Exploring Silver as an Alternative Treatment for Controlling Macrogyrodactylus congolensis Populations
Abstract
Monogeneans are ectoparasites that in high densities, can cause harm and even death to their hosts. Therefore, treatment methods are continuously evaluated. The current study aims to determine the LC50 of ionic silver and silver-engineered nanomaterials (ENMs) (nAg) in the monogenean Macrogyrodactylus congolensis and report on the toxicological effects of silver on the parasite by using their behavioural response as an indicator. Macrogyrodactylus congolensis were exposed in vivo to a range of ionic Ag and nAg concentrations for 12 h in three water media (reverse osmosis [RO], borehole, and aged tap water). The LC10, LC20, and LC50 were determined using ToxRat Professional. Behaviour was assessed using low, medium, and high concentrations (calculated from the parasites LC50) of ionic silver and nAg in the different water media. Videos were recorded with a camera for 5-min periods at 0, 1, 3, 6, and 12 h intervals. The videos were analysed using Noldus EthoVision XT software. The results demonstrated significant increases in the acceleration of movement, body contact between parasites, the distance travelled, mobility, and swimming speed when exposed to increased concentrations of silver. Changes that have been observed are most likely in response to toxicological stress and neurological damage caused by silver. Furthermore, silver and RO water being the most toxic, and aged tap water is the most optimal water medium. Furthermore, parasites showed an increase in behavioural changes as exposure concentrations increased. Lower LCx values were recorded when parasites were exposed to the ionic Ag compared to the nAg in all water media, indicating that the ionic silver was more toxic to M. congolensis than nAg, which highlights the need for the safe development of nanotechnology.
1. Introduction
Monogenean ectoparasites are commonly present on the gills or skin of marine and freshwater fishes, on the eyes of hippopotamuses, or as endoparasites of frogs [1]. They can be especially difficult to control in fish farms or aquariums, and heavy infections may cause excessive mucous secretion, damage to the epithelium, haemorrhages, osmotic problems, and atrophy of the gills [2, 3]. Parasites in the taxon Gyrodactylidae occur on their host’s skin throughout their lifecycle and thrive in nature and aquaria as their progenetic lifestyle allows rapid multiplication [4–6]. For example, Gyrodactylus salaris devastated salmon populations in Norway and thereafter spread across Europe [7–9]. Khalil [10] also documented a deadly infection of Macrogyrodactylus polypterid on Polypterus senegalus in aquaria in Sudan. Treatments such as formalin, salt, and praziquantel have been used with success to reduce such infections, but these studies were done on egg-laying monogeneans (families: Gyrodactylidae and Capsalidae) and were not effective during all life stages [6, 11]. In the current study, Macrogyrodactylus congolensis from the family Gyrodactylidae was evaluated. They are viviparous (give live birth) to fully developed mature offspring. Macrogyrodactylus congolensis is a notorious parasite of the African sharptooth catfish (Clarias gariepinus), one of the top aquaculture species in Africa, especially in Egypt, Nigeria, Uganda, and South Africa. These countries are among the continent’s leading contributors to the sector [12, 13]. Inland fish farming alleviates poverty significantly by creating jobs and ensuring food security, particularly in West Africa, where fish is a staple food [12, 13]. Arafa et al. [14] and Maduenyane et al. [3] reported M. congolensis on the skin of C. gariepinus where it causes severe alterations to the integument, including the proliferation of club cells and mucus cells and in heavy infections fatalities. Apart from consuming the host epidermis when feeding, they can cause macroscopic damage to the host’s skin, when penetrating the skin with their relatively large haptors and smaller marginal hooks and lifting of the epidermis through haptoral attachment by vacuum [3].
Silver is commonly used in several fields because its antimicrobial properties make it effective in the treatment of bacteria, viruses, and fungi, which contributes to wound healing and water sanitation. It also has various other medical and diagnostic applications [15]. Likewise, engineered nanomaterials (ENMs) have gained attention due to their multiple beneficial applications in aquaculture, such as controlling fish diseases, detoxification, and as immune stimulants [16–21]. A study by Dananjaya et al. [22] reported that chitosan–silver nanocomposites were effective antibacterial agents against Aliivibrio salmonicida. The nanocomposites disrupt bacterial cell membranes, induce oxidative stress, degrade DNA, and inhibit protein synthesis, leading to cell death. Additionally, the study found that chitosan–silver nanocomposites were non-toxic to zebrafish and fish cells at tested concentrations, suggesting potential use in aquaculture as a safer alternative to antibiotics. Tattiyapong et al. [23] developed a vaccine using chitosan nanoparticles to enhance antigen delivery and immune response in tilapia against tilapia lake virus (TiLV). The nanoparticle-based vaccine demonstrated effective mucosal absorption, reducing TiLV-induced mortality by 25% in laboratory trials and 31.88% under field conditions. The use of chitosan nanoparticles improved vaccine stability, antigen uptake, and delivery through the fish’s mucosal surfaces, making it a practical alternative to traditional injection vaccines. Similarly, Saleh et al. [24] have shown that nAg is effective against a protozoan, Ichthyopthirius multifiliis (ick), in fishes, as it disrupts the cell membranes in the free-living stages. Minimal information is available about the effect of AgNPs against metazoan parasites of fish. Abu-Elala et al. [25] reported that chitosan–silver nanocomposites were a safe alternative for controlling Lernaea cyprinacea (a parasitic crustacean) infecting goldfish (Carassius auratus) in ornamental aquaria. Their study showed that nanocomposites displayed strong antiparasitic activity, accelerated wound healing, and reduced the risk of secondary infections, making them a viable replacement for traditional chemical treatments. Furthermore, Pimentel-Acosta et al. [26] reported that AgNPs with different size diameters (ARGOVIT and UTSA) were effective against the monogenean, Cichlidogyrus spp. adults and eggs and caused swelling and disruption of the parasite’s tegument. Later, Pimentel-Acosta et al. [27] reported that exposure to high and low concentrations of AgNPs to Cichlidogyrus spp. caused DNA damage and cell death in the parasites.
Furthermore, nanotechnology has increasingly been used in aquaculture to enhance fish health and growth by controlling microbial infections and enhancing feed. For instance, isobutyl cyanoacrylate nanoparticles have demonstrated significant antibacterial activity against fish pathogens without adversely affecting fish health or growth, suggesting their potential to reduce antibiotic use in aquaculture [28]. Polymeric nanoparticles loaded with nutrients have shown promise in fish nutritional supplementation, offering lower toxicity to the environment compared to nonencapsulated nutrients [29]. It is essential to conduct comprehensive harm–benefit analyses and risk assessments to ensure the safe use of nanotechnology; nanomaterials can accumulate in fish tissues and organs, potentially leading to toxicity [30].
As aquaculture facilities use various water types, the current study included reverse osmosis (RO), boreholes, and aged tap water. The current study did not include reconstituted water as this type of water is not commonly used in aquaculture facilities. RO water is purified through filtration to remove particles, bacteria, and dissolved solids [31]. RO water is used in aquaculture facilities, particularly in hatcheries, recirculating aquaculture systems (RASs), and marine or brackish environments where precise water quality control is required [32]. RO water may lack beneficial minerals such as calcium and magnesium [32]. Natural waters are complex, containing a mixture of dissolved minerals, organic matter, gases, microorganisms, and suspended particles, all of which interact and vary depending on geographical, environmental, and biological factors. Similarly, borehole water is sourced from aquifers and can contain natural minerals but may also harbour contaminants like bacteria, heavy metals, or nitrates. Its quality varies, making regular testing important [33]. In South Africa, borehole water is the main source of drinking water in many rural areas, and it is also used in aquaculture. Aged tap water, treated and distributed by municipalities, typically contains a mix of ions, including chlorine and fluoride, and ageing infrastructure can introduce contaminants [34].
This study aimed to determine the LCx of the parasite M. congolensis to ionic Ag and nAg in different water media while assessing their behaviour as behaviour coupled with survival of the parasites could indicate the early-stage effects of the toxicant.
2. Methods and Materials
2.1. Silver-ENMs and Ionic Silver
The nAg stock (Inqaba Biotechnical Industries (Pty) Ltd, Glentham Life Sciences Ltd, product code: GX0585, MW: 107.87, Average particle size: ±35 nm, United Kingdom) was prepared using Milli-Q water with a stock concentration of 100 mg/L. The stock concentration was sonicated (Ultrasonic Cleaner PS-10A, Integral systems, Johannesburg) for an hour prior to use. To dilute to the required exposure concentrations, relevant volumes of the nAg stock solution were added to the various water media (RO water, borehole water, and aged tap water). Silver nitrate (AgNO3) (Inqaba Biotechnical Industries (Pty) Ltd, Glentham Life Sciences Ltd, product code: GK7224-100G, MW: 169.88, United Kingdom) was used for ionic exposures and was dissolved directly in the relevant water media (RO water, borehole water, and aged tap water) to make up a stock solution of 100 µg/L. The working solutions were prepared to relevant volumes and the stock solutions were added to the water media.
2.2. Characterisation of Silver-ENMs
The concentration, size distribution, zeta potential (surface charge), and dissolution were determined based on Klaine et al. [35], Stone et al. [36], and Von der Kammer et al. [37]. The stock solution was prepared in Milli-Q water and working solutions to disperse the stock solutions into the different media (RO water, borehole water and aged tap water). The size of the nAg primary particles was confirmed through transmission electron microscopy (TEM) (JEM-2100/200 KV, JEOL, Japan). A drop of each of the dispersion solutions was deposited onto a carbon-coated copper grid (Protea Laboratory Solutions (Pty) Ltd, product code: S-3630C-MB, Midrand, South Africa) and allowed to air-dry before examination at high resolution (200 kV). For each sample the hydrodynamic size distribution and zeta potential of nAg were determined with dynamic light scattering (DLS) using a folded capillary cell (Malvern Zetasizer Nano series, NanoZS). The size, shape, and element analysis of nAg was confirmed using scanning electron microscopy (SEM) (TESCAN Vega 3 LMH SEM, Brno, Czech Republic) equipped with an X-Max50 energy-dispersive x-ray spectrometer (EDS) (Oxford Instruments, Halifax, England), operated by Aztec 2.1 software (Oxford Instruments, Halifax England) for Windows.
2.3. Physico-Chemical Water Quality
Physical water quality parameters of the selected media (RO water, borehole water, and aged tap water) were measured at the start and end of each test. All water media was oxygenated using air stones connected to a compressed air pump for 48 h before the start of tests. The temperature, pH, conductivity (µS/cm), total dissolved solids (TDSs) (mg/L), dissolved oxygen (mg/L), and salinity (ppt) were measured using a multi-parameter metre (HANNA instruments Inc, Woonsocket, Rhode Island, United States of America). Chemical water quality parameters included: ammonium (Ammonium test method: photometric 0.010–3.00 mg/L NH4-N with a Spectroquant:1147520002), chlorine (chlorine test [free chlorine] method: photometric, DPD 0.010–6.00 mg/L Cl2 with a Spectroquant:1005980002), nitrite (nitrite test method: photometric 0.002–1.00 mg/L NO2-N 0.007–3.28 mg/L NO2- with a Spectroquant:1147760002), nitrate (nitrate test 0.2 20.0 mg/L NO3 N 0.9:1147730001), phosphate (phosphate test 0.5 30.0 mg/L PO4 P 1.5:1148420001) and total hardness (total hardness cell test with a Spectroquant:1009610001) were measured using Merck test kits. Water media were analysed by inductively coupled plasma–mass spectrometry (ICP-MS) (NexIONR 300 series Inductively Coupled Plasma–Mass Spectrometer, PerkinElmer, Boston, USA) for element content (Na, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Rb, Mo, Ag, Cd, Ba, Au, Pb, and U). The accuracy of the analysis was monitored and assessed using certified reference material 1640a (prepared at the National Institute of Standards and Technology in Gaithersburg, MD, USA). Limits of detection and recovery rates between 80% and 120% are presented in Table 1.
| Elements | Certified reference value (µg/L) | Measured value (µg/L) | Recovery (%) | LOD (µg/L) |
|---|---|---|---|---|
| Vanadium (V) | 15.05 | 14.40 | 96.47 | 0.23 |
| Manganese (Mn) | 40.39 | 37.38 | 88.15 | 0.15 |
| Iron (Fe) | 36.8 | 32.58 | 88.08 | 0.45 |
| Cobalt (Co) | 20.24 | 19.09 | 90.73 | 0.11 |
| Nickel (Ni) | 25.32 | 23.24 | 86.94 | 0.08 |
| Copper (Cu) | 85.75 | 79.94 | 88.02 | 0.23 |
| Zinc (Zn) | 55.64 | 52.24 | 86.76 | 0.15 |
| Molybdenum (Mo) | 45.60 | 41.39 | 88.22 | 0.17 |
| Silver (Ag) | 8.081 | 7.88 | 115.4 | 0.20 |
| Barium (Ba) | 151.80 | 144.96 | 86.68 | 0.20 |
| Lead (Pb) | 12.101 | 11.43 | 100.8 | 0.16 |
| Uranium (U) | 25.35 | 23.70 | 93.76 | 0.22 |
2.4. Acute Lethality Test for Macrogyrodactylus congolensis
All fish, along with their parasites, were maintained and cultured in the research aquarium at the University of Johannesburg. All procedures involving animals were carried out within guidelines by the South African National Animals Ethics Council and approved by the University of Johannesburg Ethics Committee (Reference Number: 2023-07-12/Latief_Oldewage). A parasite culture was maintained on multiple C. gariepinus in borehole water in a temperature-controlled room with a temperature at 22–23°C, pH (6.0–8.0), and oxygen (4–8 mg/L) and a 16-h day and 8-h night cycle. Acute toxicity tests were done using adult M. congolensis; after removing it from the skin of C. gariepinus. Parasites were collected using a microscope glass slide to scrape the parasites off the skin surface of the fish and transferred to plastic Petri dishes immediately prior to exposures. For the ionic silver, a concentration range of 0.1, 1, 2, 5, 10, 20, 50, and 100 µg/L AgNO3 was used, including a control (without AgNO3). The concentration range used for nAg was 0.1, 1, 2, 5, 10, 20, 50, and 100 mg/L, including a control (without nAg). Each concentration was tested for the different water media, which included RO water, borehole water, and aged tap water. Procedures were according to the OECD 202 guideline for testing of chemicals (Daphnia sp., acute immobilisation test). In the current study, seven parasites per concentration were placed in Petri dishes containing the exposure medium. A 16 h day and 8 h night cycle in a temperature-controlled room at 22–23°C was maintained throughout the exposure. Parasite mortality was recorded with a camera, hourly for the first 6 and 12 h thereafter. The test concluded at mortality of the parasites in the control medium per exposure media. Mortality was confirmed when no movement was observed and when parasites failed to respond to a gentle touch with a 000 Camels’ paintbrush. A flow diagram of how the lethal toxicity exposure was carried out is indicated in Figure 1.
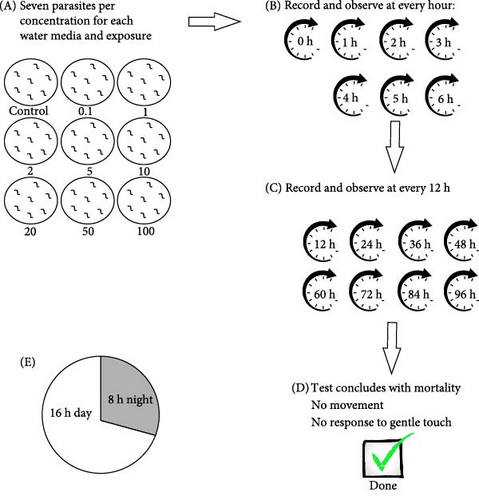
2.5. Behaviour Analysis of Macrogyrodactylus congolensis
Concentrations per exposure media were low (ionic Ag = 0.1 µg/L, nAg = 0.1 mg/L), medium (ionic Ag = 2 µg/L, nAg = 2 mg/L) and high (ionic Ag = 10 µg/L, nAg = 10 mg/L). Behaviour analysis was done for control and exposed parasites with videos recorded for 5 min every hour for the first 6 h and thereafter every 12 h. Three parasites per group (from each concentration for each water media) were randomly selected for behaviour analysis. The behaviour was recorded using a dissection microscope (Zeiss Stemi 305, Jena, Germany) equipped with a camera (Zeiss AxioCam ERc 5s). The videos were analysed using EthoVision XT software (Noldus Information Technology, Wageningen, The Netherlands), ensuring that data points per parasite did not overlap by physically viewing the videos and aligning any points by inserting any missing data points within the software. The endpoints analysed were: distance travelled, swimming speed, acceleration, mobility, and body contact.
2.6. Statistical Analysis
The LC50 of the parasite M. congolensis in different water media of ionic Ag and nAg was determined using ToxRat Professional. Graphical representations of the LC50 and violin plots, including statistical analysis for the behaviour analysis, were compiled using GraphPad Prism software (Prism 5 for Windows; Version 5.02, California, USA). Violin plots were chosen as they depict the distribution of the data using density curves. Swimming behaviour data were analysed by performing a two-way ANOVA (Kruskal–Wallis test was performed as a non-parametric alternative) with post hoc Tukey’s multiple comparisons test. The significance of the results was determined at p < 0.05 and are provided as three meaningful numbers. The principal component analysis (PCA) was constructed using R statistical software (R version 4.4.0 (2024-04-24 ucrt)—“Puppy Cup”).
3. Results
3.1. Characterisation
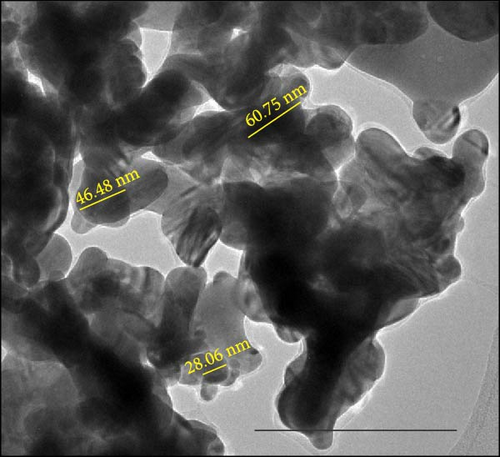
The characterisation of nAg was conducted in all water media (Milli-Q water, RO water, aged tap water, and borehole water) and was done with 0.5 mg/L. The nAg particles agglomerated in all water media (Figure 2). The average primary nAg particle size was 40.3 ± 3.04 nm; Milli-Q water had a hydrodynamic size distribution average of 211.3 nm and a zeta potential of −24.4 ± 4.65 mV. For RO water, the hydrodynamic size distribution was 298.6 nm, and the zeta potential of −17.4 ± 5.14 mV. Aged tap water had an average hydrodynamic size distribution of 255.7 nm with a zeta potential of −16.7 ± 4.13 mV. The average primary particle size in the borehole water was 38.76 ± 6.73 nm; the average hydrodynamic size distribution was 333.1 nm and had a zeta potential of −14 ± 3.7 mV. Although agglomeration of particles was observed in all water media, it was higher in the aged tap water. Minimal Ag dissolution occurred in aged tap water and was measured using dialysis tubing strips (product code: D9777, Sigma–Aldrich Ltd, Dorset, UK), with a cellulose membrane having a molecular weight of 12,000 Da. Low, medium, and high concentrations for each water media were analysed over 10 days at the following periods: 0, 6, 12, 24, 120, and 240 h. At high concentrations, there was a spike of Ag ions between 6 and 24 h.

3.2. Physico-Chemical Water Parameters
The physico-chemical parameters remained constant in each water medium during the exposures and are reflected in Table S1, while the element analysis for the RO water, borehole water, and aged tap water is displayed in Table S2. Comparing the three-water media with the South African target water quality range (TWQR) shows that the borehole water contained 133.67 ± 15.301 CaCO3 mg/L, which is above the range and indicating slightly hard water, whereas the RO and aged tap water have soft to moderately soft water. For borehole water and aged tap water Cu (borehole: 9.36 ± 1.14, aged tap water: 34.48 ± 2.30) was above the TWQR. The pH and conductivity influence the size and zeta potential of nanoparticles. RO water with an average pH of 6.4, and aged tap water with an average pH of 5.8 were slightly acidic, while the borehole water was almost neutral with an average pH of 6.9. As expected, RO water has a very low conductivity (average of 29.8 µs/cm), and borehole water and aged tap water having a higher average conductivity (228 µs/cm and 188.4 µs/cm, respectively).
3.3. Acute Toxicity Testing
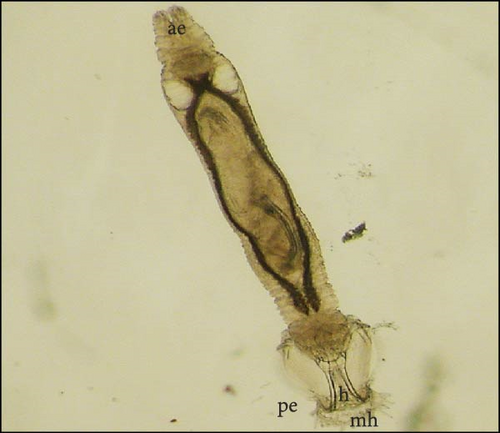
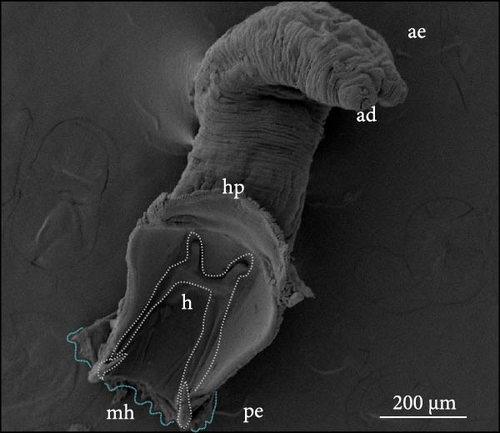
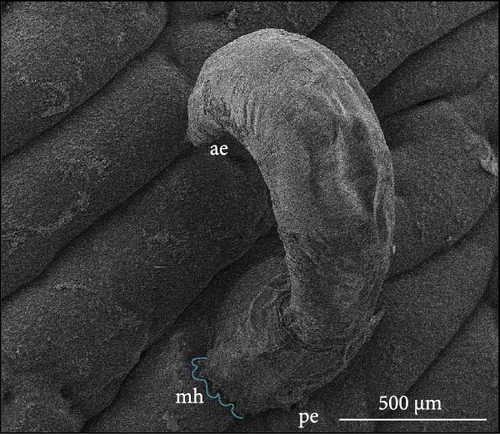
The parasites were identified by Maduenyane et al. [38] in a parallel and simultaneous study from the same laboratory, using classical morphological techniques, molecular analysis [38], and pathology [3]. Figure 3 presents a photomicrograph of specimens attached to the host C. gariepinus, a micrograph of the parasite to show morphology and their attachment haptors.

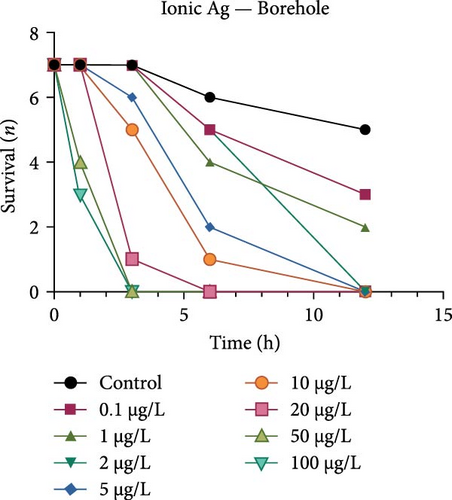
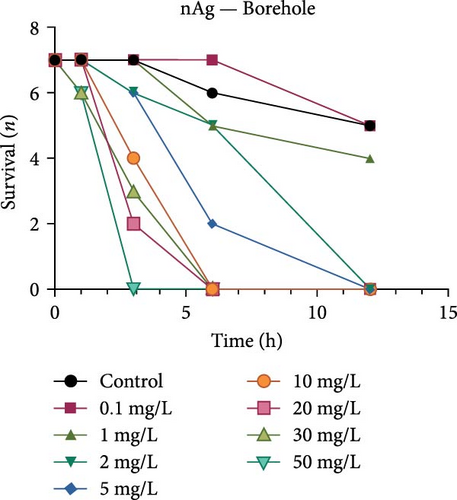
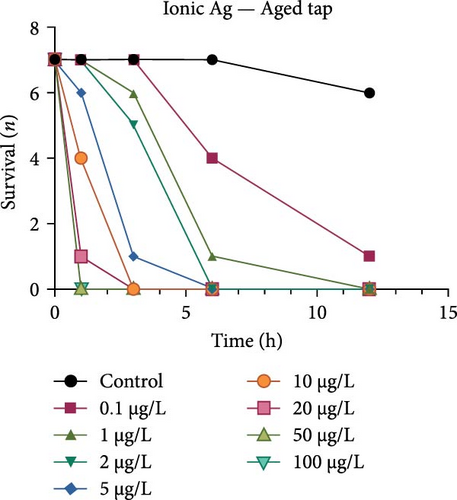
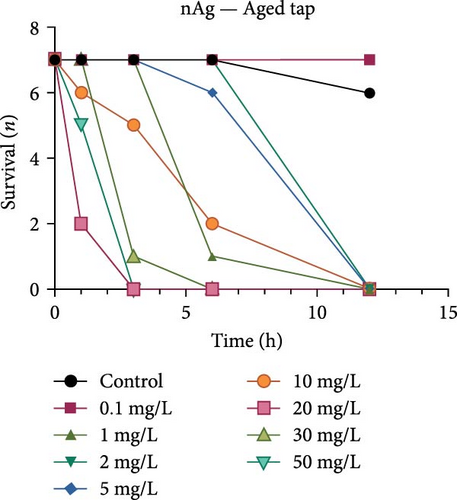
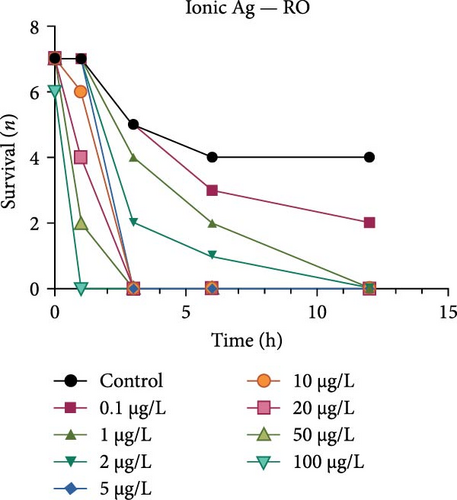
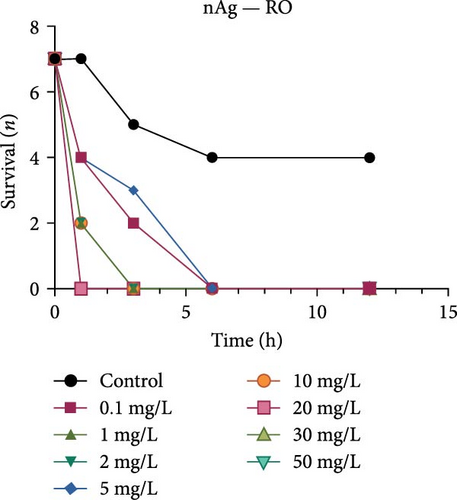
Both the ionic Ag as well as the nAg had a concentration-dependent effect on the survival of M. congolensis (Figure 4); the ionic silver had a higher acute toxicity compared to nAg in M. congolensis. The LCx values which could be calculated are listed in Table 2. The LC50 for ionic Ag in RO water was 0.101 µg/L, for borehole water 0.116 µg/L, and for aged tap water 0.171 µg/L. The LC50 for nAg for borehole water was 373.02 µg/L and 322.11 µg/L for tap water. The LC50 for nAg in RO water could not be calculated due to a 42.9% mortality rate within the control. In lower concentrations, parasites had an increase in reproductive success; in medium concentrations, the parasites reproduced, but juveniles died soon after birth or while the adult was giving birth; parasites in very high Ag concentrations died either before they reproduced or both adult and juvenile died during birth and displayed balling and stretching behaviours followed by lethargy just before dying.
| LCx | Ionic silver (µg/L) | Nanosilver (µg/L) | ||||
|---|---|---|---|---|---|---|
| RO | Borehole | Aged tap | RO | Borehole | Aged tap | |
| LC10 | 0.07 | — | 0.14 | — | 58.02 | 248.11 |
| LC20 | 0.08 | — | 0.15 | — | 109.02 | 271.11 |
| LC50 | 0.10 | 0.12 | 0.17 | — | 373.02 | 321.11 |
3.4. Behaviour
Figure 5 shows the acceleration of M. congolensis in RO water, borehole water, and aged tap water in a low (ionic Ag = 0.1 µg/L, nAg = 0.1 mg/L), medium (ionic Ag = 2 µg/L, nAg = 2 mg/L) and high (ionic Ag = 10 µg/L, nAg = 10 mg/L) concentration compared to a control over a 12 h period. The graphs show a higher acceleration in the aged tap water compared to the RO and borehole water. The medium concentration of ionic Ag in RO water caused the highest acceleration. There is a significant difference in the borehole water at a low concentration at 0 h between the control and nAg (p = 0.0022) and between the ionic Ag and nAg (p = 0.0004), there is a significant difference between the control and ionic Ag (p = 0.0278) at 1 and 3 h (p = 0.0310). Borehole water at a high concentration also showed a significant difference at 1 h between the control and nAg (p = 0.0231).
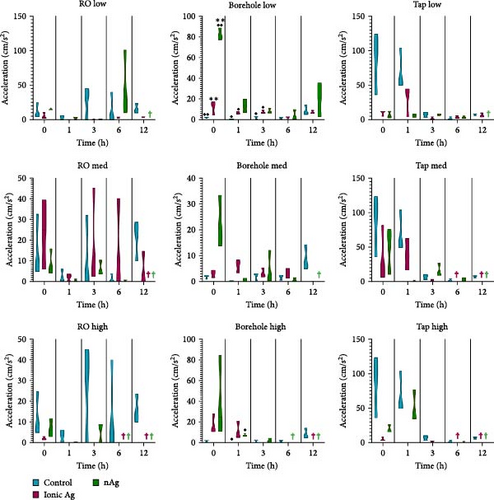
Figure 6 shows the body contact of M. congolensis in the three-water media in a low, medium, and high concentration of ionic Ag and nAg compared to the control over a 12-h period. In borehole water, the highest number of body contact between parasites occurred, while in ionic Ag even in low concentrations, an increase occurred in RO water. There is a significant difference between the control and nAg (p = 0.0272) and between the ionic and nAg (p = 0.0162) at 3 h for the tap water at a low concentration, there is a significant difference for the tap water at a medium concentration between ionic Ag and nAg at 1 h (p = 0.0297) and 3 h (p = 0.0278), and a high concentration for the aged tap water at 0 h between ionic Ag and nAg (p = 0.0041). For the borehole water, there is a significant difference in the medium concentration at 1 h between the control and ionic Ag and ionic and nAg, and the high concentration at 0 h between ionic Ag and nAg (p = 0.0201). For the RO water, there is a significant difference in the medium concentration at 3 h between the control and ionic Ag (p = 0.0437).
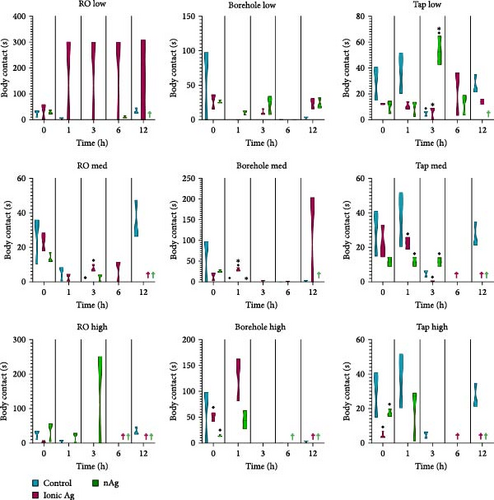
Figure 7 shows the distance travelled for M. congolensis in the three-water media in a low, medium, and high concentration of ionic Ag and nAg compared to a control over 12 h. There is a significant difference for the RO water at a low concentration at 6 h between the control and ionic Ag (p = 0.0001) and between the control and nAg (p = 0.0223), for the medium concentration there is a significant difference at 0 h between the control and ionic Ag (p = 0.0393). For the borehole water, there is a significant difference in the low concentration at 0 h between the control and nAg (p = 0.0060) and between ionic Ag and nAg (p = 0.0021), for the medium concentration there is a significant difference at 0 h between the control and nAg (p < 0.0001), and for the high concentration between the control and ionic Ag (p = 0.0001) at 0 h and at 1 h (p = 0.0391). For the aged tap water at a low concentration, there is a significant difference at 0 h between the control and ionic Ag (p = 0.0332) and between the control and nAg (p = 0.0169), for the medium concentration there is a significant difference at 0 h between the control and ionic Ag (p = 0.0367), at 1 h between the control and nAg (p = 01.0387) and at 6 h between control and nAg (p = 0.0293) and the high concentration there is a significant difference between the control and ionic Ag (p = 0.0196) at 0 and 1 h (p = 0.0407).
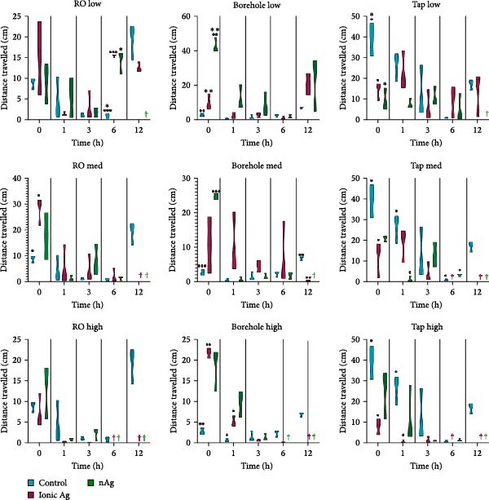
Figure 8 shows the mobility for M. congolensis in the three-water media in a low, medium, and high concentration of ionic Ag and nAg compared to a control over a 12-h period. Tap water caused the highest mobility measured by the EthoVision XT software in parasites compared to the other waters, while ionic Ag in RO water at the low concentration showed the highest mobility. For the RO water at the low concentration, there is a significant difference at 0 h between the control and ionic Ag (p = 0.0482) and ionic Ag and nAg (p = 0.0329), at 1 h between the control and ionic Ag (p = 0.0012) and ionic Ag and nAg (p < 0.0001), at 3 h between the control and ionic Ag (p = 0.0079) and ionic Ag and nAg (p = 0.0080), at 6 h between the control and ionic Ag (p = 0.0008), control and nAg (p = 0.0490) and ionic Ag and nAg (p = 0.0004) and 12 h between the control and ionic Ag (p = 0.0007). RO water at a medium exposure concentration has a significant difference between the control and ionic Ag (p = 0.0203). For the borehole water at the medium concentration at 0 h there is a significant difference between the control and nAg (p = 0.0083); at the high concentration, there is a significant difference at 0 h between the control and ionic Ag (p = 0.0070) and between ionic Ag and nAg (p < 0.0001). Tap water at a low concentration has a significant difference between the control and ionic Ag (p = 0.0182) between the control and nAg (p = 0.0154) at 0 h, and between the control and nAg (p = 0.0171) at 1 h.
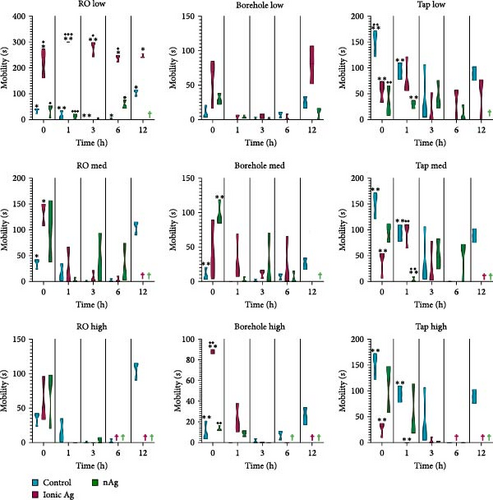
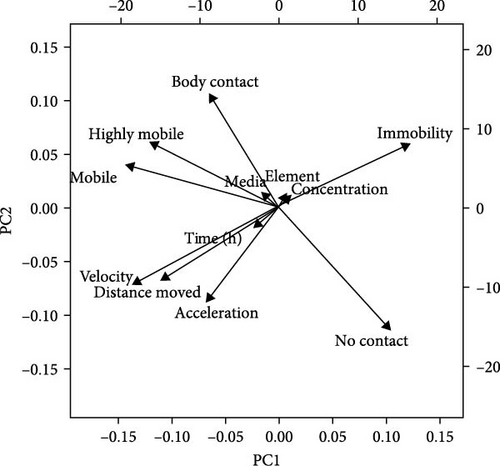
Figure 9 shows the swimming speed for M. congolensis in the three-water media in a low, medium, and high concentration of ionic Ag and nAg compared to a control over a 12-h period. Tap water showed the highest swimming speed measured by EthoVision XT software between parasites. For the RO water at a low concentration, there is a significant difference between the control and ionic Ag (p < 0.0001) and between the control and nAg (p = 0.0224) at 6 h, for the medium concentration, there is a significant difference between the control and ionic Ag (p = 0.0439) at 0 h. For the borehole water at a low concentration, there is a significant difference at 0 h between the control and nAg (p = 0.0077) and ionic Ag and nAg (p = 0.0020), at the medium concentration at 0 h there is a significant difference between the control and nAg (p < 0.0001) and at 12 h between the control and ionic Ag (p = 0.0013), at the high concentration for borehole water, there is a significant difference between the control and ionic Ag at 0 h (p < 0.0001) and 1 h (p = 0.0363). For the aged tap water at the low concentration, there is a significant difference between the control and ionic Ag (p = 0.0340) and the control and nAg (p = 0.0182) at 0 h, for the medium concentration at 1 h between the control and nAg (p = 0.0369) and between the control and nAg (p = 0.0248) at 6 h. For the high concentration in aged tap water, there is a significant difference between the control and ionic Ag at 0 h (p = 0.0215) and 1 h (p = 0.0389). The PCA (Figure 10) shows that several factors are related to the swimming behaviour of M. congolensis exposed to a range of concentrations of ionic Ag and nAg, in different water media. The figure shows body contact, mobility, velocity (swimming speed), distance moved and acceleration to have a strong correlation.
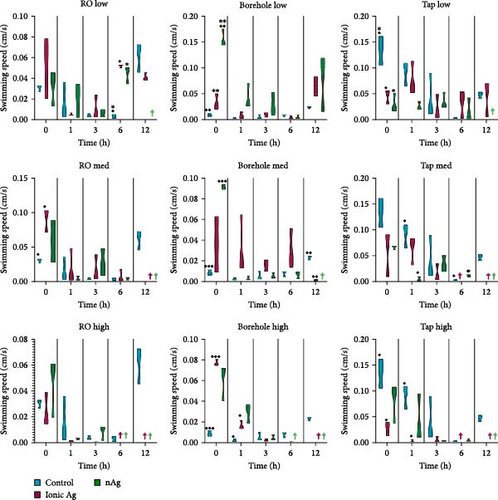
4. Discussion
ENMs in the aquatic environment are subjected to physical (pH, TDS, conductivity, and temperature), chemical (NOM), elemental (metals), and biological (organism and/or tissue culture) constituents [39]. These influence the agglomeration/aggregation, dissolution, redox potential, and biodegradation transformation processes [40–42]. In the current study, nAg was characterised using Milli-Q water, RO water, borehole water, and aged tap water. The characterisation was done in the different media to assess the transformed nanomaterial. According to Lawler et al. [43], an increase in pH results in nAg becoming more negatively charged; this trend is observed in the current study when comparing the zeta potential of RO water and aged tap water; however, the borehole water deviates from this pattern, potentially due to its higher volume of natural organic matter content, which enhances the negative charge on the surface of the nanomaterials at a near-neutral pH. Due to steric stabilisation mechanisms, negatively charged particles lead to less particle aggregation, thus making these particles readily available for distribution and biological uptake [44]; however, at higher concentrations, the increased particle density can overcome stabilisation forces, potentially resulting in aggregation. In the present study, the formation of large agglomerates and aggregates was macroscopically observed within the Petri dishes during the exposure experiments.
Parasites showed reduced survival and significant behavioural alterations, indicating that both ionic Ag and nAg affect these parasites. In laboratory settings, silver salts like AgNO3 readily release toxic Ag+ ions, but in natural waters, these ions often bind with organic matter and sulphides, reducing their toxicity, but the hardness of the water, indicated by the presence of Ca2+ and Mg2+ cations, does not reduce silver toxicity [45]. In the current study, the borehole water which (harder than RO water) but still had a higher LC50 for the parasites for AgNO3. Furthermore, pH (4.5–6.8) does not directly affect silver’s binding to fish gills but may rather influence toxicity through interactions with dissolved organic carbon. The RO water and borehole water exceed the safe range, and this may lead Ag to cause a stress response in the parasites. Anions like chloride (Cl-) provide inconsistent protection against silver toxicity, and rainbow trout and certain daphnids benefit from higher Cl- concentrations, while other species experience little to no protective effects [46]. The current study shows that M. congolensis was more sensitive to ionic silver, which was used in the form of AgNO3, compared to nAg; this trend was also reported for amphipods [47, 48]. The ionic silver concentrations used were 1000 times lower compared to nAg. The effects for silver are generally understood, while studies on long-term exposure to nAg are unknown, these could have compounding effects with nano-specific responses and ionic silver dissolution. The higher toxicity of AgNO3 is partly due to a higher ionic metal exposure compared to that of nAg [49]. Kühr et al. [50] previously reported that the release of ionic Ag from nAg is the major driving factor causing adverse effects.
A comparison of the survival curves showed that M. congolensis survival decreased rapidly as nAg concentrations increased. In a shorter amount of time in the RO water higher mortality occurred compared to the borehole and tap water. Comparing borehole and tap water, parasite numbers in the tap water declined faster over time. The LC50 of ionic Ag after 12 h for M. congolensis was 0.1 µg/L for RO water, 0.12 µg/L for borehole water, and 0.17 µg/L for tap water. The LC50 of nAg after 12 h for M. congolensis was 373.02 µg/L for borehole water and 321.11 µg/L for tap water. Saleh et al. [24] reported that 10 μg/L nAg with an average diameter of 21 nm was 100% effective against I. multifiliis after 2 h of exposure. As no mortalities occurred in the treated fish, they recommended the development of AgNPs for antiprotozoal agents for fish aquaculture. Pimentel-Acosta et al. [26] reported that nAg UTSA, with a diameter of 1–3 nm, was effective against Cichlidogyrus at a concentration of 6 μg/L after 3 h. This significantly lower concentration compared to the current study might be attributed to the smaller particle size used in their study and/or the large aggregation and agglomeration of the nAg in the current study reducing the particles’ dispersibility. Aggregation and agglomeration inhibit biological uptake due to larger cluster sizes having a reduced surface area [51]. A study by Tomar and Preet [52] reported that 25,000 μg/L of nAg with a diameter of 15–25 nm caused 87% mortality in adults of the nematode Haemonchus contortus after 24 h; this was a much higher concentration (about 60x higher) than the concentration in the current study for M. congolensis and that for Cichlidogyrus exposed to UTSA or ARGOVIT nAg [26]. The higher concentration may have been required due to the impenetrable cuticle of nematodes, a complex and varied structure resistant to toxicants [53]. The tegument of a typical monogenean, such as that described for Cichlidogyrus halli typicus, has a thinner outer membrane and a cytoplasmic epidermis (syncytium) and a basal lamina, followed by a layer of circular and longitudinal muscle fibres [54] and would provide less protection, rendering them much more vulnerable to toxicants.
ENMs may enter and accumulate in unintended aquatic organisms and could potentially have toxic effects at increased concentrations (bioaccumulation) to organisms higher up in the food chain. It can impact their behaviour, reproduction, and survival, having toxic effects on fish, invertebrates, algae, and other primary producers [55]. Behavioural alterations are accepted as highly effective endpoint measurements for assessing the impact of nanomaterials [56]. Parasites exposed to medium and high concentrations of ionic Ag and nAg produced more offspring in a shorter period of time compared to that in the low concentrations and the control. Alterations reflect toxicity effects in various aquatic species and life stages, even at sub-lethal exposure concentrations [57]. In the current study for RO and borehole water at 0 and 1 h, parasites exposed to Ag show a higher acceleration, swimming speed, mobility, and distance travelled compared to the control in low, medium, and high concentrations, which could be a response of the parasite to avoid the pollutant or toxicant. This can be regarded as a hyperactive erratic movement similar to observations of zebrafish, where this behaviour was linked to a stress response [58]. The increased erratic swimming behaviour could also be attributed to neurological abnormalities, as reported in the monogenean Cichlidogyrus when exposed to nAg [27]. At the high concentrations at 6 h in our study, the parasites decreased swimming behaviour compared to the control, as they were dying or dead. Parasites exposed in the aged tap water to both ionic Ag and nAg also showed reduced mobility, swimming speed, acceleration, and distance travelled in aged tap water and RO water compared to those in the control. The behaviour is consistent with that in zebrafish [59]. Higher mobility in the RO water when exposed to ionic Ag, could be due to the toxicity of ionic Ag and that the ions enter the body of the parasite more readily. Parasites exposed to silver in this study have higher mobility before death and could possibly also explain the higher mobility in the parasites exposed to ionic Ag in RO water. In borehole water, the distance, swimming speed, acceleration, and mobility of the parasites in nAg were higher compared to those in ionic Ag and the control, which could indicate hyperactivity in response to the Ag reacting with constituents present in the borehole water. Vogt et al. [60] reported that nAg exposure on whitefish, Coregonus lavaretus larvae produced significant hyperactivity even at low concentrations (45 µg/L). However, the behavioural response of organisms to nanomaterials varies even with the same material because of the size of the particles and/or the coatings. Pimentel-Acosta et al. [26] reported major ultrastructural changes in the tegument after exposures to 36 and 60 μg/L UTSA nAg and showed that exposure to nAg, even at low concentrations, Cichlidogyrus exhibited a physiological response. We concur that the behavioural responses observed in the current study were related to the silver exposure and conclude that the behaviour was related to stress responses caused by toxicant exposure to both ionic and nAg. A limitation of the current study was that parasites were exposed while off the host, which may have caused alternate behaviours from those feeding on a host, but these were all compared to a control in standardised uniform conditions.
5. Conclusion
Nanomaterials undergo complex transformations within aquatic media, which decrease their biological availability due to protein corona formation, agglomeration, or increased silver ion dissolution. Parasites exposed to both forms of Ag released more juveniles in higher concentrations compared to the control within the first 2–4 h, but juvenile mortality occurred during or soon after birth. Additionally, a decrease in behavioural activity was observed in high concentrations, suggesting a stress response to silver toxicity, while mortality did occur. The study of the toxicological effects on the monogenean parasite M. congolensis indicated that AgNO3 has a higher toxicity compared to nAg, which highlights the need for harm–benefit studies in nanotechnology. Further research is required to evaluate how silver and nAg exposure effects parasites while attached to their host, as well as its broader implications for the host fish health and, consequently, aquatic ecosystems.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Lutfiyya Latief: data curation, investigation, visualisation, writing–original draft. Annemariè Avenant Oldewage: conceptualisation, funding acquisition, supervision, writing–reviewing and editing. Tarryn L. Botha: conceptualisation, supervision, writing–reviewing and editing.
Funding
This study was supported by the National Research Foundation of South Africa (NRF) with a grant for PhD study to Lutfiyya Latief, the National Research Foundation of South Africa (NRF), Grant number SRUG22052013241-PR-2023 to Annemariè Avenant Oldewage, the University of Johannesburg, Faculty Research Committee (FRC), and University Research Committee (URC), for project funding to Annemariè Avenant Oldewage.
Acknowledgments
We acknowledge the University of Johannesburg, Faculty of Science, SPECTRUM analytical facility for equipment and facilities, and North-West University (NWU) for the use of the EthoVision behavioural and ToxRat Professional software.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




