Effects of Diurnal Oxygen Variation on Survival, Growth, and Stress Gene Expression in Pacific White Shrimp Litopenaeus vannamei
Abstract
Oxygen is typically one of the first limiting water quality parameters affecting the culture of aquatic animals. However, there is a limited understanding of the effects of short-term low dissolved oxygen (DO) in Pacific white shrimp reared in outdoor green-water environments exposed to natural diurnal cycles, especially less than 3 mg L−1 DO, and their impacts in commercial aquaculture settings. The present study evaluated the effects of cyclic short-term low DO conditions in outdoor green water tanks on shrimp survival, growth, nutrient retention, select blood parameters, and gene expression of the gill and hepatopancreas. The experiment was performed in 12 (800 L, 0.8 m2) round tanks stocked at 35 shrimp m−2 (1.99 ± 0.06 g) using three aeration regimens. Four tanks were assigned to each treatment which included: low, medium, and high aeration using 0.25, 0.35, and 0.7 cubic feet per second (cfs) airstones, respectively. Diurnal cycles were allowed to cause varying periodic periods of short-term low DO. We found no difference in shrimp growth, survival, feed utilization, blood parameters, or gene expression of shrimp maintained in systems where DO was maintained near saturation as compared to those experiencing diurnal shifts in DO and nonlethal short-term low DO conditions. Clearly, lethal levels of DO must be avoided but we did not find justification to maintain DO in diurnal systems near saturation throughout the diurnal cycle.
1. Introduction
Dissolved oxygen (DO) affects most chemical reactions in ponds, limits pond carrying capacity, and impacts the physiology of the cultured species. In outdoor aquaculture systems, oxygen availability and nutrient dynamics are primarily determined by phytoplankton density and metabolism of chemoautotrophic and heterotrophic bacteria [1]. Feed input directly stimulates natural productivity which is in turn responsible for photosynthetic production of oxygen during the day as well as oxygen uptake in the water column. This means that short-term low DO conditions become more prevalent as feed inputs increase during the tail end of a grow out cycle. Many aquaculture stakeholders believe that oxygen concentrations must be maintained close to saturation otherwise growth rates will be affected. Such a management protocol may be suitable for recirculating aquaculture systems but in outdoor pond-based systems this is expensive to achieve and maintain.
Aquatic organisms evolved mechanisms that allow them to tolerate periodic short-term low DO conditions (less than 3 mg L−1 DO). Poikilothermic invertebrates, such as mussels and crabs, exposed to natural diurnal oxygen fluctuation modify their metabolic rate to temperature by increasing oxygen uptake in undersaturation oxygen conditions [2]. Litopenaeus vannamei exposed to steady O2 values between 2 and 2.6 mg L−1 for 4 weeks exhibited an increase in hemocyanin, thus improving oxygen carrying capacity [3]. Some invertebrates react to hypoxia by increasing ventilation rate and perfusion of hemolymph in gill lamellae [4, 5]. However, despite physiological mechanisms that allow L. vannamei to tolerate acute hypoxia, Ferreira et al. [6] reported mortalities and a decrease in the growth performance of shrimp challenged with extended low oxygen concentrations. Exposure to hypoxia also increases the production of reactive oxygen species (ROS), which can cause DNA damage in hepatopancreas, hemolymph, and gill tissues, increasing the level of transcription factor HIF-1. HIF-1 is involved in the regulation of genes that trigger antioxidant enzymes, helping shrimp recover from oxidative stress [7, 8]. Furthermore, hypoxia may result in a reduction in feed consumption, which leads to shifts to anaerobic biochemical pathways [9, 10]. Finally, hypoxia was reported to compromise the immune system, increasing susceptibility to pathogens, and increase mortality [11].
Although many authors [11–13] report that low DO concentrations can reduce shrimp survival, growth, and disease resistance, most studies investigated the effects of prolonged hypoxia in controlled environments. In photosynthetic suspended-growth systems, such as ponds and other green water tanks [1], cultured animals experience short-term low DO conditions, typically before sunrise. Araujo et al. [14] reported that in ponds where DO was maintained above 2.0 mg L−1, no negative effects in growth performance and metabolic enzymes were observed. To better understand the potential effects of diurnal hypoxic events in outdoor aquaculture facilities on L. vannamei, we evaluated survival, growth, proximate body composition, and gene expression of shrimp cultured in outdoor green water systems where diurnal nighttime low DO conditions were experienced.
2. Materials and Methods
2.1. Experimental Set up and Growth Assessment
The experiment was performed at Claude Peteet Mariculture Center in Gulf Shores, AL, USA. Shrimp were acquired from Homegrown Shrimp USA hatchery (Indiantown, FL, USA) and reared on commercial feeds for 6 weeks. Water flow was equalized at 0.6 L min−1 per tank using restrictors. A total of 12 polypropylene tanks (0.8 m2 bottom; 800 L) filled with 15 ppt brackish water were used. Each tank was stocked with 30 shrimp (average weight 1.99 ± 0.06 g). Four tanks were assigned to each treatment which included: low-aeration using one 0.25 cubic feet per second (cfs) airstone; medium—using one 0.35 cfs airstone, and high—using two 0.35 cfs airstones (0.7 cfs total). Including medium and high aeration treatments provided us with a more comprehensive overview between treatments that can be more energy-intensive and costly to operate. During the first 2 weeks shrimp were offered a 1.5 mm commercial diet (40% crude protein and 9% crude lipid) and thereafter a 2.4 mm commercial diet (35% crude protein and 8% crude lipid) both produced by Zeigler, Inc. (Gardners, PA, USA). The experiment was terminated 42 days after stocking. Daily feed ration was calculated as per Garzade Yta et al. [15]; that is we expected growth to be 1.3 g week−1 the first 2 weeks and 2 g week−1 for week 3 and 4, then 2.5 g week−1 thereafter, and assumed a feed conversion ratio (FCR) of 1.2. The daily ration was divided into four feeding events at approximately 7:00, 11:00, 15:00, and 19:00 h. At the termination of the trial, four shrimp per tank were randomly collected for whole-body proximate analysis which was later used to calculate protein and phosphorus retention (PR) values. Whole body shrimp samples were sent to Midwest Laboratories Inc. (Omaha, NE, USA) for analysis. Four additional shrimp were sampled and the hepatopancreas, gills (fourth gill filament from one side), and distal gut samples were collected and preserved in DNA/RNA shield (ZYMO Research, Irvine, CA, USA) for gene expression analysis.
2.2. Water Quality
2.3. Hemolymph Analysis
At the end of the experiment, hemolymph was extracted from beneath the carapace at the cephalothorax-abdominal junction of three shrimp per tank using a 25 gauge needle and 1 cc syringe as described by Roy et al. [19]. The hemolymph was collected into a syringe preloaded with 0.4 mL anticoagulant and samples from three shrimp per tank were pooled together. The anticoagulant solution was prepared to contain 0.34 M sodium chloride (NaCl); 10 mM EDTA—ethylenediaminetetraacetic acid (Sigma, E9884); 30 mM sodium citrate tribasic dihydrate (Sigma S4641) in deionized (DI) water. The dilution factor of the collected hemolymph by the anticoagulant was calculated for each sample. Hemolymph samples were centrifuged (Fisher Scientific: Marathon 16 km, USA) at 1000 × g for 15 min and hematological parameters were obtained by analyzing 100 µL of hemolymph using Comprehensive Diagnostic Profile rotor (Zoetis, Union City, CA, USA) and VetScan VS2 analyzer (Abaxis, Inc. Union City, CA, USA).
2.4. Gene Expression
Samples from the gut and gill filaments of 48 shrimp were collected for gene expression analysis via bulk RNA sequencing (RNAseq). This included 8 individuals sampled from each DO condition (n = 24; low, medium, and high) from both the gill and gut tissues. The samples were collected aseptically and immediately submerged in approximately 10 volumes of RNAlater solution (Ambion, Austin, TX, USA) to stabilize and protect the RNA. Samples were then stored at −80°C until use. Total RNA was extracted from the gut and gill filament samples using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. Total RNA samples were assessed for quality and quantity using the 4200 TapeStation System with the RNA ScreenTape assay (Agilent Technologies, Santa Clara, CA, USA) and a BioTek Cytation 1 Plate Reader with the BioTek Take3 Microvolume Plate (Agilent Technologies, Santa Clara, CA). Total RNA samples were then sent to a service provider (SeqMatic, Fremont, CA, USA) for RNA sequencing on an Illumina NovaSeq X Instrument (San Diego, CA, USA) in a 2 × 150 bp paired-end configuration with a target sequencing depth of 25 M paired-end reads/sample. Sequencing libraries were made with the Illumina Stranded mRNA Ligation Prep Kit (San Diego, CA, USA) according to the manufacturer’s protocol.
2.4.1. Bioinformatics
Raw reads in FASTQ format were received from the service provider and assessed for quality using Trimmomatic v0.39 [20] and FastQC v0.11.8 [21] within the bioinformatics software OmicsBox v3.3.2 [22]. Using the default settings, reads were trimmed of Illumina adaptors and sequences <Q30 quality score, and minimum length of 36 bp. High-quality reads were then mapped to the Pacific white shrimp (L. vannamei) NCBI RefSeq genome assembly (Accession #GCF_003789085.1) using the STAR aligner v2.7.8a [23], with two-pass mapping configuration to produce BAM alignment files. BAM files were assessed for quality using RSeQC v5.0.1) [24, 25]. Mapped reads were then extracted from the resultant BAM files and quantified using HTSeq-Count v2.0.5 [26], with the following configuration: Strand specificity = strand-specific reverse; quantification level = gene; group by = parent; overlap mode = union. Pairwise differential expression analysis was then performed using the R program edgeR v4.0.16 [27]. First, genes with low counts were filtered via the “filterByExpr” function. Samples were then normalized using the Trimmed Mean of M values (TMM) method. The reference condition (baseline) was set to the “high DO” samples, and these were compared to both the “low DO” and “medium DO” samples (contrast conditions) using the Quasi Likelihood F-test with the additional parameter of robust = TRUE. Genes were considered significantly differentially expressed with a fold-change > ± 2 and a p − value < 0.05 after adjusting for multiple testing.
2.4.2. Validation of RNAseq Using Quantitative PCR
Validation of RNAseq data using reverse-transcription quantitative PCR (RT-qPCR) was performed similar as before [28]. Briefly, an aliquot of each total RNA sample used for RNAseq was normalized to 200 ng μL−1 with nuclease-free water and then cDNA was synthesized using the NEB LunaScript RT SuperMix Kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s protocol. Resulting cDNAs were diluted to 2 ng μL−1 with nuclease-free water and the NEB Luna Universal qPCR Master Mix kit (Ipswich, MA, USA) was used for RT-qPCR according to manufacturer instructions. Technical replicates were performed in triplicate. The RT-qPCR assay was performed using the Roche LightCycler 96 (Roche Diagnostics, Indianapolis, IN, USA) and analyzed with the included software that collected cycle threshold (Ct) values. Relative quantification was calculated by normalizing the geometric average of the reference genes to the housekeeping gene using the 2−ΔΔCT method [29, 30]. Primer sets used in the analyses, designed from both previous study, [31] and newly created, are listed in Table 1.
2.5. Statistical Analysis
Results were presented as mean ± standard error (SE). All statistical analyses were performed using SAS (V9.4, SAS Institute, Cary, NC, USA) and R 4.0 software for windows (Iowa State University, Ames, IA, USA). Growth performance, hemolymph parameters, and water quality data measurements were analyzed using one way analysis of variance (ANOVA). A significant level of 0.05 was used to determine statistical difference among treatments. Goodness-of-fit was determined using pooled standard error (PSE). One replicate of the medium aeration treatment was eliminated from the analysis because of miscounting during stocking. Survival values were analyzed in their natural form as well as arcsin transformation prior to analysis.
3. Results
3.1. Water Quality Parameters
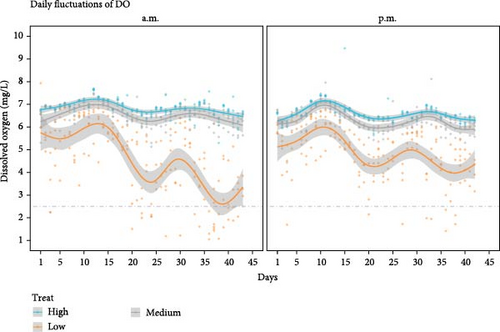
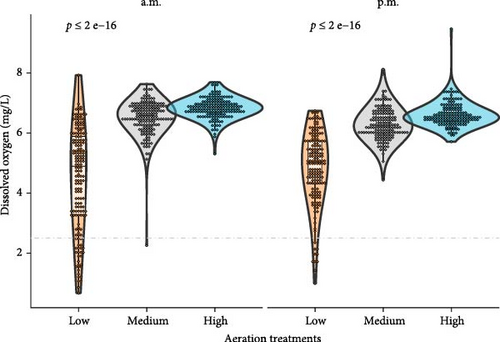
Water quality parameters were maintained within the typical range for shrimp production [34] (Table 2). Diurnal DO fluctuations as a function of AM and PM readings across the culture period are presented in Figure 1. In general, DO points for both a.m. and p.m. readings were above 6 mg L−1 in the high and medium aeration treatment. Therefore, these treatments did not experience hypoxia conditions. The occurrence and distribution of DO values are presented in Figure 2; the violin plot displayed a wider section between 6 and 7 mg L−1 for the high and medium aeration treatment during morning and afternoon. In the low aeration treatment, the density of low D.O events below <2.5 mg L−1 increased, especially before sunrise recordings (a.m.); these data points represent 14% of the total occurrence. For afternoon recordings, datapoints below 2.5 mg L−1 represent just 5% of the occurrence.
| Parameters | Values |
|---|---|
| Temperature (°C) | 29.10 ± 2.50 |
| Salinity (g L−1) | 14.81 ± 0.33 |
| Total ammonia nitrogena (mg L−1) | 0.13 ± 0.11 |
| Nitrite nitrogen (mg L−1) | 0.53 ± 0.37 |
| pH | 8.65 ± 0.31 |
| Alkalinity (mg L−1) | 109.33 ± 28.75 |
| Calcium (mg L−1) | 182.22 ± 28.14 |
| Magnesium (mg L−1) | 462.33 ± 30.47 |
| Phosphate (mg L−1) | 3.87 ± 0.34 |
- Note: Values are presented as mean ± standard deviation.
- aAverage ammonia nitrogen readings from ion electrode and WaterLink SpinTouch.
In the low aeration treatment of the experiment, DO concentrations below 1.5 mg L−1 occurred at the beginning of week 3, and the lowest DO was recorded on day 36 (0.67 mg L−1) at 5:00 a.m.. In the high and medium aeration treatment, DO was greater than 4.3 mg L−1 for the entire culture period. DO was significantly lower (p < 0.001) in the low aeration treatment both in the morning and evening (Table 3). Salinity fluctuated between 14.3 and 15.5 ppt. The maximum temperature recorded was 30.6°C and the minimum 24.3°C, pH below 8.0 were recorded during darkness hour before sunrise, average pH was 8.65. Total ammonia nitrogen mean was 0.13 mg L−1 and nitrite nitrogen was 0.53 mg L−1. Ammonia-nitrogen and nitrite-nitrogen remained within safe levels to avoid growth depression according to [35, 36]. The FOD was not significantly different amongst aeration treatments; FOD ranged from 0.88 to 0.90 kg O2 Kg−1 of feed.
| Aeration | Mean ± std | Min, max | Mean ± std | Min, max |
|---|---|---|---|---|
| Treatments | a.m. | a.m. | p.m. | p.m. |
| 1Low | 4.80 ± 1.71a | (0.67,7.93) | 5.20 ± 1.35a | (0.99, 6.74) |
| 2Medium | 6.60 ± 0.59b | (2.25,7.63) | 6.41 ± 0.59b | (4.43, 8.12) |
| 3High | 6.91 ± 0.37b | (5.3,7.69) | 6.66 ± 1.35c | (5.71, 9.47) |
| 4PSE | 0.612 | — | 0.444 | — |
| p-Value | <0.001 | — | <0.001 | — |
- Note: One-way ANOVA was run by aeration treatment. A significant level of 0.05 was used to determine statistical difference among treatments. Values with different superscripts within the same column are significantly different based on Tukey Pairwise Comparisons.
- 1Low = 0.25 cubic feet per second.
- 2Medium = 0.35 cubic feet per second.
- 3High = 0.7 cubic feet per second.
- 4PSE = Pool standard error.
3.2. Survival, Growth Performance and Proximate Composition
Shrimp reared in the high aeration treatment had significantly (p = 0.03) greater survival than the low aeration treatment, 100% versus 93%, respectively (Table 4). There was no significant difference in FW (g), FCR, final biomass (g), and weight gain (%) among treatments in the trial. Weekly weight gain was between 2.54 and 2.72 g week−1 with no difference in growth rates. No significant differences were found in crude protein (%), crude fat (%), ash, or mineral contents of the shrimp (Table 5).
| Treatments | Final biomass (g) | Final weight (g) |
Weekly gain (g) |
Weight gain (%) | 1TGC | 2FCR | Survival (%) | 3ANPR (%) | Phosphorus retention (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| High | 457.55 | 15.25 | 2.54 | 767 | 3.70 | 1.04 | 100a | 47.42 | 24.18 | |
| Medium | 473.33 | 15.96 | 2.66 | 798 | 3.87 | 0.99 | 99a,b | 50.69 | 24.53 | |
| Low | 455.55 | 16.29 | 2.72 | 813 | 3.94 | 1.03 | 93b | 49.60 | 22.98 | |
| 4PSE | 7.20 | 0.26 | 0.04 | 17.03 | 0.07 | 0.01 | 1.59 | 1.64 | 0.94 | |
| 5p-Value | 0.253 | 0.051 | 0.052 | 0.190 | 0.092 | 0.271 | 0.033 | 0.327 | 0.50 | |
- Note: One-way ANOVA, followed by Tukey’s multiple comparison tests to evaluate significant differences between treatment means. Superscript letters denote a significant statistical difference between treatments.
- 1TGC = Thermal-unit growth coefficient.
- 2FCR = Feed conversion ratio = feed offered/(final weight − initial weight).
- 3ANPR = Apparent net protein retention.
- 4PSE = Pooled standard error.
- 5One-way ANOVA.
| Treatments | Crude protein (%) | Crude fat (%) | Ash (%) | Phosphorus (%) | Copper (ppm) | Zinc (ppm) | |
|---|---|---|---|---|---|---|---|
| 1Low | 77.63 | 8.33 | 11.53 | 1.25 | 185.00 | 69.28 | |
| 2Medium | 76.78 | 7.08 | 12.43 | 1.33 | 180.25 | 72.45 | |
| 3High | 77.60 | 7.13 | 11.98 | 1.33 | 185.75 | 72.18 | |
| 4PSE | 0.60 | 0.67 | 0.25 | 0.03 | 7.87 | 1.18 | |
| 5p-Value | 0.55 | 0.38 | 0.06 | 0.12 | 1.12 | 0.14 | |
- Note: Three different levels of aeration supply (cfs). Values represent the means of four replicates.
- 1Low = 0.25 cubic feet per second.
- 2Medium = 0.35 cubic feet per second.
- 3High = 0.7 cubic feet per second.
- 4PSE = Pool standard error.
- 5One-way ANOVA.
3.3. Hemolymph Biochemical Parameters and Gene Expression
Hemolymph alanine aminotransferase (ALT) ranged from 159.92 to 189.25 U L−1 and alkaline phosphatase (ALP) ranged from 33.67 to 39.23 U L−1 (Table 6). Although, there were no significant differences in hematological parameters among treatments (p = 0.680), the data suggests an increase in hemolymph ALP in shrimp from the low aeration treatment.
| Hemolymph parameters | Low | Medium | Higha | PSEb | p-Valuec |
|---|---|---|---|---|---|
| Alkaline phosphatase (U L−1) | 39.23 | 29.95 | 33.67 | 7.36 | 0.680 |
| Alanine aminotransferase (U L−1) | 171.08 | 159.92 | 189.25 | 15.3 | 0.426 |
| Amylase (U L−1) | 400.08 | 490.16 | 394.34 | 41.0 | 0.233 |
| Total calcium (mg dL−1) | 25.24 | 24.27 | 26.05 | 2.22 | 0.853 |
| Phosphorus (mg dL−1) | 2.16 | 2.53 | 2.55 | 0.155 | 0.192 |
| Glucose (mg dL−1) | 42.86 | 37.67 | 49.05 | 7.05 | 0.543 |
| Potassium (m mol L−1) | 12.44 | 12.50 | 12.91 | 0.375 | 0.651 |
| Total protein (g dL−1) | 14.40 | 13.50 | 13.35 | 0.731 | 0.567 |
- aMean of four replicated groups (n = 4) consisting of three pooled samples of hemolymph.
- bPooled standard error.
- cOne-way ANOVA.
3.4. Differential Gene Expression via RNA Sequencing
In total, 48 samples were analyzed via RNAseq, including 24 from gut tissue and 24 from gill tissue, with six biological replicates at each of the three treatment groups (low, medium, and high DO) at the selected time-point. After QC, sequencing data from the gut tissues included libraries ranging from 27.0 to 44.6 M PE-reads analyzed. Sequencing data from the gill tissues ranged from 25.4 to 42.4 M PE-reads analyzed. Alignment rates of uniquely mapped reads to the shrimp genome were approximately 74.2% and 76.0% among gut and gill samples, respectively. RNA integrity at the transcript level was evaluated by examination of TIN (transcript integrity number) scores, which were consistent with no apparent technical outliers, ranging from a mean 34.7–40.4 in the gut samples and mean 34.2–41.6 in the gill samples.
Results of the gene expression analysis of the gut and gill filament tissues of Pacific white shrimp exposed to lowering DO conditions, identified only the hydroxyacid oxidase 1 (HAO1) gene (GenBank Accession #XM_027377402.1) as a single significant (P − adj < 0.05; fold-change ± 2) differentially expressed gene in the medium DO gut samples as compared to the control (high DO gut samples) group. HAO1 gene expression was found to be 7.58 fold-change (P − adj = 0.04)upregulated. No other significant DEGs were identified among all other pairwise comparisons, including none among gill tissues and none in the low DO gut comparison. The RT-qPCR result (fold-change 6.22; p − value < 0.05) validated this HAO1 gene expression identified through DNAseq. The raw RNA sequencing data along with processed counts information have been made available to the public at the NCBI Gene Expression Omnibus, accessible under accession #GSE281217.
4. Discussion
The investment in aeration capacity and energy to run aeration are significant costs to a farmer. There are various types of aerators with various aeration efficiencies and cost [37], and these should be selected based on the size of the pond, maximum biomass of shrimp, and feed inputs. Clearly maintaining DO above lethal levels is a necessary and primary consideration in all types of production. However, the effects of intermittent low DO levels as seen in diurnal system are not well understood and often viewed as a detriment to production. Results of the present trial suggest that short periods of diurnally low oxygen do not affect the growth rate, FW or final biomass of Pacific white shrimp reared in greenwater environments. However, we observed a significant reduction in survival in the low aeration treatment. We believe the mortality happened at week 5, close to the end of the experimental period when recorded morning oxygen concentrations were 0.67 mg L−1. This lethal D.O has been reported to decrease survival by 10% when P. monodon was exposed to 0.6 mg L−1 for 8 h of stress, followed by exposure to >12 h of normoxia during 21 days in clear water conditions, however they reported no mortality in 4 h of stress to 0.6 mg L−1 [38]. These results emphasize the ability of crustaceans to cope with cyclic hypoxia events until certain threshold.
It is clear that morning DO levels decreased as the production cycle progressed. This is likely a result of the increased amount of feed added daily to the tanks without a concomitant increase in aeration. It is important to point out that we did not have continuous recording of oxygen concentrations, and thus it is possible that DO levels might have attained concentrations lower than those observed but still not low enough to be lethal. Shrimps in estuaries are exposed to naturally intermittent periods of hypoxia, and have hence evolved behavioral and physiological mechanisms to deal with such situations [4]. In nature it is possible for shrimp to swim away from hypoxic conditions, but such an option is not available in ponds and thus we believe that physiological coping mechanisms are more important that behavioral mechanisms in pond settings. However, canal coping strategies seem to cease to be effective when shrimp are exposed to prolonged acute hypoxia [5, 11].
Aeration was enough to maintain predominantly adequate DO concentrations even in the low-level treatment. Although we observed low DO events in the low aeration treatment, these events were not prolonged enough to trigger high mortality events. Furthermore, the reduction in survival in the low-aeration treatment was not great enough to reduce population density to the point of influencing overall biomass, which would impact the value of the total harvest. McGraw et al. [39] reported that in earthen ponds, that shrimp L. vannamei and L. stylirostris co-stocked at 33 shrimp m−2 survival was significantly lower in the minimal threshold of aeration activation of 1.1 mg L−1 compared to minimal threshold of 2.8 and 4.6 mg L−1. However, shrimp growth was not affected across aeration treatments. Their pond experiment differs from our study because mortality affected biomass production, probably because aeration was inadequate to maintain specific threshold concentrations, exposing shrimp to prolonged sublethal DO. A more recent study conducted by Araujo et al. [14] in the same earthen ponds as used by McGraw et al. [39] evaluated automatic aeration setpoint of 2.5, 3.5, and 4.5 mg L−1. L. vannamei was stocked at 25 shrimp m−2 and reared using automated feeding system as well as DO control. Their results showed no significant differences in the FW (33.3 and 33.6 g) or average final yields (7500–8500 kg ha−1). However, electrical costs differed significantly among treatments, with higher DO setpoints treatments exhibiting a higher aeration cost with no discernible benefit. Similar observations were reported by Coiro et al. [40], where larvae marsh grass shrimp (Paleomon vulgaris) exposed to intermittent or constant low hypoxia had similar growth compared to shrimp maintained in DO saturated water. Moreover, they reported better growth in cyclic low DO exposure than a constant low DO treatment. These results also seem to hold true in estuarine finfish. The teleost (Fundulus grandis) exposed to an artificial daily hypoxic period for 5.0 h per day for 30 days displayed no adverse effects in growth compared to fish in oxygen-saturated water, although reproductive capacity was reduced [41].
Glucose content is considered the primary energy source for cells. In crustaceans, DO variation can shift respiration metabolism, changing glucose content to fulfill energy demands. In the current experiment, glucose levels in the shrimp hemolymph exposed to short-term low DO were not significantly different between treatment groups. This may indicate that the shrimp recovered without an impact on glucose levels after short-term low DO exposure. The re-establishment of DO during sunlight hours might allow the glucose level in shrimp hemolymph to return to glucose homeostasis after low DO periods. Cruz-Moreno et al. [42] reported no difference in glucose content in the muscle of P. vannamei under normoxia, hypoxia, and reoxygenation.
Abnormal enzyme levels of ALP and ALT are considered indicators of hepatopancreatic disorders caused by environmental stressors [43–45]. In Penaeus species, ALP are involved in digestion and detoxification process [46]. ALT can play an important role in amino acid metabolism to regulate osmotic pressure when salinity changes [47]. However, our experiment did not experience great variations in other environmental factors besides DO concentration. Hence, ALT and ALP did not display greater activity associated with cyclic short-term low DO events. The lack of significant differences in the remaining hemolymph parameters may also suggest that intermittent short-term low DO did not cause observable stress in shrimp reared in the low aeration treatment to produce metabolic disorders.
In the present study we evaluated the effects of cyclic short-term low DO conditions in outdoor green water tanks on Pacific white shrimp and gene expression. Samples from the gut and gills filaments of 48 shrimp were analyzed using RNA sequencing. After sequencing to a read-depth of >25 M paired-end (2 × 150 bp) reads on an Illumina sequencer, each sample underwent analysis for quality control, alignment of reads to the Pacific white shrimp reference genome assembly, counts data extracted, and pairwise differential gene expression analyzed, whereby low and medium aeration levels were compared to the control (high). While RNAseq is typically robust for analyzing global gene expression changes between treatment groups, we only identified a single gene as differentially expressed. We do not believe that this is due to technical limitations. The analyses were robust and included multiple QC measures throughout the workflow along with including a significant number of biological replicates. Moreover, principal component analysis (PCA) and multi-dimensional scaling (MDS) plots revealed no significant separation between treatment and control replicates (data not shown). Thus, the explanation is likely biological. One such possibility is that the “snapshot” we selected (time, treatment, and tissue) does not indicate global gene expression changes and therefore might direct us to look elsewhere. Another possibility is that the DO changes selected here induced only slight alteration in gene expression, for which low expressed genes were not detected, and further examination would be necessary. In any event, the lack of wholesale gene expression changes is highly correlated with the physiological results of this study.
The single significant (P − adj < 0.05; fold-change > ± 2) differentially expressed gene we identified is designated as the HAO1 gene from gut samples. This is an interesting finding in and of itself, as the HAO1 gene, also known as glycolate oxidase 1 (GOX1), is a gene related to the 2-hydroxyacid oxidase enzyme that is part of the oxidoreductase family and plays a role in glyoxylate and dicarboxylate metabolism. In rainbow trout (Oncorhynchus mykiss), HAO1 has been predicted to play a role in the rate of cellular antioxidant generation [48]. The complete functional capacity of hydroxyacid oxidases in shrimp needs to be further explored.
Overall, our analysis suggests that Pacific white shrimp exposed to low DO in a diurnal cycle does not induce global changes in gene expression at these levels. Moreover, the gene expression results further corroborate the findings of this study, indicating that shrimp raised in cyclic short-term low DO conditions exhibited no significant differences in growth compared to those raised under DO conditions maintained near saturation.
Present results provide a more realistic idea of shrimp adaptation to natural oxygen fluctuations in outdoor greenwater aquaculture. In the present study, oxygen concentration was affected mainly by algae biomass, respiration, and inadequate aeration. Unlike earthen ponds, there was no effect of sediment on DO concentrations. Evidence suggests that sediments may change oxygen dynamics, being one of the major oxygen consumer through the cycle [49]. However, even in earthen ponds, cyclic short-terms of low DO was reported not to affect growth and survival [14]. Results of the present trial, as well as other research on DO management in aquaculture systems suggest that periodic daily short-term low DO is not detrimental to adult shrimp survival and growth in semi-intensive conditions. We found no difference in performance, blood parameter, or gene expression of shrimp maintained in system where DO was maintained near saturation as compared to those experiencing diurnal shifts in DO. Clearly, lethal levels of DO must be avoided but we find no need to maintain DO in diurnal systems near saturation. Aquaculturists can save power and money, and reduce emissions without compromising production, by maintaining nighttime DO concentrations at less than saturation but more than lethal concentrations. We suggest that maintaining DO concentrations at or greater than 3 mg L−1 would be beneficial to farmers.
Disclosure
The current manuscript was mistakenly published as a preprint version in the SSRN, but the authors requested the removal immediately until the manuscript is peer-reviewed by the Journal. The file is unavailable on Research Gate. However, the link still appears as [50].
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was funded by the United States Department of Agriculture-Agricultural Research Service (USDA-ARS) through Specific Cooperative Agreement #58-6010-0-007 and supported by the Institute of Food and Agriculture’s Hatch program (Grant ALA016-08027).
Acknowledgments
The authors would like to thank all students, professors, and faculty members from Auburn University and Claude Peteet Mariculture Center who participated in this project. This work was supported by the United States Department of Agriculture—Agricultural Research Service (USDA-ARS) through Specific Cooperative Agreement #58-6010-0-007 and supported by the Institute of Food and Agriculture’s Hatch program (ALA016-08027).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




