Reproductive Behavior in Odontobutis potamophila (Günther, 1861)
Abstract
An animal’s behavior can directly reflect its psychological or physiological condition. The in-depth understanding of fish behavior, especially reproductive behavior, is an important safeguard to promote sustainable aquaculture. In this study, we used the Posture-Act-Environment (PAE) coding system to construct an ethogram of Odontobutis potamophila to help people better judge and identify O. potamophila behaviors; and we designed two groups of experiments to figure out the individual factors affecting the reproduction of O. potamophila. These were the selection of female O. potamophila for male O. potamophila of different sizes, with or without nests, and with or without parental care behaviors, as well as the selection of male O. potamophila for female O. potamophila of different sizes. The results showed that we recorded 14 postures, 22 movements, and 29 behaviors of O. potamophila. Based on their biological functions, the recorded behaviors of O. potamophila were divided into nine types, that is, exploration, territoriality, attack, courtship, mating, parental care, ingestion, stationary behavior, and others. O. potamophila had nocturnal habits, and all the nighttime activities were significantly higher than those during the daytime (p < 0.001). The mating system of O. potamophila was polygamous, and the average mating duration for successful spawning was 9.99 ± 1.23 h. In mate choice experiments, both females and males spent significantly more time stationary than activity (p < 0.001). Females exhibited a slight preference for males with large individuals (strength of preference [SOP] = 53.46%), no nests (SOP = 50.51%), and no parental care behavior (SOP = 55.58%). The results of male mate choice were similar. Although the standard length of females was positively correlated with fecundity (r = 0.61), there were no significant differences in the number of male-to-female choices or duration of association (p > 0.05). This indicated that males showed no preference for larger or high-fertility females (SOP = 47.10%). Therefore, it is likely that males may not be selective for females with different fecundity levels. This study enhances the current understanding of the behavioral patterns of O. potamophila. It establishes a foundation for further research on its reproductive behavior and contributes to the advancement of captive breeding for this species.
1. Introduction
Behavioral science is increasingly used in fish conservation practices, and studies on reproductive behavior can inform artificial fish reproduction [1–3]. The behavior of animals responds most directly to environmental changes. The study of animal behavior can reveal the pattern of activities and determine the relationships between animals and the environment [4]. In species-oriented behavioral studies, it is vital to accurately describe the behavioral patterns of animals by constructing an ethogram [5]. This can help in examining the functions of behaviors, transformations between behaviors, and interrelationships. An ethogram is a catalog of animal behavior based on its identification and classification. The Posture-Act-Environment (PAE) coding system is commonly used, which is based on the classification of ecological functions along the axis of posture, action, and environment [6]. Compared to traditional descriptive classification methods, this system reduces the understanding bias caused by different descriptions of the same behavior by different researchers. The PAE coding system helps researchers to understand the characteristics and functions of behaviors based on the environment, posture, and movement of various behaviors. Now, the PAE coding system has been widely applied to terrestrial animals. Aquatic animals that have used such methods to establish ethogram include Neophocaena phocaenoides asiaeorientalis [7] and Schizothorax wangchiachii [8].
Sexual choice is a prerequisite for reproductive behavior, including mate choice and competition [9]. Mate choice is important for individuals to improve their fitness. The outcome directly determines whether an individual will have reproductive success to passing on genes. Mate choice is a tradeoff process, and the associated benefits largely depend on mate quality. However, individuals have different strategies for choosing mates. This may be based on direct benefits, such as the other individual’s size and fertility or genetic material from the parental generation [10, 11]. In traditional theories of sex roles, females are often considered to make mate choices because they have a greater investment in gametes and offspring than males [12]. To pass on their genes, females may be selected by evaluating males for a certain phenotypic trait that represents the possession of a special ability, such as larger individuals, the ability to occupy resources, and the ability to provide more parental care [13]. Males are thought to be nonselective for mates and seek to maximize their reproductive success by increasing the number of mates rather than their quality [14]. An increasing number of studies have suggested the existence of mutual mate choice, including male mate choice under certain conditions in species characterized by traditional sex roles [15, 16]. However, relatively few studies have examined male mate choice preferences or the relationship between female body size and fertility.
Odontobutis potamophila is an endemic economic fish in China. However, in recent years, their numbers have declined significantly due to factors such as the construction of water conservancy projects, overfishing, and irrational artificial breeding and releasing, and the existing artificial propagation technology is not yet mature, resulting in an oversupply in the market. In order to promote the sustainable development of O. potamophila aquaculture and help it achieve ecological restoration, we constructed a reproductive ethogram of O. potamophila through behavioral classification and investigated the behavioral factors affecting mate choice before reproduction as a way of providing a basic basis for the development of its artificial reproduction technology.
2. Materials and Methods
2.1. Collection and Staging of Experimental Fish
The O. potamophila used in the experiment were collected from Weishan Lake, Jining, Shandong Province, China, and captured using ground cages (15 × 0.6 m, 4 mm mesh). The test fish were separated by sex and temporarily housed in an indoor concrete pool (170 × 170 × 80 cm) in the Modern Fisheries Park, Weishan County, Jining, Shandong Province, China. Sterilized gravel and artificial water plants were placed at the bottom of the pool to simulate a natural environment. The concrete pool was equipped with an inlet and outlet, which were kept open during the feeding period to maintain the pool with slight water flow. During the feeding period, sufficient live bait of Macrobrachium nipponense was provided. The concrete pools were exposed to the same light as in the natural photoperiod during the test period. The light-to-dark time ratio was 13:11 (daytime: 05:00–18:00; nighttime: 00:00–04:00, 19:00–23:00).
2.2. Experimental Design
2.2.1. Reproductive Behavior Collection
The behavioral activities of O. potamophila were collected from the observation pool, which has the same structure and basic facilities as the concrete pool. An artificial nest (L 8 cm × Φ 15 cm, nest opening height 6 cm) was placed in the observation pool, consisting of two tiles fastened to each other in a hollow ellipsoidal structure. An infrared camera (Hikvision HIKVISION DS-2CD3T35D-I5) was overlaid 1.5 m directly above the observation pool. An underwater camera (Hikvision DS-2XE6222F-IS) with illumination at night was placed in each of the side corners of the bottom of the pool, pointing at the artificial nests, to record the behavior inside the nests (Figure 1). A network DVR (Dahua DH-NVR808-32-HDS2) was used to record the video. A portable underwater camera (GoPro HERO7) was used to record important underwater behavioral events.
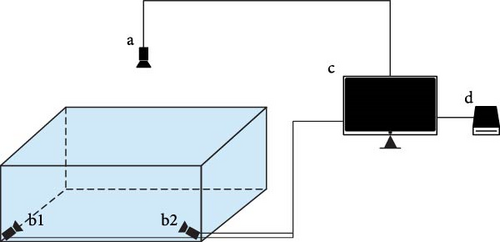
Behavioral observations were conducted on April–June 2019 and May–July 2020. The videos recorded during April–June 2019 were mainly used to construct reproductive ethograms. In this phase, 20 pairs of O. potamophila (males: standard length 98.52 ± 4.39 mm, weight 26.33 ± 4.20 g; females: standard length 87.90 ± 3.19 mm, weight 21.65 ± 2.30 g) were stocked into the observation pool. The water temperature during the experiment was 18.33 ± 0.36°C, the dissolved oxygen was 5.64 ± 0.35 mg/L, and the pH was 9.10 ± 0.02. The videos recorded during May–July 2020 were used to supplement the ethogram and study the dynamics of behavioral occurrence. In this phase, the O. potamophila was sequentially inserted into the observation pool. The number of deaths and new additions to the experimental fish were recorded daily. A total of 32 males (standard length 109.15 ± 4.47 mm, weight 33.41 ± 4.44 g) and 29 females (standard length 105.12 ± 4.26 mm, weight 27.57 ± 3.16 g) were added. The water temperature during the experiment was 26.00 ± 0.23°C, the dissolved oxygen was 4.48 ± 0.14 mg/L, and the pH was 8.33 ± 0.19.
The study employed focal following and continuous recording methods to examine the behavior of individuals present in and around the nest [17]. Behavioral status was recorded at a 1-min interval for each O. potamophila individual, and changes in behavioral status were recorded continuously [18]. The duration and frequency of occurrence of each behavior were studied using the full-event sampling method. This entailed the occurrence of a particular behavior or event serving as the sampling criterion, with the target behavior then observed and recorded. Additionally, the total length of each individual was estimated using the nest as a reference point. Behavior videos of longer duration, for example, parental care behaviors, were sampled based on periods, with 10 min randomly sampled within each hour of the selected timescale for analysis.
During behavioral observations in 2020, videos recorded using an aquatic infrared camera were primarily used to investigate the diurnal activity patterns and rhythms of important behaviors in O. potamophila, including fighting, feeding, mating, and parental care of males. The infrared camera was turned on for night recording as a criterion, defining the daytime as 5:00 am to 18:00 pm (05:00–18:00) and the remainder as nighttime (00:00–05:00, 18:00–23:00). The recorded videos were sampled and divided into two parts. The first part (Part Ⅰ) was from May 8 to June 4, 2020, a total of 28 days. Five time periods were selected for counting (daytime: 05:00–06:00, 12:00–13:00, and 17:00–18:00; nighttime: 00:00–01:00, and 22:00–23:00). The second part (Part Ⅱ) was from June 5 to July 7, a total of 33 days, for 24 h statistics. The number of individuals appearing in the field of view and the number of significant behavioral events were recorded every 10 min/h. The frequency of occurrence was the ratio of individuals that were recorded to the total number of individuals in the pool. These data are presented as the mean of the proportion of individuals that appeared each hour. The aquatic infrared camera and two underwater cameras were recorded throughout the day during the experiment. The recorded video was played using a player (Smart Player), first at 4x speed and then at normal speed when target animals or important behaviors were detected and recorded.
2.2.2. Mate Choice
Mate choice experiments for O. potamophila were conducted in a transparent glass fish tank (195 × 35 × 20 cm) divided into three sections by two transparent glass partitions (35 × 20 cm, 1 cm thick). The transparent tape was applied to the outside of the middle section, 8 cm near the partition, as a choice zone for behavioral observations. The remaining area in the middle was used as a neutral zone. Three sides of the experimental fish tanks were covered with black cloth to prevent external interference. An infrared camera (Hikvision HIKVISION DS-2CD3T35D-I5) was used on the uncovered side for videotaping, and a network DVR (Dahua DH-NVR808-32-HDS2) for video recording. The observation subjects were placed in the middle of the fish tank and the stimulus subjects were randomly placed in the left and right parts of the fish tank. These were paired with artificial fish nests for males, according to the purpose of the experiment. The experiment tank was filled to 15 cm with oxygenated water, and the water was replaced after each set of experiments. The water temperature was 18.2 ± 0.37°C, the dissolved oxygen was 5.93 ± 0.36 mg/L, and the pH was 9.06 ± 0.03.
- a.
The choice preference of females for males of different sizes was conducted in 22 replications. Females with an individual size of 40.89 ± 4.48 g were paired with males of different sizes. The weight of the large individual group was 29.71 ± 2.70 g, and the weight of the small individual group was 17.12 ± 1.75 g (analysis of variance [ANOVA], F = 15.36, p < 0.001).
- b.
The choice preference of females on whether males had the nest was conducted in nine replications because of quantity constraints. The female individuals selected were 28.24 ± 7.38 g in size. The males were divided into nest and nonnest groups and reused in nine replications.
- c.
The choice preference of females for males, whether they showed parental care behaviors, was conducted in 20 replications. The females selected were 25.67 ± 4.19 g in size. The males were divided into parental care and nonparental care groups. The nest with eggs was provided for males with parental care behaviors, and the empty nest was provided for males without parental care behaviors. In this experiment, males were removed at the end of each experiment and reused after 1 h interval.
For the male mate choice, males were placed in the middle of the fish tank, and different females were randomly placed in the left and right parts of the fish tank. This experiment was conducted in 20 replications; males with a weight of 28.86 ± 3.06 g, larger females with a weight of 22.28 ± 1.86 g, and small females with a weight of 12.27 ± 1.03 g were put in a fish tank (ANOVA, F = 22.28, p < 0.001).
2.3. Data Collection and Analysis
2.3.1. Reproductive Behavior
The data were divided into qualitative and quantitative parts. PAE coding systems were constructed primarily for qualitative data to describe and classify the behaviors that occurred. The occurrence dynamics and daily rhythms of important behaviors were converted into time proportions, event proportions, and frequency of occurrence to compare diurnal differences in the occurrence of individual behaviors.
2.3.2. Mate Choice
When processing the video data, 20 min of behavioral data of individuals within the middle of the fish tank were analyzed. The timekeeping units were seconds (s). The following parameters were recorded to characterize individual performance and choice preferences: (1) activity/stationary time: time spent by the selected individuals in the fish tank. (2) Number of choices: When 2/3 of the body length of an individual in the neutral zone entered the choice zone, it was classified as a single choice. (3) Association time: The time spent by an individual entering the choice zone and interacting with the stimulus object. The time spent interacting with potential mate objects can be considered a preference and is often used as an indicator of preference choice [19]. The strength of preference (SOP) is the proportion of an individual’s association time on a particular side to the total association time. An individual is considered to prefer a particular side when the ratio of association time is greater than 50% in each experimental group.
A one-sample t-test was used to compare the differences in SOP vs. 50% for each experimental group. A binomial test was used to test the number of groups in each experimental group that had an SOP > 50% and to determine the choice preference of each experimental group. Absolute differences in the fecundity of females of different sizes were compared, and correlation analyses of standard length and female fecundity were performed.
All the data were tested for normality and homogeneity of variance before analysis, and one-way ANOVA was performed if conditions were met. Otherwise, the data were transformed using logarithmic or square root transformation and reanalyzed, or the appropriate nonparametric test was selected (Mann–Whitney U test). All the data were expressed as (mean ± standard error, mean ± SEM), and p < 0.05 was considered significant. The data were processed and statistically analyzed using SPSS 19.0 and plotted using Origin Pro 8.0.
3. Results
3.1. Reproductive Behavioral Profile of O. potamophila
3.1.1. Behavior Coding
Postural coding: A total of 14 postures of O. potamophila were specifically recorded, that is, mounting, hanging, floating, swimming, rushing, pitching, bowing, jumping, ovipositing, turning, sinking, standing, rotating, and turning upside-down (Table 1).
| Postures | Definition | Code |
|---|---|---|
| Mounting | Lying or attaching to the bottom of a pool or other object with the body remaining stationary. | 1 |
| Hanging | The body hangs in the water with the pectoral fins swinging slightly or not moving. | 2 |
| Floating | The body floats on the surface of the water, and the pectoral fins oscillate slightly or do not move. | 3 |
| Swimming | Moving freely in the water depends on pectoral and caudal fin oscillations. | 4 |
| Rushing | The pectoral fins are close to the body and rely on the caudal fin for rapid movement through the water. | 5 |
| Pitching | The head and trunk are raised, the ventral fins are supported, and the pectoral fins are slightly swayed. | 6 |
| Bowing | The head tends to the bottom, and the trunk is pressed down, accompanied by a swing of the caudal fin. | 7 |
| Jumping | Leaping out of the body with the sudden force of the tail fin. | 8 |
| Spawning | The trunk is raised, the tail is cocked and twitching, and the genital papillae are slightly erect. | 9 |
| Turning | Rotate left and right along the dorsoventral axis of the body, swinging the caudal fin. | 10 |
| Sink | The body remains stationary and falls freely to the bottom of the water. | 11 |
| Standing | Upright with head on top and tail on bottom, generally attached to vertical pool walls, etc. | 12 |
| Rotating | The trunk rotates in the direction of the cephalocaudal axis. | 13 |
| Upside-down | The torso is turned along the dorso-ventral axis with the abdomen on top and the back on the bottom. | 14 |
Act coding: A total of 22 movements were differentiated and recorded. This included eight for the head, six for the carapace, four for the pectoral fin, one each on the ventral and dorsal fins, and two on the caudal fin (Table 2). There are four mouth-related movements in the head, namely biting, sucking, swallowing, and gaping. The pectoral and caudal fins are the main locomotor organs and have slightly more movements than the other fins. Meanwhile, the movements of the ventral and dorsal fins were similarly described.
| Act | Code | Act | Code |
|---|---|---|---|
| Head | Swinging | 13 | |
| Butting | 1 | Rubbing | 14 |
| Biting | 2 | Pectoral fin | |
| Sucking | 3 | Stretching | 15 |
| Swallowing | 4 | Retracting | 16 |
| Gaping | 5 | Paddling | 17 |
| Breathing | 6 | Fanning | 18 |
| Heading up | 7 | Pelvic fin | |
| Heading down | 8 | Supporting | 19 |
| Trunk | Dorsal fin | ||
| Extending | 9 | Erecting | 20 |
| Bowing | 10 | Caudal fin | |
| Turning left | 11 | Extending | 21 |
| Turning right | 12 | Swinging | 22 |
- Note: The bolded parts indicate the body parts of O. potamophila. This is to highlight the action codes of different body parts of O. potamophila.
Environmental coding: A total of 12 environments, 5 biotic and 7 abiotic, in which the behavior of O. potamophila occurred, were distinguished and recorded (Table 3).
| Environment | Biotic (E1) | Abiotic (E2) | Codes |
|---|---|---|---|
| In the nest | — | + | 1 |
| Outside the nest | — | + | 2 |
| Nest mouth | — | + | 3 |
| Surface | — | + | 4 |
| Day | — | + | 5 |
| Night | — | + | 6 |
| Night with light | — | + | 7 |
| Male | + | — | 8 |
| Female | + | — | 9 |
| Group | + | — | 10 |
| Single | + | — | 11 |
| Fertilized eggs | + | — | 12 |
PAE ethograms: A total of 29 behaviors recorded during reproduction were classified into 9 major categories according to the biological function of the behaviors, that is, exploration, territoriality, attack, courtship, mating, parental care, ingestion, stationary behavior, and others (Table 4).
| Behaviors | Male | Female | Number | PAE code | ||
|---|---|---|---|---|---|---|
| P | A | E | ||||
| Exploring | ||||||
| Patrolling | ++ | +++ | 1 | 4, 10, 12 | 7, 8, 9, 10, 11, 12, 17 | 1, 2, 3, 6, 7 |
| Observing | ++ | +++ | 2 | 1, 6, 7, 12 | 5, 7, 8, 9, 10, 15, 17, 19, 21 | 1, 2, 3, 5, 6 |
| Territorial behavior | ||||||
| Occupying | +++ | ++ | 3 | 1, 4, 10, 14 | 1, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 18, 19, 20, 21, 22 | 1, 5, 6, 8, 10, 11 |
| Nest cleaning | +++ | + | 4 | 1, 4, 10, 14 | 9, 10, 11, 12, 14, 17, 18, 19, 22 | 1, 2, 5, 6, 7, 8, 11 |
| Altering | +++ | — | 5 | 4, 10 | 5, 7, 8, 9, 10, 11, 12, 15, 18, 19, 20, 21 | 1, 3, 5, 6, 7, 8, 11 |
| Threatening | +++ | — | 6 | 4, 6, 7 | 5, 8, 9, 10, 11, 12, 15, 17, 19, 20, 22 | 1, 3, 5, 6, 7, 8, 11 |
| Attacking | ++ | — | 7 | 4, 5, 7 | 1, 2, 5, 8, 9, 10, 11, 12, 13, 15, 19, 20, 22 | 1, 2, 3, 5, 6, 7, 8, 11 |
| Withdrawing | ++ | ++ | 8 | 4, 7 | 8, 9, 10, 11, 12, 17, 19, 22 | 1, 3, 5, 6, 7, 8, 9, 11 |
| Avoiding | ++ | ++ | 9 | 4, 13 | 8, 9, 10, 11, 12, 17, 22 | 1, 2, 3, 5, 6, 7, 8, 9, 10, 11 |
| Leaving | ++ | ++ | 10 | 4, 5, 13 | 9, 10, 11, 12, 17, 22 | 1, 2, 3, 5, 6, 7, 8, 9, 11 |
| Sneaking | ++ | + | 11 | 4, 5 | 9, 10, 11, 12, 15, 21 | 2, 3, 5, 6, 7, 8, 9, 11 |
| Aggressive behavior | ||||||
| Attacking | +++ | ++ | 12 | 4, 5, 10, 13 | 1, 2, 5, 8, 9, 10, 11, 12, 13, 15, 17, 18, 19, 20, 22 | 1, 2, 3, 6, 7, 8, 10 |
| Fleeing | ++ | ++ | 13 | 4, 5, 10 | 9, 10, 11, 12, 17, 22 | 1, 2, 3, 5, 6, 7, 8, 9, 10, 11 |
| Courtship | ||||||
| Display | +++ | — | 14 | 4, 9, 13, 14 | 9, 10, 13, 15, 18, 19, 20, 22 | 1, 3, 6, 7, 8, 11 |
| Guiding | +++ | ++ | 15 | 1, 4, 10 | 8, 9, 10, 11, 12, 13, 17, 18, 22 | 1, 3, 5, 6, 7, 8, 9 |
| Mating behavior | ||||||
| Pairing | +++ | +++ | 16 | 1, 4, 13, 14 | 9, 10, 11, 12, 13, 14, 17, 18, 19, 22 | 1, 5, 6, 7, 8, 9 |
| Accompany swimming | ++ | ++ | 17 | 4, 10, 13, 14 | 9, 10, 11, 12, 13, 14, 17, 18, 19, 22 | 1, 5, 6, 7, 8, 9 |
| Ovulating | — | +++ | 18 | 9, 14 | 8, 9, 10, 13, 17, 22 | 1, 5, 6, 7, 9 |
| Ejaculating | +++ | — | 19 | 9, 14 | 8, 9, 10, 13, 17, 22 | 1, 5, 6, 7, 8 |
| Parental care | ||||||
| Fanning | +++ | — | 20 | 1, 4, 10, 13, 14 | 7, 8, 9, 10, 11, 12, 13, 18, 19, 22 | 1, 5, 6, 7, 8, 11 |
| Guarding | +++ | — | 21 | 4, 5, 7 | 1, 5, 7, 8, 9, 10, 11, 12, 15, 19, 20, 22 | 1, 3, 5, 6, 7, 8, 11 |
| Feeding behavior | ||||||
| Cannibalism | + | ++ | 22 | 1, 4, 5, 6, 7 | 2, 3, 4, 5, 15, 20, 22 | E2, 9 |
| Filial cannibalism | ++ | +++ | 23 | 1, 4, 5, 6, 7 | 2, 3, 4, 7, 8 | 1, 5, 6, 7, 9, 12 |
| Hunting | ++ | +++ | 24 | 1, 4, 5, 6, 7 | 2, 3, 4, 5, 15, 20, 22 | E2 |
| Stationary behavior | ||||||
| Sitting | +++ | +++ | 25 | 1, 12 | 10, 11, 12 | E2, 11 |
| Slowly swimming | +++ | +++ | 26 | 2, 3, 11, 12 | 9, 15, 17, 19, 21 | 4, 6, 7, 11 |
| Others | ||||||
| Aggregation | +++ | +++ | 27 | 1, 4, 5, 7 | 9, 10, 11, 12, 14, 15, 16 | 1, 2, 5, 10 |
| Sound production | ++ | — | 28 | 1, 6, 7, 14 | 5 | 1, 2, 3, 5, 6, 7, 8 |
| Breathing | +++ | +++ | 29 | 1–14 | 6 | E1, E2 |
- Note: + indicates the behavior is likely to occur, and more + indicates a higher frequency of the behavior.
- Abbreviation: PAE, Posture-Act-Environment.
3.1.2. Dynamics of the Occurrence of Significant Behaviors
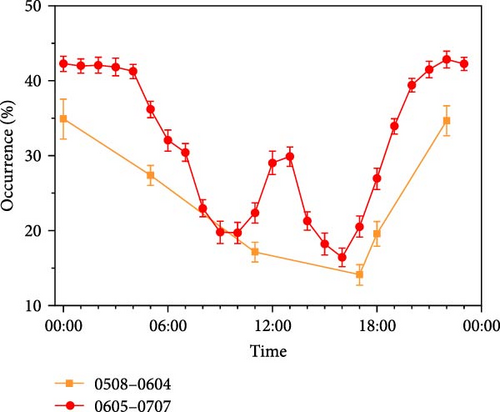
O. potamophila showed a clear difference in the diurnal activity rhythm in this experiment. Sampled observations from Part Ⅰ indicated that a significantly greater proportion of individuals came out during the nighttime than during the daytime (nighttime: 29.95 ± 1.10%; daytime: 15.84 ± 0.94%, t = 9.74, p < 0.001). Observations from Part Ⅱ showed the same pattern, with significantly more nighttime than daytime activity (nighttime: 39.38% ± 0.38%; daytime: 23.62% ± 0.46%; Mann–Whitney U, Z = −19.43, p < 0.001). The overall trend was high at both ends and low in the middle, with higher occurrence rates for 00:00–5:00 and 18:00–23:00, reaching 40.93% and 37.82%, respectively. And the occurrence of 05:00–18:00 is low, 23.62% (Figure 2A).
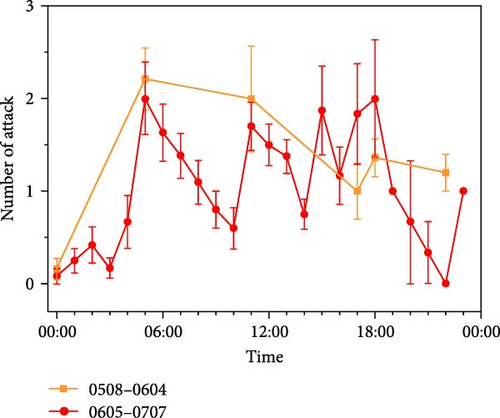

The present study focused on observing and recording the attack, predation, and mating behaviors of O. potamophila and parental care behaviors of male O. potamophila. The results from Part Ⅰ, comprising a total of 28 sampled days of observation, showed that attacks were observed on 25 days. There was a higher total number of attacks occurring at nighttime (66) than during the daytime (33). However, there was no significant difference in the number of daytime and nighttime attacks (daytime: 1.94 ± 0.37, nighttime: 1.78 ± 0.20; Mann–Whitney U, Z = −0.06, p = 0.95). Of these, the number of attacks was high at 5:00 and 12:00 (2.2 on average), with a relatively low number occurring at other times (1.4 on average). The results from 33 consecutive days of observations from Part Ⅱ showed that the occurrence of attack was not detected on only 2 days. The total number of occurrences was higher during the daytime (143) than during the nighttime (65). There was no significant difference between the number of daytime and nighttime occurrences (daytime: 1.46 ± 0.08 occurrences, nighttime: 1.55 ± 0.16 occurrences; Mann–Whitney U, Z = −0.14, p = 0.89) (Figure 2B).
Relative to the higher frequency of attacks, the occurrence of predatory behavior in O. potamophila was low. Sampling from Part Ⅰ showed that the occurrence of predatory behavior was observed on 12 days, with a slightly higher total number of nighttime predation (15) than daytime (8). However, there was no significant difference between daytime and nighttime (daytime: 2.00 ± 0.58 times, nighttime: 1.67 ± 0.29 times; Mann–Whitney U, Z = −0.51, p = 0.61)). Predation behaviors occurred more frequently at 5:00 and 11:00 (2 on average) and were relatively low at other times (1.25 on average). Observations from Part Ⅱ showed that predatory behavior was observed on 22 days. The total number of predation was lower at nighttime (14 times) than during the daytime (47 times). However, there was no significant difference between daytime and nighttime predation (daytime: 1.27 ± 0.10 times, nighttime: 1.07 ± 0.08 times; Mann–Whitney U, Z = −0.99, p = 0.32) (Figure 2C).
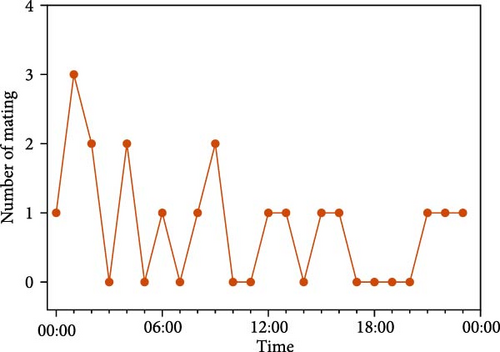
A total of 22 occurrences of mating behavior were observed and recorded during the experimental period (Table 5). Five mating events did not result in successful spawning. For four of these, the duration of mating between males and females in the nests was 0.31 ± 0.08 h. Those of the other group lasted up to 9.78 h. Mating duration for successful spawning reached an average of 9.99 ± 1.23 h, occurring slightly more frequently at nighttime (nighttime: 11 times; daytime: 8 times) (Figure 2D). Observations of the mating behavior showed that the mating system of O. potamophila was a polygamy mating system. Two types of cases were found. The first was that the males, after completing one spawning session, mated with other females and spawned at intervals. The second was that the males mated with more than one female at the same time in one nest.
| Number | Total length (cm) | Start (day hh:mm) | Duration (h) | Results (Y/N) | |
|---|---|---|---|---|---|
| Male | Female | ||||
| 1 | 16 | 9 | 5/09 18:37 | 0.38 | N |
| — | 12 | 5/09 22:00 | 0.26 | N | |
| — | 10 | 5/10 12:06 | 0.48 | N | |
| — | 10 | 5/10 22:00 | 1.34 | Y | |
| — | 12 | 5/15 01:00 | 14.4 | Y | |
| — | 13 | 5/17 23:03 | 16.38 | Y | |
| — | 10 | 5/18 04:35 | 10.84 | — | |
| — | 12 | 5/19 01:36 | 11.29; | Y | |
| — | 10 | 02:20 | 10.55 | — | |
| 2 | 14 | 10 | 5/21 01:31 | 22.78 | Y |
| 3 | 19 | 13 | 5/26 09:54 | 12.17 | Y |
| 4 | 16 | 10 | 5/28 06:06 | 7.87 | Y |
| — | 9 | 5/31 15:26 | 10.17 | Y | |
| — | 12 | 6/03 16:27 | 14.48 | Y | |
| — | 11 | 6/04 12:39 | 6.93 | Y | |
| — | 10 | 6/06 00:59 | 7.5 | Y | |
| — | 12 | 6/08 09:54 | 4.3 | Y | |
| 5 | 13 | 9 | 5/28 08:20 | 4.71 | Y |
| 6 | 10 | 14 | 5/29 13:04 | 0.13 | N |
| 7 | 15 | 13 | 5/29 17:17 | 9.78 | N |
| 8 | 12 | 13 | 5/30 04:13 | 6.47 | Y |
| 12 | 10 | 6/03 13:43 | 1.34 | Y | |
| 9 | 16 | 13 | 6/09 21:09 | 13.18 | Y |
| 10 | 15 | 13 | 6/15 02:00 | 13.03 | Y |
Experimental observations revealed that male O. potamophila had fin-flapping behavior during parental care. The daily rhythms of the percentage of temporal inputs of fanning activity show there was no significant difference in the percentage of daytime and nighttime fanning activity (daytime: 24.99% ± 1.68%, nighttime: 22.18% ± 1.61%; independent samples t-test, t = −1.20, p = 0.23) (Figure 3A).
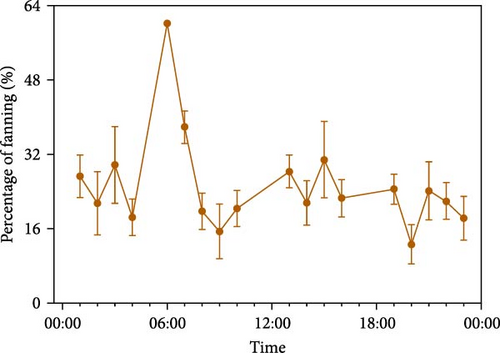

The results of the percentage of male fanning activity after one, two, and six matings, respectively, showed a decreasing trend over time after mating was completed, with the percentage of fanning activity increasing and then decreasing at the completion of each mating. The duration of males with parental care behaviors varied among mating times, with single, double, and multiple mating times until days 8, 6, and 16, respectively (Figure 3B). The percentage of male fanning activity differed significantly by mating frequency (ANOVA, F = 9.90, p < 0.001). According to Tukey’s multiple comparisons, the percentage of male fanfin activity was significantly higher in single spawning (33.87% ± 2.29%) than in two (20.91% ± 2.56%, p = 0.001) and multiple spawning (22.44% ± 1.73%, p < 0.001). Meanwhile, the other two were not significantly different (p = 0.88).
3.2. Mate Choice
3.2.1. Female Mate Choice
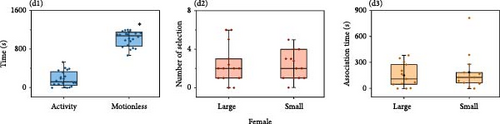
The results of females for males of different sizes showed that females spent less time active overall in the experimental aquarium. Females spent significantly more time stationary (1008.09 ± 26.34 s) than active (191.91 ± 26.34 s) (Mann–Whitney U, Z = −5.68, p < 0.001, n = 22). The number of choices for males of different sizes (large individuals: 3.29 ± 0.88 times, small individuals: 2.89 ± 0.51 times; Mann–Whitney U, Z = −0.10, p = 0.92, n = 21) and association time (large individuals: 272.38 ± 55.30 s, small individuals: 270.05 ± 69.13 s; Mann–Whitney U, Z = −0.22, p = 0.83, n = 21) were not significantly different (Figure 4A).


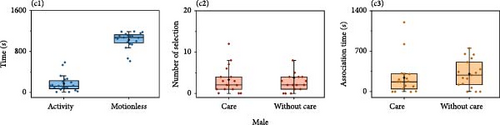
The results of females on males whether had nest showed that females were significantly more stationary (1110.89 ± 44.57 s) than active (89.11 ± 44.57 s) (Mann–Whitney U, Z = −3.58,p < 0.001). There were no significant differences in the number of choices (nest:1.13 ± 0.30 times, nonnest:1.88 ± 0.82 times, n = 8; ANOVA, F = 0.29, p = 0.30) or association times (nest: 125.50 ± 84.56 s, nonnest:151.00 ± 77.32 s, n = 8; Mann–Whitney U, Z = 0, p > 0.05) (Figure 4B).
The results of females for males whether they showed parental care behaviors showed that females spent significantly more time stationary (1028.70 ± 35.66 s) than active (171.30 ± 35.66 s) (Mann–Whitney U, Z = −5.41, p < 0.001). There were no significant differences in the number of choice (parental care: 3.29 ± 0.80 times, nonparental care: 3.82 ± 1.36 times, n = 17; ANOVA, F = 0.01, p = 0.93) or association times (parental care: 245.00 ± 76.60 s, nonparental care: 310.82.20 ± 59.79 s, n = 17; ANOVA, F = 0.73, p = 0.40) (Figure 4C).
The female SOP for large individuals, nests, and parental care were 53.46%, 49.49%, and 44.42%, respectively. None of these were significantly different from 50% (large individuals: p = 0.70; nests: p = 0.97; and parental care: p = 0.54), nor did they show a significant preference among the experimental groups (binomial test, p > 0.05) (Table 6).
| Selector | Indicator | SOP (%) | p | Large/small, nest/no nest, care/no care | |
|---|---|---|---|---|---|
| Group | Binomial p | ||||
| Female | Size | 53.46 | 0.70 | 11/10 | >0.05 |
| Nest | 49.49 | 0.97 | 4/4 | >0.05 | |
| Care | 44.42 | 0.54 | 6/11 | >0.05 | |
| Male | Size | 47.10 | 0.75 | 6/7 | >0.05 |
- Abbreviation: SOP, strength of preference.
3.2.2. Male Mate Choice
Choice of O. potamophila males for females of different sizes showed that males had significantly higher stationary times (1018.00 ± 36.50 s) than active times (182.00 ± 36.50 s) (Mann–Whitney U, Z = −5.41, p < 0.001, n = 20). The number of choice (large individuals: 2.38 ± 0.57 times, small individuals: 2.77 ± 0.66 times; ANOVA, F = 0.23, p = 0.64, n = 13) and association time (large individuals: 150.92 ± 38.59 s, small individuals: 188.31 ± 59.94 s; Mann–Whitney U, Z = −0.44, p = 0.66, n = 13) did not differ significantly (Figure 4D). The SOP of males for larger individuals was 47.10%, which was not significantly different from 50% (larger individuals: p = 0.75). No significant preference was presented in the experimental group (binomial test, p > 0.05) (Table 6).
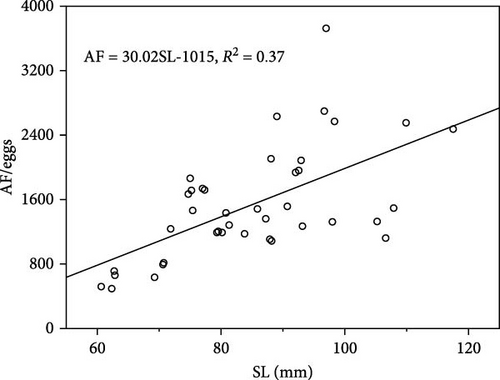
A significant difference was noted in the AF of female individuals used in male choice (large individuals: 1890 ± 158 grains; small individuals 1177 ± 101 grains; ANOVA, F = 14.52, p < 0.001). A positive correlation was noted between female body length and fecundity (r = 0.61) and a relationship of AF = 30.02SL−1,015 (R2 = 0.37, n = 40) (Figure 5).
4. Discussion
4.1. Construction of Reproductive Behavioral Profiles of O. potamophila
Identification, classification, and coding of animal behavior are important elements in the study of animal behavior [17]. The natural habitat of fish is special and is generally more difficult to observe directly for behavioral purposes. Even when observed with the help of containers such as aquariums or glass tanks, fish will not be able to display the full range of natural behaviors due to their singular environmental conditions. In this study, we simulated the natural environment of an indoor pool and recorded two consecutive reproductive periods. A total of 29 species in nine categories of O. potamophila behavior were recorded, less than 43 patterns of S. wangchiachii [8] and 45 patterns of Andrias davidianus [20]. The ethogram based on the PAE coding system of aquatic animals is simpler than that of higher terrestrial animals, with 136 behaviors for Cervus nippon sichuanicus [21] and 208 behaviors for Papio hamadryas [22]. This may be related to the evolutionary hierarchy and living environment of aquatic animals. Fish do not have facial expressions or well-developed limbs and live in simpler environments than terrestrial fish. Therefore, fish have fewer movements, postures, and types of environments to cope with than higher terrestrial animals. Among them, O. potamophila was less active on its own and lacked group social behaviors, resulting in less diversity in its ethograms.
In this study, we observed the behavior of O. potamophila, which has a diurnal activity pattern and a high rate of occurrence at night. This diurnal pattern of activity may be related to anti-predator strategies in fish [23]. The daylight period is more conducive to predator-feeding activity. Therefore, during the daytime, O. potamophila chooses nests or other coverable objects to hide from predation and comes out briefly at night [24, 25]. O. potamophila is usually weak in locomotion but swims rapidly when feeding and can also be aggressive when occupying nests and performing field behavior. There was no strong diurnal pattern of attack or predation behavior. Both occur more frequently during the daytime, with no difference in the average number of attacks during the daytime and nighttime.
However, there were differences in the behavioral characteristics of O. potamophila during the reproductive period. Females are primarily involved in mating during reproduction and begin feeding to replenish their energy after mating. Males occupy the nest during the pre-reproductive period, displaying a range of domain behaviors, including attacking, intimidating, and threatening, until the female arrives to complete the mating process. Males also exhibited parental care behaviors after mating. This sex difference in reproductive behavior reflects the different divisions of labor between males and females in the reproductive strategy of O. potamophila. Females mainly invest energy in egg production and development. Meanwhile, males invest less energy in gametes and mainly use their energy in behavioral competition and postlaying parental care behaviors to obtain more reproductive opportunities to increase offspring survival rates [14]. This idea is further validated by the social mating system of one male and multiple females presented in this study, where one male O. potamophila was able to mate with up to six different females.
Females preyed on conspecifics and ingested fertilized eggs at the end of mating. Meanwhile, males with parental care behaviors ingested fertilized eggs to a lesser extent when the clumps they guarded were destroyed by invasive fish. This type of cannibalism has been reported in many carnivorous fish [26, 27]. Cannibalism is an ecological mechanism that has adaptive value under harsh environmental conditions, resulting in the death of prey and the stabilization of the survival of the entire population [28, 29]. For males with the parental care behaviors of O. potamophila, when the egg masses they are protecting are destroyed, the return from egg care is no longer sufficient to cover the cost of egg care. Therefore, the males abandon the eggs they are currently protecting or eat them. They then start a new round of mating to obtain more fertilized eggs to improve their reproductive fitness. This tradeoff between current reproductive success and potential mating opportunities has also been reported in fishes with parental care [30, 31].
4.2. Mate Choice of O. potamophila
This study was conducted to investigate the mate choice of O. potamophila by selecting male O. potamophila based on body size, nest, and parental care behaviors and female O. potamophila based on body size. During the four experiments in mate choice, most of the females in the experiment were stationary and spent less time active, which is an important reason for avoiding predators, in addition to their own habits. Predators can influence mate choice during the reproductive period. Therefore, O. potamophila can greatly shorten the process of mate sampling and conserve the energy that can be invested in reproduction by reducing activity and selecting directly after identifying a target [32].
Body size, a trait typically associated with biological fitness, is a direct benefit of sexual choice. Having a larger body size provides an advantage in either fighting or competition for resources and also implies the potential genetic benefits of having a faster growth rate [33, 34]. Numerous studies have proved that females select larger individuals as mates [19, 35, 36]. There was no significant difference in the number of choice and association time invested by female O. potamophila for different-sized males in this study. However, more association time was invested for larger individuals than for smaller ones. This may indicate a slight preference of females for larger males to some extent.
O. potamophila lays viscous eggs requiring substrate attachment. Under natural conditions, they lay their eggs in the crevices of stone dams, empty mussel shells, or hidden caves made of other substrates. Meanwhile, traces of spawning have been found on the walls of indoor concrete pools. For O. potamophila, a finely constructed nest like that of birds is not required. Although higher-quality nests can provide cryptic protection for fertilized eggs and parents with parental care, females in the present study invested slightly more time in individuals without nests. Nests may not be determinative, and other traits may potentially influence female choice [37–39].
In this study, females invested slightly more time in individuals with non-parental care than individuals with parental care. The social mating system of O. potamophila is polygynous. Males with parental care must weigh the potential of obtaining a higher reproduction rate when investing in reproduction with the next female before discarding the eggs they are currently protecting. Although males with parental care can show that they are dominant at the beginning of the reproductive process and can provide assurance of offspring survival, this also implies that males are already involved in reproduction during the current reproductive period and have limited energy to invest in a new round of reproduction. Therefore, females may prefer individuals who have not reproduced and can obtain more input for their offspring [31].
Male mate choice occurs in two ways, and the validity ratio determines the sex of an individual [15]. When the validity ratio is female-biased, that is, there are more females than males to be bred, females compete for mates, and males act as selectors. When males invest more in reproduction than females, males become a scarce resource for females to achieve reproductive success. For example, males with parental care behaviors invest huge amounts of energy in reproduction [40]. Or, species that do not have parental care behaviors exhibit complex courtship behaviors that result in high energy expenditure [41]. Male O. pomaphila has parental care behaviors, and there is a brief period of female-biased sex ratios during the reproductive period. Therefore, male O. potamophila would theoretically be selective. In contrast, the present study showed that, under laboratory conditions, there was no significant difference in the number of choice and association times of male O. potamophila for females of different sizes. However, the number of choices and association times of small individuals were slightly greater than those of large individuals, presenting a slight preference for small individuals. This deviates from the results of male mate choice in other fish with male parental care habits [16, 42].
If the degree of correlation between body size and fecundity influences male preference for larger females, the strength of male preference will be linked to the relationship between body size and fecundity. Several studies have shown a correlation between male choice preference and female size and fecundity in species where males have parental care behaviors. For example, male Gasterosteus aculeatus prefers large females and has a high correlation between female standard length and fecundity [43]. Female standard length explained 37% of the variance in AF in the present study (Figure 5). The correlation coefficient between the two was similar to the values reported elsewhere in the literature [35, 44, 45]. Meanwhile, the coefficient of variation for female fecundity was also higher. This suggests that the potential benefits of a preference for female body size for mate choice would also be higher. However, the results of the present study did not exhibit a tendency to show a preference for body size.
5. Conclusion
In this study, we constructed a comprehensive spectrum of reproductive behaviors exhibited by O. potamophila, encompassing a detailed framework of 29 behaviors organized into nine distinct categories. These included 14 types of postural codes, 22 types of movement codes, and 12 types of environmental codes. The behavioral rhythms of the species were distinctly diurnal, with a significantly higher percentage of individuals active at night than during the day (p < 0.001). Furthermore, there was no absolute diurnal bias observed in key attack, predation, mating, and egg protection behaviors. Overall, the behavioral rhythms were clearly discernible. In the mate choice mechanism, the overall resting time of female and male individuals was significantly longer than the activity time (p < 0.001). This state was driven by habituation and enemy avoidance in order to conserve energy for rapid mate choice. The female subjects exhibited a slight inclination towards larger males, with prolonged interaction periods. However, there was no discernible preference for male nest occupancy or egg-guarding behavior. Although female body size was positively correlated with fecundity, there was no significant difference in male preference for female size (p > 0.05), a result that differed from that observed in other male egg-guarding species.
Ethics Statement
The experiments were conducted under the approval of the research committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
Disclosure
A preprint has previously been published in ResearchGate and withdrawn from the website SSRN (DOI:10.2139/ssrn.4795609).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the Major Science and Technology Project of Hubei Province (grant number 2022BBA009), Huai’an Major Science and Technology Project (grant number HAN202213), Huai’an Natural Science Research Program (grant number HAB202377), and Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection (grant number HSXT30322).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




