provided by histopathology. Therefore, general health assessment using histopathology should be conducted routinely in addition to targeted or passive surveillance with molecular techniques. If a new disease is suspected using histopathology, molecular analysis may be performed to supplement and validate histopathological findings. This would enable rapid identification of an emerging pathogen or a new genotype of an existing pathogen.
Currently, routine diagnosis of diseases of penaeid marine shrimps is carried out routinely for VpAHPND, EHP, WSSV, IMNV, IHHNV, TSV, YHV, MrNV, NHP-B, BP, MBV, LSNV, HPV, CMNV, PvNV, MoV, DIV1, and PvSV. Considering this is a long list and it is quite expensive to screen animals for all the diseases, stakeholders should prioritize screening of those diseases that are being most relevant for their operations in maturation, hatchery, and grow out ponds.
WOAH outlines diagnostic techniques for WOAH-listed and others significant diseases, providing guidance for aquatic animal health laboratories worldwide, enhancing efficiency and advancing global aquatic animal health. Adopting these methods, along with adherence to accredited international standards such as ABNT NBR ISO/IEC 17025 : 2017, ensures diagnostic accuracy and reliability [18].
In this study, the detection of IHHNV (in 2 of 42) and PvSV (in 5 of 42) in small juvenile shrimp from the nursery area located outside the indoor maturation, qualified as “suspected” cases, as only histopathology identified then, under WOAH Aquatic Manual [18] criteria, not “confirmed”. These findings, classified as severity grade G-Trace, indicate infection levels detectable only by well-trained histopathologists, as they may involve isolated cells with small or larger viral stroma in the nucleus or cytoplasm, Molecular pathology did not detect either virus in these juveniles, likely due to the very low level, preventing confirmation of infection. We usually find a different scenario in your experience where PvSV can easily be detected by PCR in shrimp. Importantly, neither virus appeared to cause disease in these small juveniles of P. monodon.
Historically, IHHNV is linked to high mortality in P. stylirostris; it prompted the development of specific pathogen-resistant (SPR) lines. In P. vannamei and some populations of P. monodon, it is associated with runt deformity syndrome (growth suppression). There are speculations on whether IHHNV should remain as a WOAH-listed pathogen [52] however, the reality is that further independent studies need to be conducted to determine the true economic impact of IHHNV on different Penaeid species and different genetic lines to inform such decision making process. Survival, growth, impact of deformity severity and frequency on processing, and impact of multi-factorial pathogen and environmental impacts, all need to be taken into account.
PvSV is an emerging, non-WOAH-listed pathogen that has been called attention due to its prevalence and it may be globally distributed [9, 53]. It is a systemic/enteric disease that is more easily detected in hepatopancreas than in other organs through histopathology. However, molecular pathology can detect PvSV in various tissues, with the hepatopancreas been the most reliable. While PvSV does not cause mortality in shrimp on its own (Andrade TPD, unpublished data), its potential role in morbidity and mortality, particularly through interactions with other viruses like IMNV and WSSV, remains to be clarified.
PCR-based methods remain the primary tools for disease diagnosis and monitoring in shrimp farming due to their high sensitivity, specificity, and ability to detect low pathogen levels. Despite their advantages, they have limitations, highlighting the need to complement them with other diagnostic approaches.
Climate change directly impacts environmental parameters such as temperature and salinity, altering the distribution and the distribution and prevalence of pathogens, influencing shrimp health and productivity. For instance, the incidence of WSSV increases at lower temperature, while IMNV is more prevalent at higher temperatures. The native P. monodon in Brazil, which is genetically well-adapted to the region, may perform better due natural adaptation to the local climate and endemic diseases. Therefore, genetic optimization, combined with application of disease diagnostics, should be prioritized to select desirable traits in these native P. monodons populations. This approach would enhance their health and productivity, ultimately benefiting farmers.
Based on the information presented, the P. monodon lines assessed here are free of the 19 pathogens using both molecular diagnostics and histopathology. Domestication over 10 generations may have cleared pathogens from the population and further disease screening of animals from the wild would provide germplasm that may be infused into existing breeding programs developed from locally adapted stock. Further diagnostics using both molecular diagnostics and histopathology and formal quarantine protocols may be put in place to prevent problems with pathogen dispersion to prevent increases in disease incidence.
4.2. Diversity and Inbreeding
Genetic diversity within and among populations may be shaped by many different factors, such as inbreeding, which may be quantified using genetic markers. Inbreeding results from the mating of related individuals and its associated reductions in genetic variation may lead to negative consequences such as inbreeding depression or a reduction in fitness due to increased homozygosity [54, 55]. Inbreeding in domesticated populations is managed by directing mating between unrelated animals and infusing new germplasm into populations to increase genetic diversity. A recent review of Australian P. monodon populations found inbreeding coefficients (F) in Australian wild caught animals were similar to that found in many of the populations included in this study [49]. As well, Vu et al. [49] found North Queensland and Northern Territory P. monodon populations exhibited F values greater than 0.10. Many of the populations in the current study had higher inbreeding coefficients, with domesticated animals from the global breeding programs and the Mozambique Channel exhibiting F values that were much greater. There were no inbreeding coefficients greater than the high F values found for wild L. vannamei collected from Panama to Mexico (F = 0.53) [56] or from the Mexican coast (F = 0.36) [57]. While the impacts of inbreeding in shrimp are not widely reported, Moss et al. [58] associated F values greater than 0.10 with inbreeding depression in the form of slower growth and survival in Pacific white shrimp (L. vannamei).
4.3. Genetic Distance Among Populations
Various global scale studies of P. monodon genetic diversity involving wild populations distributed across eastern Africa, Southeast Asia, Australia, and South Pacific islands have been undertaken [46–48, 59]. Comparisons of wild and domesticated populations have been made [60], which demonstrated marked differences between wild and domesticated populations. The large genetic distance between the Mozambique Channel animals and the other populations has also been noted by You et al. [47], Benzie et al. [46], and Duda and Palumbi [59]. Our findings for the animals for Fiji align with Waqairatu et al. [48], who found a genetically distinct population that has been developed.
Within Australia, a similar pattern of population differentiation was noted, with the Western Australian (WA) population identified as distinct from various other Australian populations. This has been attributed to population separation by lower sea levels that occurred during the Holocene by Sloss et al. [61]. Vu et al. [49] also found P. monodon populations pairwise Fst values within Australia were low (0.001 to 0.107) and similar to what was found in this study, with the exception of the most geographically separated site in Western Australia, which exhibited the largest significant pairwise Fst (0.107) value when compared with populations from Queensland. While this Fst estimate is higher than what was found in the current study, our results support the findings of others that have found there is a restriction in gene flow between geographically disparate Australian P. monodon populations. The relatively low levels of inbreeding indicate there are few restrictions to breeding strategies to domesticate this population.
5. Conclusions
The source of the populations that have become established in Brazil is unknown and may result from various introductions of different populations. The process of developing maturation and hatchery systems to domesticate this population has produced broodstock that are not inbred compared to other populations. The current domesticated Brazilian P. monodon population is most closely related to the Indonesian and Vietnamese populations that were sampled in this study. Large genetic differences observed between the Brazilian population and those from the Mozambique Channel and Western Australia make these regions desirable for providing additional genetic diversity should this be required in the future. Given the low to moderate level of inbreeding and the freedom from specific pathogens, this population would provide a suitable starting point to initiate a breeding program for P. monodon in the north of Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Thales P.D. Andrade and Jeremy Brawner conceived the study and produced a draft of the manuscript, and all authors improved the draft and approved for submission. Thales P.D. Andrade and Amanda Gomes managed the sampling, literature review to determine occurrences of P. monodon in Brazil that was used to produced maps, preformed pathogen detection using real-time RT-PCR/PCR, standard RT-PCR/PCR, and histopathology. Jeremy Brawner analyzed the marker data.
Funding
This research was provided by the Crustaceans Diseases Diagnostic Laboratory (LAQUA-UEMA) of the State University of Maranhão from diagnostics services. Funding for travel sampling and shipping to pathogen testing and genotyping was received from Marine Aquacrusta Ltd. & Flavors of the Coast. Funding to complete the research was provided by Genics and CSIRO.
Acknowledgments
The authors thank Marine Aquacrusta Ltd. & Flavors of the Coast for producing and collaborate in this study.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




