Green Boost for Nile Tilapia, Oreochromis niloticus: Unveiling the Multifaceted Effects of Chenopodium album Leaves Powder on Growth, Hematology, Antioxidant Defense, Nonspecific Immunity and Tolerance Against Aeromonas hydrophila
Abstract
This study assessed the impact of dietary Chenopodium album leaf powder on various aspects of Nile tilapia (Oreochromis niloticus), including growth performance, hematological and biochemical profiles, antioxidant status, nonspecific immune response, and resistance to Aeromonas hydrophila infection. A 60-day feeding trial was conducted with 180 Nile tilapia fingerlings, with an initial average weight of 11.79 ± 0.23 g, fed diets supplemented with increasing levels of C. album at 0, 10, 20, and 30 g/kg. After feeding, the fish underwent an experimental challenge with the pathogen A. hydrophila. Following the challenge, the fish survival was monitored for 14 days. The results demonstrated that as the level of C. album supplementation increased, there were significant (p < 0.05) improvements in growth performance and a reduction in the feed conversion ratio (FCR). The hematological and biochemical profiles showed significant (p < 0.05) enhancements in the groups that received C. album-supplemented diets. Additionally, the antioxidant status in the fish serum significantly (p < 0.05) improved, as evidenced by increased activities of superoxide dismutase (SOD), catalase (CAT), and reduced glutathione. The nonspecific immune response, including lysozyme activity, phagocytic activity, and fish survival, also exhibited significant (p < 0.05) enhancements, particularly in the groups receiving 20–30 g/kg of C. album. Furthermore, there was a significant decrease in serum glucose and cortisol levels in the groups fed with 20–30 g/kg of C. album. In conclusion, C. album supplementation at 20–30 g/kg improved growth, immune response, and disease resistance in Nile tilapia. This diet also reduced stress markers, enhancing overall health.
1. Introduction
Aquaculture is a pivotal player, and its significance is poised to persist, addressing the global surge in demand for aquatic products [1]. Tilapia (Oreochromis sp.) stands out as an extensively cultivated freshwater aquaculture species, and there is an expectation that its production will rise to meet the growing demand for fish driven by an expanding population [2]. Its widespread global cultivation can be attributed to its swift growth, efficient feed utilization, natural spawning, and excellent edible characteristics [3]. However, aquaculture development has given rise to several challenges, including slow growth, weakened health, and enhanced vulnerability to infectious diseases, particularly bacterial ones. These diseases exhibit opportunistic traits and can impose substantial adverse economic consequences on freshwater and marine aquaculture [4]. Additionally, fish undergo constant exposure to a range of stresses within their intensive production systems. These exposures can include fluctuations in water quality, overcrowded conditions, and temperature variations [5, 6].
Applying traditional medications and vaccines for disease prevention and treatment carries notable drawbacks [7]. The use of antibiotics in aquaculture to address bacterial infections contributes to the growing issue of antibiotic resistance, driven by misuse, overuse, and the transfer of resistance genes among aquatic bacteria. This trend threatens treatment efficacy and poses risks to public health through the potential transfer of resistant pathogens or residues in fish for human consumption [8, 9]. Consequently, it becomes imperative to devise safe and cost-effective alternatives to conventional disease control methods, aiming to uphold the principles of eco-friendly aquaculture. Over the years, remarkable strides in fish nutrition have resulted in the formulation of specialized feeds and innovative, well-balanced commercial diets. These advancements contribute significantly to achieving optimal growth and the production of top-quality, healthy fish [10, 11]. Numerous endeavors have been undertaken to incorporate different plants as feed additives, presenting innovative strategies to mitigate disease risks and enhance the immune system when fish are exposed to stressors like poor water quality, transport, and rough handling in aquaculture settings [12–15]. Among these plants, Chenopodium album Linn., belonging to the Chenopodiaceae family, originates from Western Asia and falls within the category of explored plants. It is a member of the broader genus Chenopodium, which boasts a global distribution and encompasses ~250 species [16]. C. album has grown organically as a weed in wheat, gram, mustard, barley, and other crop fields [17]. This species has been cultivated as a leafy vegetable and a significant secondary grain crop, serving as a valuable source of high-protein and well-balanced amino acids for human and animal consumption [18]. The leaves of C. album are abundant in proteins, featuring a notable concentration of essential amino acids like lysine, leucine, and isoleucine. Additionally, they contain substantial quantities of calcium, iron carotenoids, and vitamins A and C [19]. Phytochemicals such as polyphenols, flavonoids and isoflavonoids, saponin, phenolic amide, alkaloid chenoalbicin, xyloside, apocarotenoids, etc., have sparked considerable interest due to their potential contributions to maintaining human health, particularly in significantly reducing the risk of cancer [20, 21]. C. album has demonstrated potent antioxidant properties, effectively preventing or retarding the pace of oxidation [20]. C. album contains seven distinct free phenolic acids, including vanillic acid, gallic acid, syringic acid, protocatechuric acid, protocatechuric acid, protocatechuric aldehyde, caffeic acid, and vanillin [22]. Apart from the many benefits of C. album in aquaculture, it also poses certain risks and limitations, including variability in its bioactive compounds, potential toxicity from secondary metabolites, and the risk of nutritional imbalances when overused in diets [23]. Studies have also revealed that the leaves of C. album possess antifungal, antibacterial, antiviral, and immunostimulant properties [24, 25]. Therefore, incorporating the leaves of C. album into the diet could potentially enhance immune function and reduce the susceptibility of fish to pathogens. However, the exploration of its full potential as a dietary supplement in aquaculture remains incomplete. Hence, the primary goal of this study was to assess the impact of incorporating different concentrations of C. album leaves powder into the diets xof Oreochromis niloticus. The evaluation focused on growth, hemato-biochemical indicators, antioxidant levels, and fish resistance to Aeromonas hydrophila infection in Nile tilapia.
2. Materials and Methods
2.1. Plant Preparation
Leaves from C. album were gathered in Khyber Pakhtunkhwa province, Pakistan, from the village area. Subsequently, a botanist at the Faculty of Botany, University of Sargodha, Pakistan, identified and authenticated the specimens. The leaves were washed with tap water and cleaned thoroughly with distilled water. Following this, they were dried at 45°C. The plant material was finely powdered using an electronic grinder and then stored in a sealed plastic container for later use [26]. The proximate composition of the analyzed feed was determined following the AOAC [27] protocol. The identification of bioactive components within C. album was conducted through gas chromatography–mass spectrometry (GC–MS) analysis (Table 1). The GC–MS analysis was performed using a PerkinElmer TurboMass Spectrophotometer (Norwalk, CT, USA, model CTO 6859). The system was equipped with a capillary column measuring 30 m in length and 0.25 mm in diameter, coated with 95% dimethylpolysiloxane as the stationary phase. Helium was used as the carrier gas at a constant flow rate of 0.5 mL/min to ensure optimal separation and detection. The resulting chromatographic peaks were analyzed and quantified using TurboMass OCPTVS-Demo SPL software, ensuring precise evaluation of the compounds.
| Compounds | Molecular formula | RT (min) | PA (%) |
|---|---|---|---|
| Ascaridole | C10H16O2 | 2.81 | 0.32 |
| Cryptomeridiol | C15H28O | 9.86 | 0.48 |
| Carvacrol | C10H14O | 12.10 | 0.63 |
| Chenoalbicin | C36H34N2O7 | 13.64 | 7.54 |
| p-Cymene | C10H14 | 15.51 | 1.22 |
| α-Terpinene | C10H16 | 15.80 | 9.60 |
| Phytic acid | C6H18O24P6 | 17.23 | 5.33 |
| Methyl 10-methyldodecanoate | C14H28O2 | 18.06 | 1.64 |
| Isophytol | C20H40O | 18.23 | 0.87 |
| Ethyl palmitate | C18H36O2 | 18.27 | 0.72 |
| Dibutyl isophthalate | C16H22O4 | 18.36 | 1.22 |
| Ethyl linoleate | C20H36O2 | 18.45 | 0.97 |
| Kaempferol | C15H10O6 | 18.22 | 0.82 |
| Gallic acid | C7H6O5 | 17.73 | 0.74 |
| Ascorbic acid | C6H8O6 | 17.04 | 0.68 |
- Abbreviations: GC–MS, gas chromatography–mass spectrometry; PA, peak area; RT, retention time.
2.2. Fish Rearing Conditions
Fingerling Nile tilapia, O. niloticus were procured from a local fish hatchery in Faisalabad, Pakistan, and underwent examination by CCoA [28] guidelines. Subsequently, 15 fish were allocated to each of the 12 glass tanks (80 L; 60 × 30 × 45 cm), resulting in 180 fish. The fish were initially housed for 2 weeks, during which they were provided with the basal diet and maintained a 12/12 light–dark cycle. Concurrently, constant observation was carried out to monitor any diseases or instances of mortality. Afterward, the weights of individual fish were recorded, with an average initial weight of 11.79 ± 0.23 g at the onset of the experiment.
2.3. Experimental Design
After the acclimation period, the fish were randomly assigned to four treatments, each with three replicates (45 fish per treatment, 15 fish per replicate). Each tank served as a replicate unit and was provided with continuous aeration. Four experimental diets were formulated by incorporating leaf powder into the mixture at concentrations of 0 g/kg (control), 10 g/kg diet (1%), 20 g/kg diet (2%), and 30 g/kg diet (3%). The doses were selected based on common practices in aquaculture for plant-based additives [26, 29]. These concentrations were typical for preliminary studies to assess a range of effects on growth, immunity, and health, providing a basis for future research. The diet ingredients underwent mechanical mixing, resulting in the formation of pellets with a diameter of 1.5 mm through the use of a pellet machine. After being prepared, the diets underwent a 24-h air-drying period at room temperature and were stored at 4°C until use. The composition of the basal and experimental diets, along with their respective ingredients, is presented in Table 2. The fish were given the experimental diets at a rate equivalent to 3% of their total biomass, administered twice daily at 9:00 a.m. and 3:00 p.m., over 60 days. Adjustments were made to the dietary percentage every 2 weeks to align with fluctuations in fish weight. Additionally, any unconsumed feed was gathered, dried, and deducted from the initially provided amount to ensure precise calculation of feed intake (FI) [26].
| Basic ingredients (g/kg) | Concentration of C. album diet (g/kg) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| Fish meal | 120 | 120 | 120 | 120 |
| Soybean meal | 410 | 410 | 410 | 410 |
| Maize powder | 280 | 270 | 260 | 250 |
| Wheat bran | 110 | 110 | 110 | 110 |
| Sunflower oil | 40 | 40 | 40 | 40 |
| Cod liver oil | 10 | 10 | 10 | 10 |
| Vitamin premixa | 10 | 10 | 10 | 10 |
| Mineral premixb | 20 | 20 | 20 | 20 |
| C. album leaves powder | 0 | 10 | 20 | 30 |
| Chemical examination | ||||
| Crude protein (N × 6.25) | 307 | 304 | 306 | 308 |
| Crude fiber | 54 | 54 | 56 | 56 |
| Crude lipids | 74 | 75 | 78 | 78 |
| Ash | 72 | 73 | 74 | 74 |
| Nitrogen-free extractc | 493 | 494 | 486 | 484 |
| Gross energy (kcal/kg)d | 445.66 | 445.87 | 446.16 | 447.47 |
- Vitamin premixa (per kilogram): vitamin A (88,000 IU), vitamin E (7000 mg), vitamin D3 (2,000,000 IU), vitamin K3 (1500 mg), biotin (50 mg), folic acid (700 mg), nicotinic (20,000 mg), pantothenic acid (7000 mg), vitamin B1 (700 mg), vitamin B2 (3500 mg), vitamin B6 (1000 mg), and vitamin B12 (7 mg).
- Mineral premixb (per kilogram): ZnSO4 (4.0 g), FeSO4 (20 g), MnSO4 (5.3 g), CuSO4 (2.7 g), CaI2 (0.34 g), Na2SeO3 (70 mg), CoSO4 (70 mg), and CaHPO4•2H2O up to 1 kg.
- Nitrogen-free extract (NFE)c 100 − (protein % + fat % + crude fiber % + ash %).
- Gross energy (GE)d was calculated as kcal/g for protein (5.65), lipid (9.45), and NFE (4.11).
Daily monitoring of water temperature, dissolved oxygen, and pH levels was conducted to maintain the recommended parameters and sustain optimal conditions for growth and survival throughout the experiment. The water temperature varied between 26.02 and 26.86°C, dissolved oxygen levels were 6.2–6.7 mg/L, pH levels were between 7.1 and 7.6, and the ammonia concentration ranged from 0.21 to 0.26 mg/L. All parameters remained within the acceptable limits for fish aquaculture [30]. Biweekly, three-fourths of the tank water and accumulated fish excrement were replaced with fresh, aerated water sourced.
2.4. Fish Growth Performance
Here, FW refers to the final weight. IW refers to the initial weight. DW stands for dry weight. WW stands for wet weight. ln represents the natural logarithm. N indicates the number of culture days.
2.5. Blood Sampling
After the 60-day feeding experiment, blood samples were obtained from four randomly chosen fish in each tank (16 samples/group). Before sample collection, the fish were anesthetized using 85 mg/L clove oil [32]. Blood was collected from the caudal vessels in heparin-containing vials to assess hematological parameters. For white blood cell (WBC) count and differential, blood was collected in EDTA-containing vials to preserve leukocyte morphology and ensure accurate results. Aseptic syringes, devoid of anticoagulants, were employed to collect additional blood samples. After sampling, the fish were transferred to clean, aerated water for recovery (7.20 ± 0.16 mg/L). Once they had fully recovered, they were moved to their respective tanks. These samples were centrifuged at 5000 rpm for 15 min to obtain serum, which was then stored in the refrigerator at −20°C until needed. The biochemical and immunological assays were carried out using the serum samples.
2.6. Hematological Analyses
Hematological analyses include the assessment of red blood cell (RBC) and WBC counts using an upgraded Neubauer hemocytometer [33]. Hemoglobin (Hb) concentration was determined through the cyanmethemoglobin method, which involved measuring optical density at 540 nm using spectrophotometry. Hematocrit (Hct) levels were determined by centrifuging micro-Hct tubes at 3000 rpm for 5 min. The blood smears were prepared on glass slides and stained using the May–Grünwald Giemsa process [34].
2.7. Biochemical Analysis
Serum albumin (catalog No. SB 028 500) and total proteins (catalog No. SB 0250 500) were analyzed spectrophotometrically (Lambda EZ201, PerkinElmer USA), employing methodologies outlined by Reinhold [35] and Henry [36], respectively. Moreover, serum globulins were measured using the procedure of Coles [37]. Serum glucose levels were determined utilizing a colorimetric diagnostic kit (Siemens Healthineers, Germany). The assessment of serum cortisol levels was conducted using the procedure of Tunn et al. [38].
2.8. Serum Antioxidant Enzymes Status
Antioxidant activities in serum were assessed through the utilization of a colorimetric commercial kit (Suzhou Comin Biotechnology Co. Ltd). Catalase (CAT, catalog No. CT 25 20) and superoxide dismutase (SOD, catalog No. SD 25 21) activity was observed through the methodology given by Aebi [39] and Nishikimi, Rao, and Yagi [40], respectively. The quantification of glutathione dehydrogenase (GSH, catalog No. GH 25 14) through colorimetric analysis was carried out in accordance with the protocol of Beutler [41].
2.9. Analysis of Nonspecific Immune Response
2.10. Challenge Test
To assess the resilience of O. niloticus to A. hydrophila bacteria (obtained from the Microbiology Department, Punjab University Pakistan), 15 fish (five from each treated tank) were randomly selected and collectively placed in a separate tank (one tank per treatment). After a 60-day trial involving feeding and blood sampling, each fish received an intraperitoneal injection of 0.1 mL containing a suspension of A. hydrophila at a concentration of “1.5 × 108 cells/mL.” The chosen concentration for the intraperitoneal injection of A. hydrophila was selected to ensure a consistent and controlled exposure to the pathogen, sufficient to induce a measurable immune response in the fish without causing immediate mortality [26]. The fish were attentively observed for any signs of illness, including unusual behavior. Any deceased fish were gently removed from the tanks to minimize stress. Survival rates were closely monitored in all groups throughout the 14-day postinfection period. Bacterial infection was confirmed by extracting bacteria from deceased fish. Upon the termination of the experiment, random fish were captured from the surviving group and examined for the presence of bacteria. It was found that the fish had successfully resolved the bacterial infection, as no bacteria were detected in the live specimens. Throughout the postchallenge phase, the fish were provided with experimental diets and maintained under the identical rearing conditions implemented during the initial feeding period.
2.11. Relative Percentage Survival (RPS)
2.12. Statistical Analysis
The statistical analysis was performed using a one-way analysis of variance (ANOVA) in SPSS version 28.0. Before conducting ANOVA, the normality of the data was assessed using the Shapiro–Wilk test and visual inspection through Q–Q plots to ensure the data followed a Gaussian distribution. Tukey’s multiple comparison post hoc test was used to compare differences between groups. Additionally, segmental nonlinear regression was conducted using Prism GraphPad version 9.0 to determine the optimal dose for specific factors such as weight gain, feed conversion ratio (FCR), protein efficiency ratio (PER), and specific growth rate (SGR). The threshold for statistical significance was set at p < 0.05.
3. Results
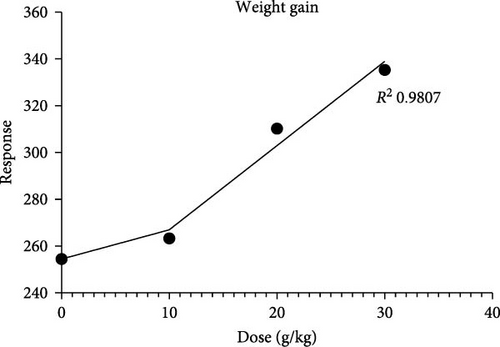
Results of the growth performance of Nile tilapia fed on C. album-supplemented diet is given in Table 3. A significant impact (p < 0.05) was observed across all growth parameters. Diets supplemented with C. album demonstrated a significant enhancement (p < 0.05) in final weight, weight gain (%), SGR, FI, and PER, with the most pronounced effects seen in the 30 g C. album diet, followed by 20, 10 g, and the control group, respectively. The 30 g plant-based diet exhibited a significant reduction (p > 0.05) in the FCR, followed by the other groups. From the regression analysis of the weight gain of the fish, it was found that all three experimental groups showed higher weight gain than the control group. Figure 1 provides detailed results.
| C. album leaves powder g/kg diet | ||||
|---|---|---|---|---|
| Parameters | 0 | 10 | 20 | 30 |
| Initial weight (g) | 11.77a ± 0.19 | 11.83a ± 0.26 | 11.76a ± 0.21 | 11.81a ± 0.22 |
| Final weight (g) | 41.72b ± 0.35 | 42.97b ± 0.28 | 48.22a ± 0.76 | 51.41a ± 1.29 |
| Weight gain (%) | 254.46b ± 10.23 | 263.32b ± 5.24 | 310.22a ± 2.35 | 335.28a ± 2.74 |
| Feed intake (g) | 51.26c ± 0.52 | 51.86c ± 0.42 | 55.05b ± 0.46 | 59.03a ± 0.52 |
| Specific growth rate (%) | 2.10b ± 0.03 | 2.15b ± 0.02 | 2.35a ± 0.003 | 2.43a ± 0.04 |
| Feed conversion ratio | 1.74a ± 0.04 | 1.60b ± 0.03 | 1.39c ± 0.02 | 1.35c ± 0.01 |
| Protein efficiency ratio | 1.87c ± 0.03 | 2.02b ± 0.02 | 2.32a ± 0.03 | 2.36a ± 0.02 |
- Note: Data represent means ± pooled SEM. Values with different letters are significantly different (p < 0.05). Values with the same letter are not significantly different (p > 0.05).
Furthermore, in the experimental groups, it was found that weight gain exhibited a linear relationship with the dose of C. album, indicating that increasing the extract dosage resulted in increased weight gain. The highest weight gain was recorded for the 30% dose. This finding was further supported by the SGR, FCR, and PER analysis (Figure 2). The results revealed that the lowest FCR was recorded for the experimental group fed with 30% of the plant extract. PER was also highest in the group fed with 30% of the plant extract.
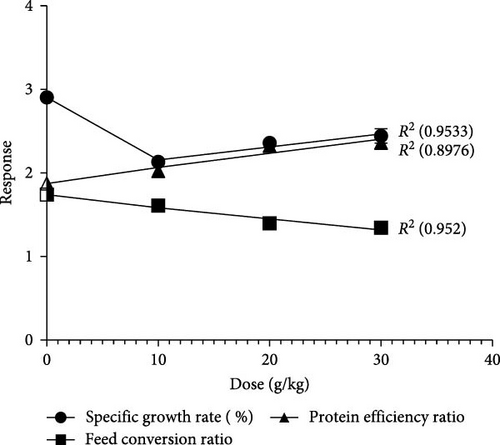
Table 4 presents the hematological indices of Nile tilapia that were provided with a diet fortified with varying concentrations of C. album. Compared to the control group, hematological indices were significantly improved (p < 0.05) in fish diets fortified with C. album. In a dose-dependent fashion, the hematological parameters, including RBCs, Hb, Hct, and WBC levels, exhibited a significant (p < 0.05) increase. Additionally, the inclusion of C. album powder in Nile tilapia diets led to a substantial increase in lymphocytes (LYM), heterophils (HET), eosinophils (EOS), and monocytes (MONO) values.
| C. album leaves powder g/kg diet | ||||
|---|---|---|---|---|
| Parameters | 0 | 10 | 20 | 30 |
| RBCs (106/mm3) | 2.37b ± 0.13 | 2.64ab ± 0.06 | 2.75a ± 0.09 | 2.90a ± 0.05 |
| Hb (g/dL) | 7.31d ± 0.06 | 7.56c ± 0.04 | 7.73b ± 0.08 | 7.96a ± 0.07 |
| Hct (%) | 20.87c ± 0.27 | 21.23b ± 0.32 | 22.74a ± 0.35 | 22.93a ± 0.42 |
| WBCs (103/mm3) | 5.34d ± 0.02 | 5.54c ± 0.04 | 5.86b ± 0.03 | 6.03a ± 0.05 |
| LYM (103/mm3) | 2.93c ± 0.01 | 3.02b ± 0.03 | 3.14a ± 0.03 | 3.18a ± 0.02 |
| HET (103/mm3) | 1.42c ± 0.02 | 1.49b ± 0.01 | 1.58a ± 0.03 | 1.62a ± 0.06 |
| EOS (103/mm3) | 0.32d ± 0.005 | 0.38c ± 0.002 | 0.46b ± 0.001 | 0.50a ± 0.003 |
| MONO (103/mm3) | 0.64c ± 0.003 | 0.63c ± 0.004 | 0.67b ± 0.007 | 0.72a ± 0.003 |
- Note: Data represent means ± pooled SEM. Values with different letters are significantly different (p < 0.05). Values with the same letter are not significantly different (p > 0.05).
- Abbreviations: EOS, eosinophils; Hb, hemoglobin; Hct, hematocrit; HET, heterophils; LYM, lymphocytes; MONO, monocytes; RBCs, red blood cells; WBCs, white blood cells.
The serum biochemical indices of Nile tilapia, fed on an experimental diet with varying levels of C. album, are presented in Table 5. The albumin, total protein, and globulin levels exhibited a significant enhancement (p < 0.05) in the diets fortified with 30 and 20 g of C. album, as opposed to the control group. The serum glucose and cortisol levels showed a significant decrease (p < 0.05) in fish-fed diets enriched with 30 g C. album compared to the other groups.
| C. album leaves powder g/kg diet | ||||
|---|---|---|---|---|
| Parameters | 0 | 10 | 20 | 30 |
| Albumin (g/dL) | 2.31d ± 0.05 | 2.63c ± 0.03 | 2.87b ± 0.06 | 3.08a ± 0.03 |
| Total proteins (g/dL) | 5.29c ± 0.14 | 6.17b ± 0.05 | 6.59a ± 0.12 | 6.95a ± 0.06 |
| Globulin (g/dL) | 2.97c ± 0.12 | 3.53b ± 0.01 | 3.70ab ± 0.03 | 3.86a ± 0.02 |
| Glucose (mg/dL) | 74.53a ± 1.25 | 72.09a ± 1.08 | 67.31b ± 1.13 | 62.26c ± 1.03 |
| Cortisol (ng/L) | 52.45a ± 0.92 | 50.38a ± 0.85 | 45.31b ± 1.27 | 42.22c ± 0.52 |
- Note: Data represent means ± pooled SEM. Values with different letters are significantly different (p < 0.05). Values with the same letter are not significantly different (p > 0.05).
Table 6 presents the outcomes of the serum antioxidant enzyme activities in Nile tilapia that were nourished with a diet enriched with C. album. The study uncovered notable enhancements (p < 0.05) in the serum concentrations of CAT, SOD, and GSH, with the most significant improvement observed in the group receiving 30 g of C. album supplementation. This was followed by the 20, 10 g, and control group.
| C. album leaves powder g/kg diet | ||||
|---|---|---|---|---|
| Parameters | 0 | 10 | 20 | 30 |
| CAT (U/L) | 68.54d ± 1.82 | 76.82c ± 0.67 | 88.72b ± 2.53 | 96.85a ± 1.87 |
| SOD (U/mL) | 6.09c ± 0.62 | 7.51c ± 0.39 | 9.49b ± 0.48 | 13.45a ± 0.76 |
| GSH (µmol/mL) | 11.39c ± 0.48 | 13.33c ± 0.27 | 15.71b ± 0.48 | 18.48a ± 0.76 |
- Note: Data represent means ± pooled SEM. Values with different letters are significantly different (p < 0.05). Values with the same letter are not significantly different (p > 0.05).
- Abbreviations: CAT, catalase; GSH, reduced glutathione; SOD, superoxide dismutase.
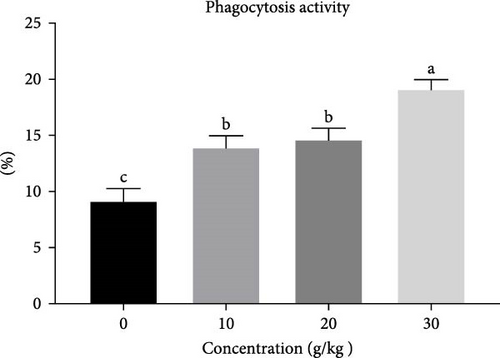
Nile tilapia that were given diets containing C. album exhibited a significantly elevated (p < 0.05) nonspecific immune response compared to fish fed the control diet. Significant enhancements (p < 0.05) in phagocytic activity were observed, with the most pronounced effects seen in fish fed 30 g of C. album, followed by those consuming 20 and 10 g (Figure 3).
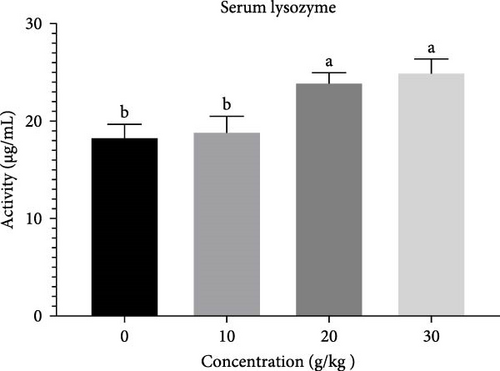
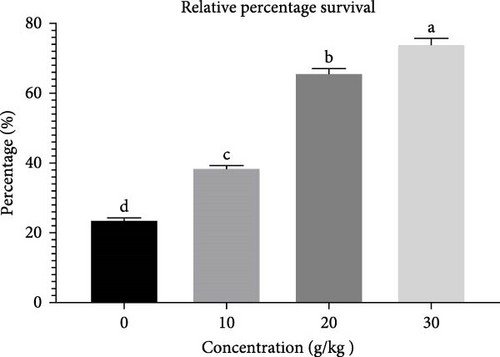
The lysozyme activity exhibited a considerable increase (p < 0.05) in fish that consumed 30 and 20 g of C. album (Figure 4). The RPS showed a significant increase (p < 0.05) in fish that were fed 30 g of C. album, followed by those fed 20, 10 g, and the control group (Figure 5).
4. Discussion
Aquaculture offers a sustainable source of cost-effective and nutritious protein for human consumption, contributing to improved human health [44]. Unfortunately, the ongoing challenge of disease outbreaks significantly impedes achieving sustainable production through advanced intensification methods. Numerous environmentally friendly and safe compounds are currently under investigation in aquaculture, aiming to modulate immune responses, enhance growth performance, and prevent diseases in fish [45, 46]. Hence, our study seeks to explore the potential of C. album leaf powder in enhancing the health, immune response, and growth performance of Nile tilapia. The results of this study highlighted the successful enhancement of several health indicators in Nile tilapia through the dietary replacement of C. album. The most favorable outcomes were observed in the group of fish that received the 30 g C. album diet, showing a notable enhancement in final weight, weight gain (%), SGR, FCR, and PER compared to those fed the control diet. These observations align with the findings of Amhamed et al. [47], who explored the impact of C. album on the growth rate of Cyprinus carpio. To the best of our knowledge, no existing study has explored the effects of C. album on tilapia species. There is a scarcity of studies focusing on C. album as a dietary source for the health and growth of fish. The utilization of plant-based diets in aquaculture has gained significant attention and prominence. Herbs and plants, known for their rich bioactive compounds, can enhance the overall health, growth, and immune response of fish [12]. These natural additives often contain antioxidants, antimicrobials, and anti-inflammatory agents, which promote disease resistance and enhance the overall robustness of aquatic species [48]. Javanmardi et al. [49] demonstrated that ascorbic acid (6 mg/L) enhanced the growth performance of Nile tilapia. Similarly, C. album also contains ascorbic acid, contributing to improved growth performance in Nile tilapia. Furthermore, a study by Fazio et al. [50] revealed that fruit extract of Withania coagulans enhances the Labeo rohita weight gain, SGR, and FCR. SGR is a fundamental parameter in assessing the growth dynamics of fish in aquaculture. Its role extends beyond a simple measurement, encompassing health, nutrition, environmental conditions, and economic considerations [51]. FCR in aquaculture emphasizes its importance in assessing feed efficiency, economic viability, and environmental sustainability [52]. Aquaculturists can enhance production practices by focusing on efficient feed conversion and contribute to the industry’s long-term sustainability. The FCR in our study significantly decreased in groups fed the 30 g/kg C. album diet. Several studies have demonstrated the positive effects of plant-based diets on fish growth. For instance, Elabd et al. [53] reported improved growth in O. niloticus fed a moringa leaf diet, while Hamid et al. [54] observed enhanced performance in Oreochromis mossambicus and O. niloticus fed a diet fortified with Carica papaya leaf extract. Similarly, Lamin et al. [55] found better weight gain, FCR, SGR, PER, and FI in C. carpio when supplemented with a Zingiber officinale-based diet. Our findings align with Habib et al. [26], who reported a significant improvement in growth performance and reduced FCR in C. carpio-fed 2.5% Withania somnifera root powder. PER provides a valuable measure of the nutritional quality of the feed. It assesses how effectively the dietary protein supports the growth of the fish. Higher PER values suggest better utilization of protein for growth, indicating a more nutritionally balanced diet [56]. Further, optimum FI contributes to the overall success of aquaculture operations by promoting growth, ensuring nutritional requirements are met, and supporting the health and well-being of the fish population [57]. Also, elevated levels of protein, essential oils, fiber, carbohydrates, vitamins, and minerals contributed to an enhanced nutritional profile in the diet [56]. The fish’s health and physiological conditions can be assessed by examining hematological parameters [58]. The present study verified that Nile tilapia fed with C. album significantly elevated all blood indices when the diets contained 30 g/kg of plant leaf powder. C. album contains many phytochemicals comprising flavonoids, glycosides, proteins, alkaloids, carbohydrates, saponins, and phenolics [59]. Due to its elevated protein content, C. album offers human and animal nutritional benefits [22]. Several studies have consistently reported enhanced hematological indices in Nile tilapia. These improvements were observed with the administration of Moringa oleifera extract [60] and diets supplemented with Laurus nobilis [61]. Elevated levels of RBCs, Hb, and HCT in fish typically indicate improved oxygen-carrying capacity in the blood [62]. This can suggest enhanced oxygen transport to tissues, which is crucial for various physiological functions, including metabolism and overall health. These hematological parameters in aquatic organisms like fish are vital indicators of their adaptation to environmental conditions and overall well-being [58, 63]. Increased WBC counts and favorable changes in the differential leukocyte count in fish resulting from an improved diet can enhance immune system activity and overall health [64]. A better diet can contribute to a more robust immune response in fish, helping them resist infections and maintain optimal well-being [65]. This study significantly enhanced the WBC count and differential leukocytes in fish fed on a C. album-based fortified diet.
In this study, the albumin level was markedly enhanced in Nile tilapia fed on 30 g C. album, followed by 20 and 10 g, followed by the control group. This is according to the findings of Fereidouni, Akbary, and Soltanian [66], who reported a higher level of albumin in Mugil cephalus fed on Allium sativum. Albumin is a protein crucial for various physiological functions, including maintaining osmotic balance, transporting hormones and nutrients, and contributing to the fish’s overall protein profile [67, 68]. Therefore, an elevated albumin level is often associated with a well-balanced and nutritious diet. In this investigation, fish receiving diets supplemented with C. album exhibited elevated total serum proteins and globulin concentrations. This finding implies that C. album can potentially enhance protective proteins, activating the immune system. Increased blood protein levels, especially globulins, serve as a positive indicator of improved liver function and a heightened innate immune response [69]. Similar findings were reported by Hassaan et al. [70], who observed enhanced survival and health in Nile tilapia fed a diet fortified with Silybum marianum seeds. Key stress markers in fish, such as serum glucose and cortisol levels, typically fluctuate in response to environmental changes or dietary interventions [56]. In typical conditions, cortisol plays a regulatory role in various physiological processes in fish, facilitating rapid physiological adjustments in response to stress [71]. Cortisol stimulates various components of intermediary energy metabolism, enhancing oxygen absorption, promoting gluconeogenesis, and inhibiting glycogen synthesis [72]. In the present study, including C. album in the diet they have significantly reduced blood glucose and cortisol levels. The abundance of flavonoids and essential minerals in C. album plays a crucial role in regulating glucose uptake and lipid metabolism [59]. Other studies also revealed lower cortisol and glucose levels in Nile tilapia fed on a fortified diet with Citrus bergamia peel oil [73] and supplemented diet with olive leaf extract [51].
Oxidative stress can potentially damage essential biological components, including proteins and DNA. The body possesses a protective mechanism to counteract oxidative damage to its tissues [74]. CAT and SOD are antioxidant enzymes capable of neutralizing reactive oxygen free radicals. Glutathione, as a nonenzymatic antioxidant, can also mitigate the impact of these radicals through enzymatic reactions. In the present investigation, notable elevations above the control levels were observed in the activities of CAT, SOD, and GSH in fish serum when fed a diet supplemented with C. album. This is because the C. album contains bioactive compounds, including flavonoids, terpenoids, alkaloids, polyphenols, glycosides, steroids, and vitamins known for their antioxidant and anti-inflammatory properties [22]. Further investigations have consistently shown parallel findings, illustrating an elevated antioxidant status in fish subjected to a plant-based fortified diet like Amoush et al. [75], who observed increased antioxidant levels in Oncorhynchus mykiss with the inclusion of cherry stem extract, and the study conducted by Hamed and El-Sayed [76], which reported comparable results in Nile tilapia through the utilization of M. oleifera leaf extract.
The challenge test is commonly employed as the standard assay for evaluating the overall health of the immune system [77]. The enhanced resistance of fish to pathogenic microorganisms serves as a key indicator of the effectiveness of immunostimulants [78, 79]. As per the results of this study, the inclusion of C. albumin in the diet exhibited a protective effect against A. hydrophila infection in the fish. This potential association could be attributed to the enhanced levels of nonspecific immune parameters, such as increased lysozyme levels, phagocytic activity, and antioxidant enzyme activities. Moreover, the observed reductions in glucose and cortisol levels may have played a role in reinforcing the fish’s defense against infection. C. album has been reported for its exceptional pharmacological attributes, showcasing antibacterial capabilities among its diverse medicinal properties [20]. Lone et al. [24] highlighted the positive effects of C. album leaf extract on antibacterial and antioxidant activities. In the current study, fish survival rates significantly improved with the 30 g/kg C. album-supplemented diet, likely due to its beneficial properties. This might be because of their wound healing, anti-inflammatory, antifungal, and antibacterial properties [80, 81]. This study, conducted under controlled laboratory conditions, may not fully reflect the complexities of natural aquaculture environments. Additionally, the long-term effects of C. album supplementation remain unclear and require further investigation. Future research should validate these findings in various conditions and over extended periods while identifying the specific bioactive compounds responsible for the observed benefits.
5. Conclusion
In conclusion, incorporating C. album into the diet of Nile tilapia at a concentration of 30 g/kg has yielded positive outcomes, enhancing various aspects of their health and performance. The observed improvements in growth, blood parameters, antioxidant status, and immunity suggest that C. album can play a beneficial role in bolstering the overall well-being of Nile tilapia, particularly in enhancing their resilience against bacterial challenges, such as those posed by A. hydrophila. These findings underscore the potential of C. album as a valuable dietary supplement for promoting the robust health and immune response of Nile tilapia in aquaculture settings. Further research and exploration of the mechanisms underlying these positive effects could contribute to optimizing the use of C. album in aquaculture practices.
Ethics Statement
The research undertaken received ethical clearance from the Department of Biology at Gomal University Tank, with approval granted by the ethical committee. The trial registration number for this study is GUT/BIO/123, and the initiation date was June 28, 2022. The research adhered strictly to ethical considerations outlined in European Legislation concerning Animal Rights (Supporting File).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Syed Sikandar Habib and Javed Ahmed Ujan conceived the idea and designed the experiment. Samples collection and analysis were performed by Syed Sikandar Habib, Samrah Masud, and Khalid Hussain Rind. Interpretation of data and statistical analyses for the study carried out by Osman Sabri Kesbiç, Francesco Fazio, Khalid Hussain Rind, and Mohamed Mohany. The first draft of the manuscript was written by Samrah Masud, Javed Ahmed Ujan, Osman Sabri Kesbiç, and Francesco Fazio. The manuscript was reviewed and edited by Francesco Fazio, Osman Sabri Kesbiç, Salim S. Al-Rejaie, and Mohamed Mohany. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project (RSPD2025R758), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (RSPD2025R758), King Saud University, Riyadh, Saudi Arabia. We also acknowledge the use of ChatGPT-4 for language editing and improving readability.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




