Expression and Localization of Piwil1 During Gonadal Development in Monopterus albus
Abstract
PIWI proteins and Piwi-interacting RNAs (piRNAs) are crucial for gametogenesis, embryogenesis, and stem cell maintenance in animals. The Piwil1 (Piwi-like RNA-mediated gene silencing 1) gene has rapidly evolved in the protogynous hermaphrodite ricefield eel (Monopterus albus) compared to other teleosts. Notably, there is a significant reduction in DNA methylation levels and an increase in the expression levels of the Piwil1 gene in testes compared to those in ovaries. However, the role of Piwil1 in the gonadogenesis of ricefield eel remains unclear. This study elucidated the histomorphological characteristics of ricefield eel gonads at different developmental stages and analyzed the expression and localization of Piwil1 using qRT-PCR, western blot, and immunofluorescence. Histological analysis showed that the female gonad contained oocytes at different developmental stages, the intersex gonad had both ovarian and testicular tissues, and the male gonad included spermatogenic cells and testicular lobules. Quantitative real-time PCR and western blot indicated significantly higher Piwil1 expression in the ovary, intersexual gonad, and testis compared to other tissues, with levels increasing during gonadal differentiation and peaking in the testis. Immunofluorescence analysis revealed that the PIWIL1 protein was abundantly localized in the cytoplasm of immature oocytes, granulosa cells surrounding the oocytes, spermatogonia, and primary spermatocytes. Importantly, in both the ovary and testis, the expression of Piwil1 initially increased but then decreased at the mRNA and protein levels. These results suggest that Piwil1 might play a crucial role in gonadal development and gametogenesis in ricefield eels.
Summary
- •
Elucidating the characteristics of gonads at different developmental stages of Monopterus albus based on histomorphology.
- •
Piwil1 expression is significantly higher in the ovary, intersexual gonad, and testis, peaking in stage III of the testis.
- •
PIWIL1 protein is primarily located in the cytoplasm of immature oocytes, surrounding granulosa cells, spermatogonia, and primary spermatocytes.
1. Introduction
The PIWI protein, a member of the Argonaute subfamily first identified in the Drosophila germline, is characterized by the conserved PAZ and PIWI domains [1]. It plays a crucial role in individual development through the PIWI-interacting RNA (piRNA) pathway [2–5]. PIWI/piRNAs are important regulators of gametogenesis, involved in transposon silencing, DNA methylation, transcriptional silencing, and post-transcriptional control [6]. In Drosophila melanogaster, PIWI proteins were initially identified as regulators of germline stem cell division [7]. Piwi mutations abolish the proliferation ability of both female and male germline stem cells [8]. In mammals such as Mus musculus, three piwi homologous genes were identified: Miwi (piwil1), Mili (piwil2), and Miwi2 (piwil4). Miwi and Mili have been demonstrated to perform essential roles in spermatogenesis [9, 10].
In addition, piwi homologous genes have been reported in teleosts. In zebrafish (Danio rerio), two homologous genes of piwi, Ziwi (piwil1) and Zili (piwil2), have been studied. Loss of Ziwi function results in a progressive loss of germ cells due to apoptosis during larval development [11]. Zili is required for germ cell differentiation and meiosis [12]. In medaka (Oryzias latipes), both Olpiwi1and Olpiwi2 are germ cell-specific, and may play important roles in germ cell development and gametogenesis [13]. Opiwi (Olpiwi1) knockdown significantly reduced the number of primordial germ cells (PGCs) both in vivo and in vitro, and it affected the distribution of PGCs in developing embryos [14]. In common carp (Cyprinus carpio), Piwil1 and Piwil2 were cloned and play a crucial role during ovarian differentiation and development [15]. In Nile tilapia (Oreochromis niloticus), Piwil1 and Piwil2 are essential roles in male germline, especially in spermatogenesis [16]. In pufferfish (Takifugu fasciatus), Piwil1 and Piwil2 were related to the differentiation of germ cells [17]. In turbot (Scophthalmus maximus), dark sleeper (Odontobutis potamophila), and Japanese flounder (Paralichthys olivaceus), Piwil regulated by hormones might play a crucial role during gonadal differentiation and development, and gametogenesis [18–20]. In Dabry’s sturgeon (Acipenser dabryanus), both AdPiwil1 and AdPiwil2 mRNAs were restricted exclusively to germ cells of both sexes [21]. These researches indicate that PIWI proteins are essential for the germline stem cell maintenance, gametogenesis and gonadal development in diverse organisms, including teleosts.
Ricefield eel (Monopterus albus), an important aquaculture species in China, is a protogynous hermaphroditic Synbranchiform teleost that commonly changes sex from functional female to functional male after ovulation [22]. Previously, it has been shown that many sex-determination- and differentiation-related genes, such as DEAD-box helicase 4 (vasa) [23], growth differentiation factor 9 (gdf9) [24], forkhead box L2 (foxl2) [25], growth hormone (GH) [26], gonadal soma derived factor (gsdf) [27], gonadotropin-releasing hormone 2 (GnRH2) [28], and murine double minute 2 (mdm2) [29], were related to sex change of ricefield eels. The whole-genome DNA methylation and transcriptomes of ovary and testis from ricefield eel were analyzed in our prior study. Our findings revealed a significant reduction in DNA methylation levels and an increase in expression levels of the Piwil1 gene in testes compared to ovaries [30]. Moreover, the piRNA pathway genes, including Piwil1, evolved faster in the ricefield eel, a kind of protogynous hermaphrodites, than the other teleosts [31]. However, the function of Piwil1 in the sex change of ricefield eel is unclear.
This study first elucidates the characteristics of gonads at different developmental stages of the ricefield eel based on histomorphology. Next, the expression profiles of Piwil1 were investigated by examining relative expression levels in various tissues, and at different stages of embryonic and gonadal development using quantitative real-time PCR (qRT-PCR). A polyclonal antibody for PIWIL1 was then produced to analyze its protein expression and subcellular localization. Finally, western blot analysis confirmed the tissue expression of Piwil1, while fluorescent immunochemistry revealed its subcellular localization. Overall, this study analyzes the spatial–temporal expression and subcellular localization of Piwil1 during gonadal development, providing insights into its role in sex change and establishing a foundation for understanding the gametogenesis of the ricefield eel.
2. Materials and Methods
2.1. Animals and Sampling
Ricefield eels used in this study were purchased from the Wuhan Baishazhou Aquatic Products Wholesale Market. All the treatments involving ricefield eels in this study were reviewed and approved by the Animal Ethics Committee of the Yangtze River Fisheries Research Institute, Chinese Academy of Fisheries Sciences (Wuhan, China). Sampling was conducted in accordance with the guiding principles for the Protection and Utilization of Experimental Animals. Three female ricefield eels were randomly selected for tissue collection. The body length (49.8 ± 2.46 cm) and body weight (95.91 ± 6.91 g) of those adult individuals were measured. The eels were first anesthetized by a 0.05% solution of 3-aminobenzoic acid ethyl ester methanesulfonate-222 (MS-222) (Sigma, USA) before dissection. The following tissue were collected: heart, liver, spleen, kidney, muscle, gonad, intestine, skin, telencephalon, midbrain, and cerebellum. Each tissue sample was collected in triplicate. These tissues were preserved in RNAlater (Biological Industries, Israel) and subsequently stored at −80°C. Additionally, ricefield eels at different developmental stages (unfertilized egg, fertilized egg, gastrula, eye capsule, hatching stage, 3 days post-hatching [dph], 8 dph, 16 dph, 30 dph, ovarian stages II–IV, and intersexual gonad stages [early, metaphase, late], as well as testis stages II–V) were collected and frozen directly at −80°C. Each sample from these stages was collected in triplicate. Meanwhile, a portion of the gonadal tissues was fixed overnight in Bouin’s solution at 4°C and then transferred to 70% ethanol for immunohistochemistry experiments.
2.2. Histological Sections of the Gonads
After fixation with Bouin’s solution, gonadal tissue samples at various developmental stages were dehydrated in a graded series of ethanol (70%, 80%, 90%, 95%, and 100%) and subsequently embedded in paraffin. Sections measuring 4.5 µm were cut and utilized for hematoxylin–eosin (HE) staining and immunofluorescence analysis, respectively.
2.3. Quantitative Real-Time PCR
Total RNA extraction was performed from embryos at various developmental stages and different tissues of the rice field eel using the RNeasy Plus Mini Kit (Qiagen, Düsseldorf, Germany) according to the manufacturer’s instructions. The integrity and concentration of the RNA were assessed using a Nanodrop-2000 spectrophotometer (Thermo Fisher Scientific, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
cDNA synthesis from total RNA was performed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan). Ricefield eels Piwil1 (GenBank number KF663555) and reference gene β-actin (GenBank number AY647143.1) sequences were acquired from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). Primers for each gene were designed by Primer 3 (https://primer3.ut.ee/). Primer sequences for β-actin were 5-GTCGCCCCTGAAGAGCACCCT-3 and 5-TCACACCATCACCGGAGTCCA-3′. Primers for Piwil1 were 5-ACCCGTCGTAACAGCACCGAA-3′ and 5-CTTGACACCGCTGTACTTGTCC-3. qRT-PCR was conducted on a QuantStudio6 Flex real-time PCR system (Thermo Fisher Scientific) with TBPremix Ex Taq TM Ⅱ kit as instructed, using a reaction volume of 20 μL containing 1 µL cDNA sample, 10 µL TB Green Premix Ex Taq II, 0.40 μL Rox Reference Dye II, 0.40 µL of each primer, and 7.80 µL ddH2O. Relative Piwil1 mRNA levels were calculated based on the 2−ΔΔCT method using the PCR R package [32]. Data were presented as means ± SD with three samples in each group. The significant differences between the means were analyzed using the one-way analysis of variance (ANOVA). All statistical differences were considered significant when p < 0.05.
2.4. Western Blot Analysis
The polyclonal antibody specific to Piwil1 of ricefield eel was produced against the peptide of 15 amino acids (KLGERGGRRQDFHDC). The peptide was conjugated to the KLH peptide (GenScript Company), and then the peptide-KLH conjugate was used to immunize the rabbit to get the polyclonal antibody.
Tissues were lysed using RIPA buffer (Beyotime, Shanghai) supplemented with a protease inhibitor cocktail (Roche, Shanghai, China). The extracted proteins were separated using a 12% SDS-PAGE gel and subsequently transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk in phosphorus-buffered saline (PBS, pH 7.4) at room temperature for 1 h. They were then incubated overnight at 4°C with polyclonal antibodies against PIWIL1 or β-Actin (Biosharp, Wuhan), followed by incubation with horseradish peroxidase-labeled secondary antibodies (Biosharp, Wuhan) at room temperature for 1 h. Finally, the signals were visualized by enhanced chemiluminescence (ECL) Western-blotting Analysis Kits (Pierce Co., Rochford, USA).
2.5. Immunofluorescence Analysis
Immunofluorescence analysis was conducted using the following method: paraffin sections of tissues were prepared, dewaxed, and subjected to antigen retrieval using Histo VT One solution (Nacalai Tesque, Kyoto, Japan). After blocking with 5% bovine serum albumin (BSA) at room temperature for 1 h, the samples were incubated overnight at 4°C with PIWIL1 antibody. Following three washes with phosphate-buffered saline with tween 20 (PBST), the samples were incubated for 1 h at room temperature with the fluorescein isothiocyanate (FITC)-conjugated goat antirabbit secondary antiserum (Thermo). Nuclei were stained with 500 μL DAPI staining solution (10 µg/mL) for 40 min in the dark. After washing with PBS again, the sections were sealed with SlowFade Gold antifade reagent (Invitrogen). Images were taken under a fluorescent microscope (BX51-34 FL, Olympus) with a digital camera (DP-73, Olympus).
3. Results
3.1. Histological Analysis of Ricefield Eel Gonads
Ricefield eel is a protogynous hermaphrodite with a natural ability for sex change. The histological characterization of different gonadal stages in the ricefield eel was analyzed. Immature females exhibited ovaries containing oocytes in the previtellogenic stage, which is the initial stage of oocyte growth. In stage Ⅱ ovaries, oocytes of varying sizes were observed, including primary oocytes with a small spherical shape (phase Ⅰ), and larger cortical–alveolar oocytes, which displayed cortical alveoli at the peripheral zone of the ooplasm (phase Ⅱ) (Figure 1A,A’). Vitellogenic oocytes, characterized by eosinophilic yolk granules and oil vesicles accumulated in the cytoplasm, were the primary marker in stage Ⅲ of the ovary (Figure 1B,B’). Subsequently, the nuclear area began to shrink and gradually migrated to the peripheral region. In stage Ⅳ of the ovary, characterized by the presence of yolk and large oocytes, the oocytes reached their maximum sizes (Figure 1C,C’).
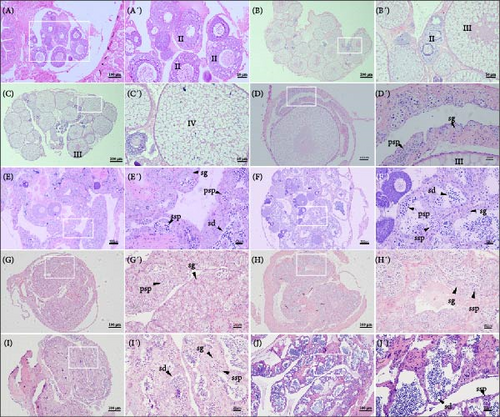
In the gonads of rice field eels at the intersexual stage, testicular lobules and oocytes were observed simultaneously. In the early stage, vitellogenic oocytes, along with spermatogonia and primary spermatocytes, which are characteristic of typical testicular cells, were present (Figure 1D,D’). As the gonads continued to develop, the oocytes gradually degenerated, while the number of primary spermatocytes increased, accompanied by the emergence of secondary spermatocytes and spermatids. This was considered to be the middle period of the intersexual stage (Figure 1E,E’). In the late stage of intersexual development, the gonads were predominantly filled with testicular cells, including spermatogonia, primary spermatocytes, secondary spermatocytes, and spermatids, with very few oocytes remaining (Figure 1F,F’).
The testes primarily consist of testicular lobules and spermatogenic cells. In stage II of testis, cleft cavities were observed within individual seminiferous tubules, and a significant number of spermatogonia and primary spermatocytes were dispersed along the lobule walls (Figure 1G,G’). When testis developed to stage III, secondary spermatocytes began to differentiate (Figure 1H,H’). In the subsequently developed testis at stage IV, secondary spermatocytes and spermatids were the predominant types of differentiated cells, while primary spermatocytes were scarcely observed (Figure 1I,I’). Finally, in testis at stage V, the lobular lumen was filled with a substantial number of mature sperm (Figure 1J,J’).
3.2. Transcription Profile of Piwil1 in Ricefield Eel
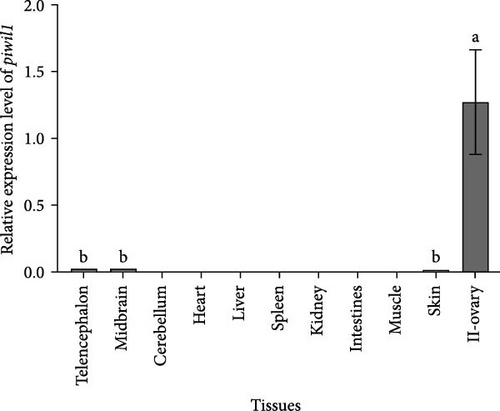
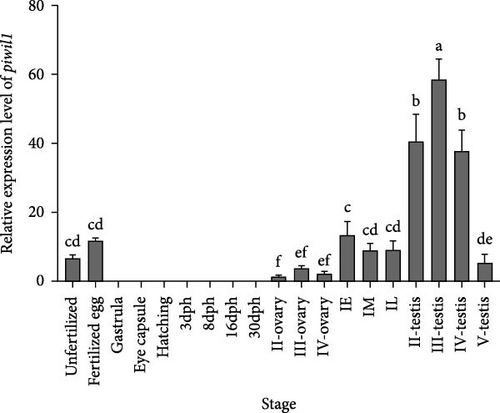
Tissue distribution analysis by qRT-PCR showed that Piwil1 was predominantly expressed in the gonad, followed by the telencephalon, midbrain, and skin. No expression was detected in the cerebellum, heart, liver, spleen, kidney, intestines, and muscle (Figure 2A). The time-dependent expression profiles of Piwil1 were detected during embryonic and gonadal development. In the early embryo developmental stages, Piwil1 transcript signals were only found in unfertilized egg and fertilized egg, whereas no transcription was detected in the following developmental stages of gastrula, eye capsule, and hatching stages (Figure 2B). During the various developmental stages of gonad, Piwil1 transcription levels increased from stage II to stage III of the ovary, and then decreased in stage IV. When the ricefield eel entered the sex change period, Piwil1 transcription levels increased ~4-fold in the early stage of intersexual gonad compared to stage IV of the ovary. In the middle and late stages of intersexual gonad, Piwil1 transcription levels showed a slight decrease but remained higher than all stages of the ovary. The expression level of Piwil1 significantly increased during testis development, peaking in stage III, followed by stages II and IV, before sharply decreasing in stage V (Figure 2B).
3.3. Western Blot Analysis of the PIWIL1 Protein in Ricefield Eel
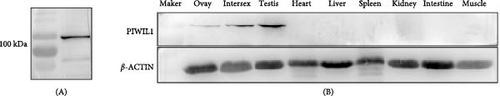
The specificity of the polyclonal antibody against PIWIL1 (~100 kDa) was confirmed by western blot analysis (Figure 3A). The tissue expression pattern of PIWIL1 was further detected with the specific polyclonal antibody. As shown in Figure 3B, PIWIL1 protein was mainly expressed in the ovary, intersexual gland, and testis; however, the expression of PIWIL1 protein was undetected in other tissues, including the heart, liver, spleen, kidney, intestine, and muscle.
3.4. Subcellular Localization of PIWIL1 in Gonads
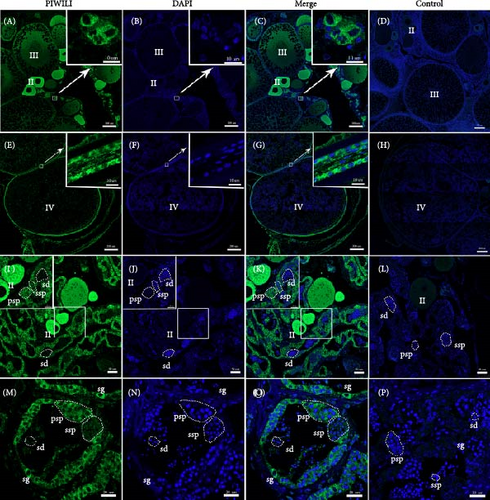
The subcellular localization of PIWIL1 at different stages of gonadal development was analyzed using immunofluorescent (Figure 4). The results indicated that PIWIL1 was primarily localized in germ cells. In the ovary, PIWIL1 protein was concentrated in the cytoplasm of oocytes at stages II and III (Figure 4A–C). At stage IV of oocytes, the expression signal of PIWIL1 protein decreased and shifted to the surrounding granulosa cells (Figure 4E–G). In the intersexual gonads, strong green fluorescent signals were observed in the cytoplasm of oocytes, spermatogonia, and primary spermatocytes, together with weaker signals present in secondary spermatocytes (Figure 4I–K). In the testis, PIWIL1 protein expression was detected in the cytoplasm of spermatogonia and primary spermatocytes, but it weakened in the cytoplasm of secondary spermatocytes and was not detected in spermatozoa (Figure 4M–O). No positive signals were observed in the negative control (Figure 4D,H,L, and P).
4. Discussion
The ricefield eel, a protogynous hermaphrodite teleost, is considered as a model system for vertebrate sexual development. Many studies have been aimed to identify factors contributing to the sex-changing phenomenon of ricefield eels [33, 34]. To date, a series of studies have demonstrated that PIWIs are essential in gene silencing and transposon regulation during germ differentiation and gonadal development in animals [35–39]. The Piwil1 gene has rapidly evolved in the protogynous hermaphrodite ricefield eel compared to other teleosts [31]. Moreover, our previous study indicated that there is a significant reduction in DNA methylation levels and an increase in the expression levels of the Piwil1 gene in testes compared to those in ovaries [30]. These findings suggest that Piwil1 is likely involved in the gonadal development of the ricefield eel.
To further understand the function of Piwil1 during gonadal development in the ricefield eel, we analyzed the expression pattern of Piwil1. In this study, the gonadal sexual stages of collected gonadal tissues from individual ricefield eels were carefully verified by histological analysis, which are classified as ovary (stage I to IV), intersexual (early stage, metaphase stage, and late stage), and testis (stages II to V) (Figure 1). Our findings from qRT-PCR (Figure 2) and western blot analysis (Figure 3) showed that Piwil1 were predominantly expressed in the gonads of the ricefield eel. We also noticed that Piwil1 have only a slight expression in some somatic tissues, such as telencephalon, midbrain and skin (Figure 2A), which is similar to tilapia [16], dark sleeper [19], pufferfish [17], Japanese flounder [20], and Dabry’s sturgeon [21]. Somewhat different from the Piwil1 homologs genes found in zebrafish [11], medaka [13], common carp [15], tilapia [16], and turbot [18], which tissue-specific expressions were present in testis and ovary whereas almost no expression was detected in other tissues. Thus, the result of tissue expression in our study indicates that Piwil1 has an important role in gonadal tissues, but it is also likely involved in other biological processes within somatic tissues.
Piwil1 is abundantly expressed in the gonads, its overall expression trend increased gradually during the sexual change process. However, the difference in relative expression of Piwil1 between the testes and ovaries is striking. Consistent with previous studies on tilapia [16], pufferfish [17], Japanese flounder [20], and Dabry’s sturgeon [21], higher expression of Piwil1 was detected in testis than in the ovary (Figure 2B), indicating that it is essential for spermatogenesis in the male germline. In contrast, the dark sleeper Piwil1 [19] expressed higher in the ovaries than in the testes. Moreover, the mRNA expression of Piwil1 in the ricefield eel was low in fully mature testes and ovaries, which is similar to findings in crucian carp [15], tilapia [16], and pufferfish [17]. Piwil1 transcription levels rose from stage II to stage III of the ovary, followed by a decrease in stage IV. Piwil1 in the intersexual gonad and testis showed similar mRNA expression pattern across developmental stages, initially increasing and then decreasing with the process of gonad development (Figure 2B). The transcription profile characteristics of Piwil1 in the gonads imply that Piwil1 is essential for germ cells differentiation and gonadal development in the ricefield eel. Previous studies revealed that the medaka Opiwi RNA is maternally inherited and expressed in embryonic and adult germ cells and central nervous system (CNS). Opiwi knockdown affects early embryonic development [14]. In our study, during the early developmental stages of the ricefield eel embryo, Piwil1 transcript signals were present only in unfertilized and fertilized eggs. No transcription was detected in subsequent developmental stages, including gastrula, eye capsule, and hatching (Figure 2B). The finding suggests that Piwil1 may play a crucial role during the early embryogenesis in the ricefield eel.
Due to the role of Piwil1 in gonadal development, its subcellular distribution was investigated in the ricefield eel. In this study, immunofluorescence analysis revealed that the PIWIL1 protein was primarily localized in germ cells. Furthermore, the PIWIL1 protein was abundantly localized in the cytoplasm of immature oocytes, granulosa cells surrounding the oocytes, spermatogonia, and primary spermatocytes (Figure 4). These results are similar to findings in zebrafish [11], medaka [13, 14], dark sleeper [19], and Dabry’s sturgeon [21], where Piwils are expressed in the cytoplasm of oocytes, spermatogonia, and spermatocytes, as well as in some granules. Thus, the combined findings of mRNA expression, protein expression, and subcellular localization strongly indicate that Piwil1 may play an important role in the differentiation of germ cells during gonadal development. Furthermore, for the PIWIL1 protein in ricefield eels to fulfill its role, its localization in the cytoplasm is essential.
In summary, the results presented here provide some preliminary yet essential insights into the expression patterns and subcellular localization of Piwil1 in the ricefield eel gonads. The findings indicate that Piwi11 is predominantly distributed in the ovary, intersexual gonad, and testis. Furthermore, Piwil1 expressed throughout the various development stages of the gonad, with the highest expression level was observed in stage Ⅲ of the testis. In contrast, the expression level of Piwil1 is low during embryonic development after fertilization. PIWIL1 protein was dominantly localized in the cytoplasm of immature oocytes and granulosa cells surrounding the oocytes, as well as in the cytoplasm of spermatogonia and primary spermatocytes. Notably, in both the ovary and testis, the expression of Piwil1 initially increased but subsequently decreased at mRNA and protein levels, indicating that Piwil1 is highly enriched during the early stages of germ cell development. These findings might enrich the foundational knowledge of PIWI proteins in the fish gonadal development.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jing Zhang and Yongzhi Li equally contributed to this work.
Funding
This work was supported by the Central Public-Interest Scientific Institution Basal Research Fund, CAFS [grant number YFI20240503, YFI202420, 2023TD23] and the Key Research Project of Chongqing Fishery Technology Innovation Union [grant number CQFTIU2024-08].
Open Research
Data Availability Statement
Data will be made available on request.




