Influence of Physical Activity and Nutritional Limitation on Amino Acid, Fatty Acid Metabolism, and Biochemical Responses in Juvenile Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792)
Abstract
This study investigates the synergistic effects of swimming activity and dietary restriction on the metabolic and nutritional characteristics of muscle tissue in juvenile rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). During a 6-week study, four groups of juvenile rainbow trout, each starting with an average weight of 26.54 ± 0.36 g, were analyzed: the first group was allowed to feed freely in static water (SW group), the second experienced a dietary limitation (25% feed restriction) (LF group), the third was required to swim at a speed of one body length per second (SE group), and the fourth group faced a combination of dietary restriction (25% feed restriction) and enforced swimming activity (SELF group). Swimming activity was implemented using a water flow rate of one body length per second (1 BL s−1), ensuring a standardized exercise intensity. Comprehensive analysis revealed significant alterations in biochemical parameters, amino acid composition, and fatty acid profiles in rainbow trout muscle tissue. The results indicate a decrease in histidine levels (p < 0.05) with the combined effect of both swimming and feeding restrictions. Additionally, cysteine and semi-essential amino acids (EAAs) showed a decrease (p < 0.05) solely due to the influence of swimming. As for fatty acid outcomes, linolenic acid exhibited a reduction with the combined impact of both swimming and feeding restrictions (p < 0.05), while margaric acid significantly decreased (p < 0.05) only with the influence of swimming. Crucial shifts in antioxidant defense mechanisms, including glutathione (GSH) and lipid peroxidation (LPO) levels, were identified, highlighting the roles of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) as biochemical parameters. Detailed examination further clarified modifications in glucose-6-phosphate dehydrogenase (G6PD) activities, reactive oxygen species (ROS) levels, and lactate dehydrogenase (LDH) activity, serving as pivotal indicators of oxidative stress and tissue damage. These findings contribute to a holistic understanding of nutritional dynamics within rainbow trout muscle tissue, offering insights crucial for optimizing fish health and productivity in aquaculture.
1. Introduction
Aquaculture practices play a pivotal role in meeting the increasing demand for fish, a critical protein source for human consumption [1]. Among cultivated fish species, rainbow trout (Oncorhynchus mykiss) is notable not only for its economic significance but also as a model organism for studying physiological responses to environmental stimuli [2]. In 2023, the total global production of rainbow trout, including both freshwater and marine cultivation, was approximately 977,600 metric tons. This total combines the freshwater production, predominantly led by Iran and Türkiye, and marine cultivation, mainly driven by Chile and Norway [3]. Rich in omega-3 fatty acids and high-quality proteins, it aligns with the escalating demand for nutritionally dense seafood. Furthermore, the sustainability of rainbow trout farming practices enhances its market desirability, meeting the discerning preferences of environmentally conscious consumers [1, 4].
Optimizing the health and growth of rainbow trout is paramount in the context of aquaculture. Physical exercise and dietary management are two critical factors profoundly influencing the metabolic and nutritional aspects of fish. In recent years, a growing interest has emerged in understanding the synergistic effects of swimming activity and dietary restriction on fish physiology, particularly within aquaculture systems [5–7]. To contribute to this body of knowledge, our study investigates the combined impacts of swimming exercise and feed restriction on biochemical parameters, amino acid composition, and fatty acid profiles in juvenile rainbow trout.
Swimming exercise is recognized as an essential aspect of fish physiology, influencing biochemical parameters and metabolic processes [8]. Similarly, dietary composition plays a crucial role in providing necessary nutrients for growth and overall well-being [9]. Although there are numerous studies on the effects of diet-derived amino and fatty acids in fish, such as those by Ghosi Mobaraki et al. [10] and Roohani et al. [11], research focusing on the combined effects of swimming activity and dietary restrictions remains scarce. The intricate interplay between swimming activity and dietary restriction and its comprehensive effects on the metabolic and nutritional characteristics of rainbow trout remain to be fully elucidated. The present study endeavors to unravel the intricate interplay between environmental perturbations, specifically water flow dynamics, and dietary modulation, aiming to discern their collective impact on the biochemical profile of fish tissues. This gap highlights the novelty and significance of our investigation, seeking to bridge the knowledge gap in understanding how physical exercise and nutritional limitations concurrently affect fish health and metabolism.
Dietary restriction in trout may lead to decreased protein synthesis, potentially causing a deficiency in essential amino acids (EAAs) and negatively impacting growth and development due to reduced protein availability [12]. Similarly, dietary restriction can alter lipid metabolism, with changes in fatty acids affecting energy production and cellular functions, and a deficiency in essential fatty acids, like omega-3, disrupting cell membranes and hormonal balance [13]. Conversely, swimming activity increases trout’s energy demands, possibly enhancing muscle protein synthesis and requiring more specific amino acids, along with boosting fatty acid oxidation to meet energy needs. This study explores the effects of dietary restriction and swimming on trout’s nutrient profiles, crucial for growth, energy metabolism, and health, examining beyond conventional physiological markers to include oxidative stress indicators (such as glutathione (GSH) and lipid peroxidation (LPO)), antioxidants (catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), GR, and GST), and metabolic enzymes (glucose-6-phosphate dehydrogenase (G6PD) and lactate dehydrogenase (LDH)), linking environmental and dietary factors to amino and fatty acid metabolism, and highlighting their roles in enzymatic activities and the synthesis of amino acids and polyunsaturated fatty acids (PUFAs), underscoring their regulatory importance in biochemical parameters.
The ensuing sections of this study will expound upon these intricate relationships, elucidating the integrated role of amino acids, fatty acids, and enzymatic activities in shaping the biochemical milieu of fish tissues. Through this inquiry, the goal is not only to advance the understanding of fundamental physiological processes but also to contribute substantively to the refinement of aquaculture practices through the assimilation of environmental and nutritional considerations. The outcomes of this research are expected to contribute valuable insights to the aquaculture community, offering a nuanced understanding of the interplay between exercise and dietary factors in rainbow trout. By unraveling the complex interactions within the metabolic and nutritional pathways, our study may have practical implications for the optimization of aquaculture practices, ultimately enhancing the health and growth of rainbow trout in a controlled environment.
2. Materials and Methods
2.1. Experimental Guidelines
This study was conducted at Istanbul University (IU) Aquatic Vertebrate Experimental Unit (IUSUCAN), Sakarya, Türkiye, which holds authorization for the production, supply, use, and research on aquatic vertebrate species as specified in the “Regulation on the Welfare and Protection of Aquatic Vertebrates Used for Scientific Purposes” issued by the Turkish Ministry of Agriculture and Forestry, dated 13/02/2020 with the number 10004. The experimental procedures were carried out in accordance with the ethical principles established by the IU Local Ethics Committee for Animal Experiments, as evidenced by the ethical approval certificate dated 06.12.2019 with reference numbers 2019/10 and 2019/19.
2.2. Fish, Rearing Conditions, Experimental Settings, and Sampling
Juvenile rainbow trout (O. mykiss, Walbaum 1792) used in this study were produced at the IU Sapanca Inland Fisheries Production Research and Application Unit, Sakarya, Türkiye. The culture water temperature was maintained at 16°C. Fish were acclimatized to tanks for 1-week prior to beginning the feeding and swimming trials. After the acclimation period, 432 fish with an average body mass of ~27 g were randomly divided into into groups. The fish used in this study were selected from the same broodstock and maintained under identical conditions; no statistically significant differences were detected among the groups (p > 0.05) in terms of weight and length.
Four recirculated aquaculture systems (RASs) consisted of three circular fish tanks with a volume of 450 L were constructed with a total volume of 1700 L. Each system consists of three parallel fish tanks, one balance tank (sump), one external filter (Eheim Professional 3 1200xL, 1700 L h−1, Germany), one chiller (Teco TK2000, Italy), and one aeration motor (blower). Each fish tank is equipped with a separate pump (Eheim Compact On 5000, Germany) to ensure circulation of water within the corresponding balance tank. To generate controlled water flow within the fish tank, the methodology outlined by McKenzie et al. [14] was adopted. The speed of 1 body lengths per second (BL s−1) used in the exercise groups was selected based on its proven effectiveness in supporting salmonid species such as rainbow trout. This speed has been shown in previous research to mimic natural swimming conditions, thereby fostering uniform growth, enhanced physiological welfare, and efficient energy mobilization, and robustness in fish populations [6]. Additionally, swimming at this speed has been demonstrated to improve metabolic efficiency and optimize protein synthesis in muscle tissues, which are critical for assessing both fish health and aquaculture productivity. The choice of this flow rate also aligns with the study’s objectives of examining the synergistic effects of exercise and dietary restrictions on metabolic and nutritional outcomes in rainbow trout. This setup not only ensures standardized conditions across experimental groups but also enables a deeper understanding of the physiological responses induced by exercise. Fish were hand-fed twice daily (9:00 and 16:00 h) to apparent satiety with commercial juvenil trout pellets (Ozpekler Yem, Türkiye) containing 45% crude protein, 20% crude fat, and 9% ash in dry matter. The study aimed to examine the synergistic impact of water flow dynamics and dietary restrictions, with groups organized as outlined below:
Stationary water (SW) group: This group was maintained in static water (without any water flow) at a rate of 0 BL s−1 and received food until satiation, approximately equal to 2% of the fish’s body weight.
Limited feeding (LF) group: This group was kept in static water (0 BL s−1) with a 25% reduction in food intake compared to the SW group.
Swimming exercise (SE) group: This group engaged in swimming exercises under a water current of 1 BL s−1 and was fed until satiation, around 2% of the fish’s body weight.
Swimming exercise and limited feeding (SELF) group: This group participated in swimming exercises under a water current of 1 BL s−1 and experienced a 25% reduction in food intake compared to the SE group.
At the end of the trials, 72 fish from each group (6 fish from each replicate) were randomly selected and anesthetized in clove oil: ethanol mixture (1:10; clove oil concentration 40 mg L−1) according to Tunçelli and Pirhonen [15] for determination of amino and fatty acid compositions. Fish were beheaded, gutted, and filleted. Then, each fillet was deskinned, homogenized with a laboratory homogenizer (Retsch, Grindomix GM200, Germany).
Dissolved oxygen levels were continuously monitored throughout the experiment to maintain optimal metabolic conditions for rainbow trout. The oxygen saturation level was consistently kept above 90%, with dissolved oxygen concentrations ranging from 9.02 to 9.80 mg L−1. These levels were achieved using a combination of aeration and water circulation systems, ensuring consistent and comparable experimental conditions across all groups. Maintaining these oxygen levels was particularly critical for the swimming groups, where enforced swimming exercises were expected to result in higher metabolic activity. The data pertaining to fish growth and water quality have been previously published in a prior study [16].
2.3. Amino Acid Analysis of Rainbow Trout
Amino acid analysis was performed using high-performance liquid chromatography (HPLC) equipment (Shimadzu (Kyoto, Japan)), following the modified method by Erkan et al. [17]. The HPLC system includes a system controller (SCL-10 A), two liquid chromatography pumps (LC-10AT and LC-20AT), a column oven (CTO-10AC), a fluorescence detector unit (RF-10AXL), a liquid–gas extractor (DGU-14 A), a column (silica base banded with C18, dimensions: 150 × 3.9 mm, particle size: 4 μm, EMR HPLC Technologies, Germany), and an autoinjector unit (SIL-10AD). Muscle tissue samples were hydrolyzed in in glass sample containers with tightly sealed lids by adding 6 M HCl solution at 120°C for 24 h. After dilution with ultrapure water, the hydrolysates were filtered through teflon membrane filters with a pore size of 0.22 μm. Amino acids were identified by comparing retention times with reliable/true standards (Sigma–Aldrich Amino Acid Standard 1 mL Product Number: AAS18) and quantified based on weight. The analysis employed the proprietary software Class-VP 6.14 (Shimadzu, Japan). All analyses were performed in triplicate, and the chemicals used were of analytical purity.
2.4. Fatty Acid Analysis of Rainbow Trout
Fatty acid methyl esters (FAMEs) were determined using a Perkin–Elmer Autosystem/Claurus 500 gas chromatography (GC) equipped with an integrated autosampler, a column (BPX70 GC capillary column, 60 m x0.25 mm x0.25 µm, SGE Analytical Science, Australia), and a flame ionization detector (FID) (Awenud Shelton, DE, USA) to analyze the composition of fatty acids. Lipids extracted from homogenized fish fillets according to the AOAC [18] 991.36 method were converted into their respective FAMEs, following the procedure outlined by Ichihara et al. [19]. It has been determined as a percentage (%) by comparing the retention times of FAMEs with a known reference material (Menhaden Fish Oil, 47116 Supelco) and authentic standards (Supelco 37 Component FAME Mixture, 47885-U Supelco).
2.5. Tissue Preparation and Biochemical Parameter Responses of Rainbow Trout
To prepare these samples for biochemical analysis, 10% (weight/volume) tissue homogenates were created using a cold physiologic saline solution. Subsequently, these homogenates were subjected to centrifugation at 10,000 × g for 10 min at + 4°C. The clear supernatants were then collected for further biochemical analysis.
The levels of reduced GSH and LPO were assessed according to the methods outlined by Beutler [20] and Ledwozyw et al. [21], respectively. Additionally, the activities of CAT, SOD, GPx, GR, GST, G6PD, and LDH in all homogenates were measured using the methodologies described by Aebi [22], Paglia and Valentine [23] with modifications by Wendel [24], Beutler [25], Habig and Jakoby [26], Beutler [27], and Wroblewski [28], respectively. Furthermore, reactive oxygen species (ROS) levels in the supernatants were quantified following the procedures outlined by Zhang et al. [29]. Protein content was calculated using the Lowry method as described by Lowry et al. [30].
2.6. Statistical Analyses
This study was structured using a randomized design that included four different treatments and three repetitions for each. To investigate the impact of diet and swimming activity, a two-way analysis of variance (ANOVA) was employed. Prior to analysis, the data underwent tests for normality and variance homogeneity. To assess the effects of feed restriction and swimming on various parameters across the treatment groups, four independent sample t-tests were conducted. Biochemical parameter responses of the gill and muscle tissue of rainbow trout were analyzed using GraphPad Prism Software, version 6.01 (San Diego, USA). Statistical significance was defined as p < 0.05. The data were based on mean ± standard deviation.
3. Results
3.1. Amino Acids Composition of Rainbow Trout
The composition and content of amino acids in muscle tissues of rainbow trout are shown in Table 1. When swimming activity was combined with feed restriction, changes in histidine levels were clearly observed (p < 0.05). Based on the results for cysteine, semi-EAAs, and the EAA/total amino acid (TAA) ratio, it has been statistically shown that rainbow trouts significantly differ when they are subjected to swimming compared to being kept in static water (p < 0.05).
| Amino Acids (µg/g protein) | Groups | Two-way ANOVA p value | |||||
|---|---|---|---|---|---|---|---|
| SW | LE | SE | SELF | Feeding effect | Swimming effect | Interactions | |
| Lysine | 1909.6 ± 27.5 | 2056.3 ± 52.2 | 1936 ± 129.9 | 1859.7 ± 123.2 | 0.54 | 0.16 | 0.07 |
| Methionine | 673.9 ± 14.6 | 656.2 ± 87.9 | 684.3 ± 19.2 | 667.5 ± 8.2 | 0.53 | 0.69 | 0.99 |
| Threonine | 820.3 ± 41.2 | 787 ± 153.6 | 626.3 ± 242.5 | 821.8 ± 14.8 | 0.36 | 0.37 | 0.21 |
| Isoleucine | 990.1 ± 38.7 | 1041.2 ± 54.2 | 1176.8 ± 327.1 | 962.7 ± 2.9 | 0.42 | 0.59 | 0.21 |
| Leucine | 1568.5 ± 74.9 | 1646 ± 75.3 | 1563.9 ± 78.2 | 1539.1 ± 24.9 | 0.52 | 0.19 | 0.22 |
| Phenylalanine | 835.4 ± 36.8 | 892.3 ± 57.8 | 852.7 ± 14.3 | 826.4 ± 23.4 | 0.49 | 0.29 | 0.09 |
| Valine | 1284.2 ± 31.5 | 1290.8 ± 105.6 | 1271.8 ± 37 | 1254.3 ± 7.1 | 0.88 | 0.49 | 0.73 |
| Total essential | 8082 ± 253.2 | 8369.9 ± 484.1 | 8111.7 ± 271.5 | 7931.5 ± 97.8 | 0.77 | 0.29 | 0.23 |
| Histidin | 535.5 ± 7.7 | 606.8 ± 39.2 | 608.2 ± 5.2 | 582.9 ± 17.4 | 0.11 | 0.09 | <0.01 |
| Serine | 745.5 ± 26.8 | 772.3 ± 64.2 | 762.5 ± 66.8 | 782.2 ± 27.6 | 0.44 | 0.65 | 0.91 |
| Arginine | 1275 ± 56.9 | 1298.1 ± 102.1 | 1256.2 ± 73.4 | 1276.5 ± 13.6 | 0.6 | 0.63 | 0.97 |
| Cysteine | 3870.2 ± 1200.8 | 1812.5 ± 1296.8 | 4420.1 ± 1172.4 | 4361.8 ± 483.1 | 0.13 | 0.04 | 0.15 |
| Tyrosine | 866.2 ± 20.5 | 818.1 ± 88.2 | 878.3 ± 2.2 | 868.1 ± 12.6 | 0.3 | 0.27 | 0.49 |
| Semi essential | 7292.4 ± 1122.2 | 5307.8 ± 1486.3 | 7925.3 ± 1036.3 | 7871.5 ± 530.4 | 0.15 | 0.04 | 0.17 |
| Alanine | 3692.4 ± 305.9 | 3805.7 ± 41.8 | 3727.2 ± 20.2 | 3696.4 ± 186.9 | 0.7 | 0.73 | 0.51 |
| Aspartic acid | 1925.4 ± 52.9 | 2055.6 ± 87.7 | 1923.7 ± 157.2 | 1904.9 ± 58.7 | 0.36 | 0.22 | 0.23 |
| Glutamic acid | 2813.6 ± 50.4 | 2971.6 ± 129.3 | 2732.5 ± 246.5 | 2775.4 ± 60.7 | 0.26 | 0.14 | 0.51 |
| Glycine | 984.9 ± 67.1 | 1038.9 ± 95.8 | 1095.3 ± 25 | 1066.6 ± 54.7 | 0.75 | 0.11 | 0.31 |
| Proline | 768 ± 52.8 | 735.2 ± 92.5 | 666.6 ± 151.9 | 768.1 ± 19.2 | 0.54 | 0.54 | 0.25 |
| Nonessential | 10,184.3 ± 273.9 | 10,607 ± 361.3 | 10,145.4 ± 442.6 | 10,211.3 ± 178.3 | 0.23 | 0.29 | 0.38 |
| TAAs | 25961.1 ± 652.9 | 24729.5 ± 2082.8 | 26594.5 ± 694.6 | 26418.1 ± 408.9 | 0.33 | 0.12 | 0.45 |
| EAA/TAA | 0.31 ± 0.02 | 0.34 ± 0.02 | 0.31 ± 0.01 | 0.30 ± 0.01 | 0.23 | 0.03 | 0.1 |
- Note: Values are means ± SD. The bold values indicate statistical significance.
- Abbreviations: ANOVA, analysis of variance; EAA, essential amino acid; LF, limited feeding group; SD, standart deviation; SE, swimming exercise group; SELF, swimming exercise and limited feeding group; SW, stationary water group; TAA, total amino acid.
The results of the two-way ANOVA analysis using SPSS for the measured factors indicate the significance of certain effects. For cysteine (measured in µg/g protein), the interaction effect was not significant (p = 0.15), and the independent effects of feeding and swimming were examined. While the feeding effect was not significant (p = 0.13), the swimming effect was significant (p = 0.04), with mean values of 4390.97 µg/g protein for swimming and 2841.37 µg/g protein for not swimming. For semi-EAAs (measured in µg/g protein), the interaction effect was also not significant (p = 0.17). The feeding effect remained nonsignificant (p = 0.15), but the swimming effect was significant (p = 0.04), with mean values of 7898.43 µg/g protein for swimming and 6300.07 µg/g protein for not swimming. Regarding the EAA/TAA ratio, the interaction effect was not significant (p = 0.10), and while the feeding effect was not significant (p = 0.23), the swimming effect was significant (p = 0.03), showing a mean ratio of 0.30 for swimming and 0.33 for not swimming.
The histidine level was observed to increase under the influence of both swimming activity and feed restriction practices. A significant difference was noted between the SW and SE groups based on the results of the independent sample t-test (p < 0.05). The histidine levels varied depending on experimental conditions, indicating the combined effects of swimming exercise and feed restriction.
3.2. Fatty Acids Composition of Rainbow Trout
The fatty acid composition of muscle tissues from rainbow trout, subjected to feed restriction and swimming activity practices, is provided in Table 2. When examining the effects of feed restriction and swimming on margaric acid values, it is observed that the effect of swimming significantly contributes to the variance (p < 0.05). Additionally, it has been observed that the interaction between feed restriction and swimming activity practices has a statistically significant effect on the levels of C18:3 (n-6) (γ-Linolenic acid) (p < 0.05).
| Fatty acids (%) | Groups | Two-way ANOVA p value | ||||||
|---|---|---|---|---|---|---|---|---|
| SW | LE | SE | SELF | Feeding effects | Swimming effects |
Interactions | ||
| C14:0 (Myristic acid) | 1.71 ± 0.22 | 1.57 ± 0.03 | 1.64 ± 0.07 | 1.79 ± 0.06 | 0.94 | 0.31 | 0.08 | |
| C16:0 (Palmitic acid) | 16.46 ± 0.87 | 16.36 ± 1.38 | 16.15 ± 1.14 | 17.41 ± 0.11 | 0.35 | 0.54 | 0.27 | |
| C17:0 (Margaric acid) | 0.29 ± 0.2 | 0.05 ± 0.07 | 0.03 ± 0.04 | 0.02 ± 0.02 | 0.08 | 0.05 | 0.10 | |
| C18:0 (Stearic acid) | 4.73 ± 1.43 | 5.52 ± 0.25 | 5.88 ± 0.44 | 5.47 ± 0.42 | 0.69 | 0.26 | 0.22 | |
| C20:0 (Arachidic acid) | 0.27 ± 0.07 | 0.27 ± 0.12 | 0.2 ± 0.14 | 0.34 ± 0.08 | 0.29 | 0.99 | 0.27 | |
| C22:0 (Behenic acid) | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.01 | 0.10 | 0.31 | 0.79 | |
| C24:0 (Lignoceric acid) | 0.16 ± 0.12 | 0.15 ± 0.07 | 0.13 ± 0.03 | 0.2 ± 0.07 | 0.53 | 0.79 | 0.37 | |
| SFA | 23.76 ± 2.19 | 24.05 ± 1.77 | 24.18 ± 1.67 | 25.36 ± 0.29 | 0.46 | 0.39 | 0.65 | |
| C14:1 (Myristolic acid) | 0.04 ± 0.02 | 0.07 ± 0.06 | 0.34 ± 0.3 | 0.1 ± 0.07 | 0.28 | 0.11 | 0.17 | |
| C16:1 (Palmitoleic acid) | 3.57 ± 0.45 | 3.23 ± 0.36 | 3.38 ± 0.4 | 3.57 ± 0.14 | 0.72 | 0.73 | 0.23 | |
| C17:1 (Heptadecenoic acid) | 0.15 ± 0.08 | 0.07 ± 0.09 | 0.18 ± 0.15 | 0.13 ± 0.11 | 0.33 | 0.50 | 0.80 | |
| C18:1 (n-9) (Oleic acid) | 30.48 ± 3.27 | 31.61 ± 3.15 | 31.2 ± 4.2 | 28.14 ± 0.2 | 0.60 | 0.46 | 0.28 | |
| C18:1 (n-7) (Vaccenic acid) | 0.8 ± 0.58 | 1.23 ± 0.55 | 0.43 ± 0.33 | 0.51 ± 0.07 | 0.34 | 0.06 | 0.50 | |
| C20:1 (Eicosenoic acid) | 0.19 ± 0.05 | 0.17 ± 0.13 | 0.26 ± 0.01 | 0.25 ± 0.06 | 0.73 | 0.16 | 0.90 | |
| C22:1 (n-9) (Cetoleic acid) | 0.65 ± 0.09 | 0.48 ± 0.22 | 0.54 ± 0.16 | 0.64 ± 0.15 | 0.70 | 0.79 | 0.18 | |
| C24:1 (n-9) (Nervonic acid) | 0.13 ± 0.12 | 0.12 ± 0.03 | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.64 | 0.52 | 1.00 | |
| Mono unsaturated fatty acid (Total) | 36.02 ± 3.25 | 36.97 ± 3.41 | 36.42 ± 3.71 | 33.42 ± 0.31 | 0.57 | 0.39 | 0.29 | |
| C18:2 (n-6) (Linoleic acid) | 22.32 ± 2.14 | 21.28 ± 1.63 | 21.82 ± 1.32 | 23.23 ± 0.15 | 0.84 | 0.43 | 0.19 | |
| C18:3 (n-6) (γ-Linolenic acid) | 2.83 ± 0.19 | 2.62 ± 0.3 | 2.54 ± 0.14 | 2.96 ± 0.11 | 0.38 | 0.87 | 0.02 | |
| C18:3 (n-3) (α-Linolenic acid) | 2.83 ± 0.3 | 2.62 ± 0.04 | 2.98 ± 0.26 | 2.72 ± 0.28 | 0.14 | 0.39 | 0.87 | |
| C20:2 (n-6) (Eicosadienoic acid) | 0.21 ± 0.03 | 0.18 ± 0.03 | 0.22 ± 0.04 | 0.21 ± 0.01 | 0.22 | 0.22 | 0.37 | |
| C20:3 (n-6) (DGLA) | 1.62 ± 0.11 | 1.56 ± 0.08 | 1.65 ± 0.11 | 1.63 ± 0.06 | 0.55 | 0.40 | 0.70 | |
| C20:3 (n-3) (Eicosatrienoic acid) | 0.79 ± 0.07 | 0.75 ± 0.08 | 0.81 ± 0.05 | 0.83 ± 0.02 | 0.78 | 0.19 | 0.44 | |
| C20:4 (n-6) (Arachidonic acid) | 1.02 ± 0.09 | 1.01 ± 0.07 | 1.04 ± 0.06 | 1.06 ± 0.04 | 0.93 | 0.40 | 0.80 | |
| C20:4 (n-3) (Eicosatetraenoik acid) | 0.38 ± 0.04 | 0.34 ± 0.07 | 0.37 ± 0.08 | 0.36 ± 0.05 | 0.45 | 0.81 | 0.71 | |
| C20:5 (n-3) [Eicosapentaenoic acid (EPA)] | 0.98 ± 0.14 | 0.94 ± 0.05 | 0.62 ± 0.49 | 0.71 ± 0.57 | 0.91 | 0.22 | 0.77 | |
| C22:4 (n-6) (Docosatetraenoic acid) | 0.14 ± 0.04 | 0.09 ± 0.05 | 0.09 ± 0.02 | 0.15 ± 0.06 | 0.71 | 0.91 | 0.08 | |
| C22:5 (n-6) (4,7,10,13,16-Docosapentaenoic acid) | 0.35 ± 0.07 | 0.31 ± 0.03 | 0.41 ± 0.06 | 0.32 ± 0.06 | 0.11 | 0.37 | 0.42 | |
| C22:5 (n-3) [Docosapentaenoic acid (DPA)] | 0.47 ± 0.06 | 0.45 ± 0.02 | 0.48 ± 0.03 | 0.48 ± 0.01 | 0.73 | 0.26 | 0.68 | |
| C22:6 (n-3) [Docosahexaenoic acid (DHA)] | 4.72 ± 0.39 | 4.92 ± 0.44 | 4.65 ± 0.14 | 4.83 ± 0.51 | 0.42 | 0.73 | 0.98 | |
| PUFAs | 38.64 ± 3.23 | 37.06 ± 2.72 | 37.66 ± 1.83 | 39.49 ± 1.46 | 0.93 | 0.62 | 0.26 | |
| Non-identified | 1.58 ± 1.29 | 1.93 ± 1.64 | 1.74 ± 0.54 | 1.72 ± 1.36 | 0.83 | 0.98 | 0.81 | |
| EPA + DHA | 5.7 ± 0.5 | 5.85 ± 0.49 | 5.26 ± 0.61 | 5.54 ± 0.99 | 0.59 | 0.37 | 0.88 | |
| Omega 3 (Total) | 10.16 ± 0.64 | 10.01 ± 0.61 | 9.91 ± 0.59 | 9.94 ± 1.32 | 0.91 | 0.75 | 0.86 | |
| Omega 6 (Total) | 28.48 ± 2.59 | 27.05 ± 2.13 | 27.75 ± 1.59 | 29.56 ± 0.26 | 0.86 | 0.43 | 0.17 | |
- Note: Values are means ± SD. The bold values represent statistically significant results.
- Abbreviations: ANOVA, analysis of variance; DGLA, dihomo-γ-linolenic acid; LF, limited feeding group; PUFAs, polyunsaturated fatty acids; SD, standart deviation; SE, swimming exercise group; SELF, swimming exercise and limited feeding group; SFA, saturated fatty acid; SW, stationary water group.
The results of the two-way ANOVA analysis using SPSS for the measured factors indicate the significance of certain effects. For C17 : 0 (margaric acid, measured as fatty acid %), the interaction effect was not significant (p = 0.10), and the independent effects of feeding and swimming were examined. While the feeding effect was not significant (p = 0.08), the swimming effect was significant (p = 0.05), with mean values of 0.028% for swimming and 0.171% for not swimming.
The linolenic acid level was significantly influenced by the interaction of swimming activity and feed restriction practices. Both factors were observed to elevate the linolenic acid level, with a significant variance identified between the SE and SELF groups based on the results of the independent sample t-test (p < 0.05).
3.3. Biochemical Parameter Responses of Gill and Muscle Tissues of Rainbow Trout
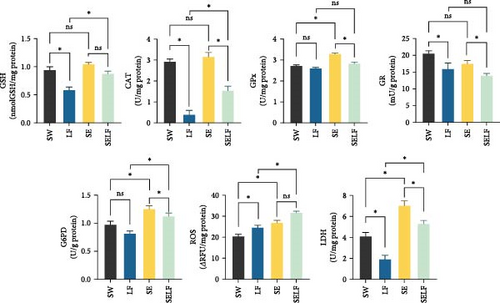
In gill tissues, significant alterations were observed in the levels of GSH, CAT, SOD, GPx, GR, GST, G6PD, ROS, and LDH due to the effect of feeding (p < 0.05), while significant variations were detected in the levels of LPO, CAT, GPx, and GST as a result of the effect of swimming (p < 0.05). Furthermore, interactions were noted in the levels of GSH, CAT, GPx, GR, G6PD, ROS, and LDH (p < 0.05) (Table 3).
| Biochemical parameters of gill tissue | Groups | Two-way ANOVA p value | |||||
|---|---|---|---|---|---|---|---|
| SW | LF | SE | SELF | Feeding effects |
Swimming effects | Interaction | |
| GSH (nmol GSH/mg protein) | 0.58 ± 0.15 | 0.87 ± 0.18 | 1.01 ± 0.1 | 0.94 ± 0.04 | <0.01 | 0.08 | <0.01 |
| LPO (nmol MDA/mg protein) | 8.97 ± 0.89 | 10.98 ± 0.91 | 8.4 ± 0.12 | 11.07 ± 0.49 | 0.48 | <0.01 | 0.35 |
| CAT (U/mg protein) | 0.38 ± 0.04 | 1.53 ± 0.04 | 3.14 ± 0.05 | 2.91 ± 0.52 | <0.01 | 0.04 | <0.01 |
| SOD (U/mg protein) | 80.57 ± 6.6 | 72.56 ± 8.65 | 91.22 ± 7.28 | 94.51 ± 3.48 | <0.01 | 0.45 | 0.08 |
| GPx (U/mg protein) | 2.59 ± 0.17 | 2.82 ± 0.04 | 3.28 ± 0.14 | 2.71 ± 0.11 | <0.01 | 0.03 | <0.01 |
| GR (mU/g protein) | 15.52 ± 1.36 | 13.9 ± 2.31 | 17.45 ± 1.32 | 20.46 ± 2.62 | <0.01 | 0.44 | 0.02 |
| GST (U/g protein) | 36.16 ± 2.66 | 48.97 ± 7.1 | 49.74 ± 2.52 | 53.05 ± 8.01 | <0.01 | <0.01 | 0.11 |
| G6PD (U/g protein) | 0.81 ± 0.14 | 1.12 ± 0.05 | 1.25 ± 0.11 | 0.96 ± 0.15 | 0.03 | 0.81 | <0.01 |
| ROS (RFU/mg protein) | 24.51 ± 2.04 | 31.5 ± 2.12 | 26.83 ± 3.9 | 20.36 ± 1.08 | <0.01 | 0.81 | <0.01 |
| LDH (U/mg protein) | 1.89 ± 0.48 | 5.28 ± 1.11 | 7.01 ± 1.43 | 4.08 ± 0.71 | <0.01 | 0.58 | <0.01 |
- Note: Values are means ± SD. The bold values indicate statistical significance at p < 0.05.
- Abbreviations: ANOVA, analysis of variance; CAT, catalase; G6PD, glucose-6-phosphate dehydrogenase; GPx, glutathione peroxidase; GSH, glutathione; LDH, lactate dehydrogenase; LF, limited feeding group; LPO, lipid peroxidation; ROS, reactive oxygen species; SD, standart deviation; SE, swimming exercise group; SELF, swimming exercise and limited feeding group; SOD, superoxide dismutase; SW, stationary water group.
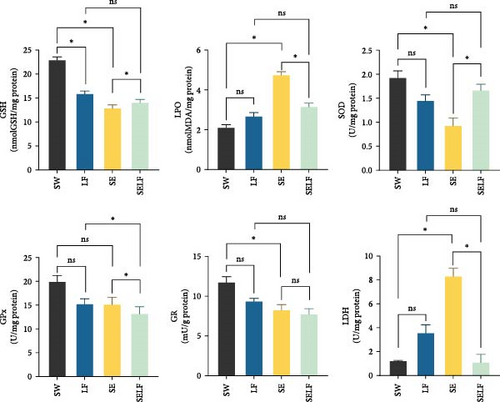
Upon examining the effects of dietary restriction and swimming on muscle tissues, variations were observed in the levels of GSH, CAT, GPx, GR, GST, ROS, and LDH due to the impact of dietary restriction (p < 0.05), while significant effects were also identified in the levels of GSH, LPO, CAT, SOD, GST, and LDH as a result of swimming activity (p < 0.05). Additionally, interactions were noted in the levels of GSH, LPO, SOD, GPx, GR, and LDH (p < 0.05) (Table 4).
| Biochemical parameters of muscle tissue | Groups | Two-way ANOVA p value | |||||
|---|---|---|---|---|---|---|---|
| SW | LF | SE | SELF | Feeding effects |
Swimming effects | Interaction | |
| GSH (nmol GSH/mg protein) | 22.86 ± 1.89 | 13.98 ± 1.65 | 12.83 ± 0.02 | 15.8 ± 1.52 | <0.01 | <0.01 | <0.01 |
| LPO (nmol MDA/mg protein) | 2.09 ± 0.55 | 2.65 ± 0.31 | 4.73 ± 0.03 | 3.15 ± 0.21 | 0.02 | <0.01 | <0.01 |
| CAT (U/mg protein) | 770.56 ± 62.53 | 477.69 ± 61.43 | 602.57 ± 2.22 | 437.95 ± 58.32 | <0.01 | <0.01 | 0.06 |
| SOD (U/mg protein) | 1.92 ± 0.33 | 1.44 ± 0.29 | 0.92 ± 0.31 | 1.66 ± 0.27 | 0.39 | <0.01 | 0.02 |
| GPx (U/mg protein) | 18.98 ± 5.37 | 15.15 ± 1.86 | 15.1 ± 0.56 | 13.14 ± 0.68 | 0.03 | 0.35 | 0.03 |
| GR (mU/g protein) | 11.4 ± 2.35 | 9.08 ± 0.78 | 8.04 ± 0.67 | 7.71 ± 1.32 | <0.05 | 0.17 | <0.01 |
| GST (U/g) protein) | 12.29 ± 0.27 | 8.76 ± 1.22 | 10.32 ± 0.38 | 11.62 ± 1.31 | 0.03 | <0.01 | 0.36 |
| G6PD (U/g protein) | 0.72 ± 0.25 | 0.67 ± 0.25 | 0.84 ± 0.25 | 0.92 ± 0.25 | 0.93 | 0.54 | 0.11 |
| ROS (RFU/mg protein) | 27.79 ± 12.58 | 9.47 ± 1.05 | 21.73 ± 7.48 | 6.28 ± 3.62 | <0.01 | 0.91 | 0.39 |
| LDH (U/mg protein) | 1.19 ± 0.02 | 3.54 ± 1.68 | 8.28 ± 2.25 | 1.07 ± 0.02 | <0.01 | <0.01 | <0.01 |
- Note: Values are means ± SD. The bold values indicate statistical significance at p < 0.05.
- Abbreviations: ANOVA, analysis of variance; CAT, catalase; G6PD, glucose-6-phosphate dehydrogenase; GPx, glutathione peroxidase; GSH, glutathione; LDH, lactate dehydrogenase; LF, limited feeding group; LPO, lipid peroxidation; ROS, reactive oxygen species; SD, standart deviation; SE, swimming exercise group; SELF, swimming exercise and limited feeding group; SOD, superoxide dismutase; SW, stationary water group.
The results of the two-way ANOVA analysis using SPSS for the measured factors indicate the significance of certain effects. For LPO levels in the gill tissue, the interaction effect was not significant (p = 0.35), and the feeding effect was not significant (p = 0.48). However, the swimming effect was significant (p < 0.01), with mean values of 8.68 for swimming and 11.02 for not swimming. For SOD levels in the gill tissue, the interaction effect was not significant (p = 0.08), but the feeding effect was significant (p < 0.01), with mean values of 76.57 for ad libitum feeding and 92.86 for restricted feeding.
For GSH levels in the gill tissue, the interaction effect was not significant (p = 0.11), but both the feeding effect (p < 0.01) and the swimming effect (p < 0.01) were significant. The mean GSH levels were 42.56 for ad libitum feeding, 51.39 for restricted feeding, 42.95 for swimming, and 51.01 for not swimming. For CAT levels in muscle tissue, the interaction effect was not significant (p = 0.06), but both the feeding effect (p < 0.01) and the swimming effect (p < 0.01) were significant. The mean CAT levels were 457.82 for ad libitum feeding, 686.57 for restricted feeding, 520.26 for swimming, and 624.12 for not swimming.
For GST levels in muscle tissue, the interaction effect was not significant (p = 0.36), but both the feeding effect (p = 0.03) and the swimming effect (p < 0.01) were significant. The mean GST levels were 10.19 for ad libitum feeding, 11.31 for restricted feeding, 9.54 for swimming, and 11.96 for not swimming. For G6PD levels in muscle tissue, none of the effects were significant (p > 0.05). For ROS levels in muscle tissue, the interaction effect was not significant (p = 0.39), and the swimming effect was not significant (p = 0.91). However, the feeding effect was significant (p < 0.01), with mean values of 7.87 for ad libitum feeding and 22.97 for restricted feeding.
The interaction effects for GSH, CAT, GPx, GR, G6PD, and ROS in gill tissue, as well as for GSH, LPO, SOD, GPx, GR, and LDH in muscle tissue, were significant and are further detailed in other figures.
In the analysis of biochemical parameters within gill and muscle tissues, the study delineated significant differences among the experimental groups utilizing the independent sample t-test (p < 0.05). In gill tissue, GSH levels were discernibly elevated in SW over LF, with SELF also showing increased levels compared to LF (p < 0.05). CAT activity analysis revealed that LF exhibited lower levels than SW, whereas SE surpassed SELF, which in turn exhibited higher levels than LF (p < 0.05). GPx concentrations were significantly higher in SE relative to SW and SELF (p < 0.05), indicating enhanced antioxidant response. GR and G6PD levels followed a similar pattern of significant variation (p < 0.05), with SE showing elevated levels compared to other groups, and SW outpacing LF in GR levels. LDH levels were notably higher in SE compared to SW and SELF, with both SELF and SW showing increased levels over LF (p < 0.05), reflecting metabolic adjustments (Figure 1).
Muscle tissue exhibited analogous trends, with GSH concentrations significantly higher in SW than LF and SE, and SELF presenting elevated levels compared to SE. LPO outcomes showed SE with higher levels than SW and SELF (p < 0.05). Conversely, SOD levels were lower in SE than in SW and SELF (p < 0.05), suggesting a differential oxidative stress response. Similarly, GPx levels in SELF were lower than in SE and LF, underscoring the impact of environmental and physiological stressors on antioxidant mechanisms (p < 0.05). Notably, GR levels in SW were elevated compared to SE (p < 0.05), and LDH levels were significantly increased in SE in relation to SW and SELF (p < 0.05), highlighting metabolic shifts across the conditions (Figure 2).
These findings provide insight into the biochemical shifts within gill and muscle tissues under various aquaculture scenarios, highlighting the complex interaction between stress exposure, antioxidant defense, and metabolic activity.
4. Discussion
The application of dietary restriction and swimming exercise, either simultaneously or independently, revealed intricate effects on the growth, metabolic processes, and overall health of juvenile rainbow trout. This study underscores the importance of meticulously balancing dietary plans and exercise regimes to optimize nutrient utilization and enhance fish welfare. Previous studies have explored the effects of swimming exercise and dietary restriction on fish metabolism [6, 31, 32]. However, our findings provide new insights into the biochemical responses and metabolic adaptations of rainbow trout subjected to combined physical and nutritional stressors.
Key findings highlight the influence of swimming exercise and dietary restriction on amino acid and fatty acid metabolism, antioxidant defense mechanisms, and tissue repair processes. These results suggest that rainbow trout adapt through dynamic shifts in metabolic pathways and antioxidant responses, emphasizing the interplay between energy metabolism, oxidative stress management, and tissue health.
4.1. Amino Acid Composition of Rainbow Trout
Under conditions of dietary restriction, fish may experience reduced nutrient intake, potentially leading to deficiencies in EAAs. Concurrently, swimming exercise, which elevates energy expenditure, can trigger the utilization of amino acids as an energy source, potentially altering the balance of essential and semi-EAAs. Both dietary restriction and swimming exercise influence amino acid synthesis pathways, leading to either reduced synthesis or increased utilization of specific amino acids [31, 33].
The analysis of amino acid composition in this study revealed significant changes in response to swimming activity and dietary restriction. Notably, histidine levels increased under these conditions, particularly in the swimming groups (p < 0.05). Histidine, an EAA, plays a critical role in buffering intramuscular pH by neutralizing excess hydrogen ions during anaerobic metabolism, mitigating acidosis, and preserving muscle function during sustained physical activity [34]. This highlights histidine’s vital role in supporting metabolic adaptations to the increased energy demand induced by exercise.
Beyond its buffering role, histidine serves as a precursor to carnosine, a dipeptide with potent antioxidant properties that neutralizes ROS and protects muscle tissues from oxidative stress [34]. Additionally, histidine-derived histamine is crucial in modulating immune responses and inflammatory processes. Fluctuations in histidine levels can directly affect histamine synthesis, influencing the fish’s ability to mount effective immune responses and maintain resilience under environmental and physiological stressors [35].
Prolonged swimming also increases energy demands, leading to the utilization of proteins and amino acids for energy production. This metabolic shift underscores histidine’s multifunctional role in antioxidation, immune modulation, and energy metabolism. Inadequate histidine levels, whether due to stress or suboptimal feeding programs, may impair protein synthesis, growth, and tissue repair, emphasizing the importance of dietary supplementation to sustain fish health and growth [36, 37].
Cysteine, another pivotal amino acid, demonstrated elevated levels in swimming groups (p < 0.05). As a precursor for GSH synthesis, cysteine plays a central role in redox balance by neutralizing ROS and supporting cellular integrity during oxidative stress. The observed changes in amino acid metabolism, particularly the increase in histidine and cysteine levels during swimming and dietary restriction, reflect metabolic reprogramming in response to heightened energy demand and nutritional stress. These adaptations involve the rerouting of metabolic pathways to prioritize essential functions such as antioxidation and energy production. For example, the elevated histidine levels likely contribute to carnosine synthesis, enhancing antioxidant capacity and buffering intracellular pH to support sustained muscle function under physical stress [38]. Similarly, increased cysteine availability supports the synthesis of GSH, a crucial antioxidant, aligning with the demands of oxidative stress during exercise [39]. The observed upregulation of cysteine levels during swimming suggests an adaptive mechanism to meet the heightened demand for GSH synthesis under physical stress [39]. Furthermore, cysteine contributes to tissue repair and muscle remodeling, facilitating recovery and resilience against physiological stressors [35].
The study found that swimming significantly influenced semi-EAAs and cysteine levels, while EAA/TAA ratios were higher in non-swimming groups. These observations reflect distinct metabolic adaptations to physical activity, where increased energy expenditure promotes the utilization of amino acids for both energy production and antioxidation.
Together, the observed changes in histidine and cysteine levels reveal their central roles in metabolic adaptation, antioxidative defense, and immune function in rainbow trout. These findings suggest that dietary restriction combined with controlled swimming can optimize amino acid metabolism, enhancing both fish welfare and production quality in aquaculture settings. Furthermore, the metabolic reprogramming observed in response to swimming underscores the importance of integrating physical activity into aquaculture practices to improve antioxidant capacity, tissue repair mechanisms, and overall health outcomes.
4.2. Fatty Acid Composition of Rainbow Trout
The fatty acid analysis in this study illuminated a significant impact of swimming activity on the fatty acid profile, particularly noting the variances in margaric acid (C17:0) and γ-linolenic acid (C18:3 n-6) levels, underscoring the complex metabolic adaptations occurring in response to physical activity.
The observed reduction in linolenic acid levels under these conditions may indicate its utilization as a key substrate for energy production, as linolenic acid is a precursor in the synthesis of essential long-chain PUFAs (LC-PUFAs) like EPA and DHA. These changes underline the metabolic shifts necessary for meeting the heightened energy demands of fish under combined exercise and dietary restriction [40]. LC-PUFAs, such as EPA and DHA, are critical for maintaining cell membrane fluidity, regulating inflammatory responses, and supporting neural development [41].
γ-Linolenic acid, in particular, exhibited significant elevations in the context of swimming and dietary restriction. This fatty acid is known for its anti-inflammatory properties, and its role in maintaining cell membrane composition and fluidity. Enhanced γ-linolenic acid levels may reflect an adaptive mechanism to improve membrane stability and resilience against stress-induced damage. Its elongation to dihomo-γ-linolenic acid (DGLA) has been linked to bioactivity, including anti-inflammatory effects [42]. These findings suggest that the combination of swimming and feed restriction amplifies metabolic responses beyond those observed in isolation [42].
Interestingly, swimming activity influenced fatty acid metabolism differently from amino acid metabolism. For instance, higher EAA/TAA ratios in non-swimming groups suggest that swimming promotes the utilization of amino acids for immediate energy production, while prioritizing fatty acids for long-term energy storage and structural roles. This dichotomy underscores the complexity of metabolic regulation in fish, where diet, exercise, and their interactions play crucial roles in shaping physiological adaptations.
The comprehensive analysis of fatty acid compositions, particularly focusing on the effects of swimming and dietary restriction, provides valuable insights into the adaptive mechanisms of fish under environmental challenges. Aligning with published literature, these findings corroborate the significant role of exercise in modulating fatty acid profiles [43, 44]. This metabolic reprogramming supports energy homeostasis, antioxidative capacity, and membrane stability, highlighting the resilience of rainbow trout to combined stressors.
Additionally, the increase in γ-linolenic acid observed under swimming and dietary restriction conditions highlights its role as an adaptive mechanism to maintain membrane stability and fluidity, protecting cells from stress-induced damage [42]. Its elongation to DGLA further contributes to cellular stability and anti-inflammatory responses [45]. The critical involvement of elongation enzymes, such as ELOVL5, in this process further emphasizes the importance of metabolic regulation in supporting these physiological adaptations [42].
The effects of swimming on fatty acid metabolism also underscore the pivotal roles of LC-PUFAs like EPA and DHA in energy metabolism and inflammatory responses. Linolenic acid’s role as a substrate for energy production and the synthesis of these LC-PUFAs further highlights its importance [40].
The dynamic changes in fatty acid metabolism induced by swimming and dietary restriction showcase adaptive mechanisms that support energy efficiency, antioxidative defense, and membrane stability. Swimming and dietary restriction collectively induce metabolic reprogramming by altering substrate prioritization to meet heightened energy demands and mitigate oxidative stress. Amino acids, particularly histidine and cysteine, are redirected toward antioxidant functions, such as carnosine and GSH synthesis, while also serving as supplementary energy sources during prolonged exercise [46]. Similarly, fatty acids such as linolenic acid are metabolized into LC-PUFAs like EPA and DHA, which play critical roles in maintaining membrane integrity and modulating inflammation [47]. The observed reduction in linolenic acid levels and the increase in γ-linolenic acid during swimming reflect a shift in lipid metabolism to meet the dual demands of energy production and membrane stability. This metabolic reprogramming involves the activation of fatty acid oxidation pathways and the biosynthesis of LC-PUFAs such as EPA and DHA, which are critical for maintaining cellular function under stress. Enhanced γ-linolenic acid levels may signal a prioritized synthesis of anti-inflammatory lipids to mitigate exercise-induced inflammation [47]. These findings lay the groundwork for further exploration of their implications in aquaculture and stress physiology.
4.3. Biochemical Parameter Responses of Gill and Muscle Tissues of Rainbow Trout
The biochemical responses of rainbow trout to dietary restriction and swimming activity highlight the complex adaptive mechanisms to environmental and physiological stressors. These adaptations are characterized by the nuanced alterations in antioxidant defense mechanisms and metabolic enzyme activities within gill and muscle tissues. Significant changes in antioxidant enzyme levels, including SOD and CAT, may reflect physiological adaptations to oxidative stress and are likely regulated by signaling pathways such as the Nrf2-Keap1 axis and HIF-1 α.
The Nrf2-Keap1 axis plays a pivotal role in regulating redox balance by enhancing the transcription of genes encoding antioxidant enzymes. During swimming, increased ROS production likely triggers the release of Nrf2 from Keap1, allowing it to translocate to the nucleus and upregulate genes such as CAT, SOD, and GPx [48]. This mechanism ensures an effective antioxidative response to exercise-induced oxidative stress. Additionally, the hypoxia-inducible factor HIF-1 α may contribute to these adaptations by modulating metabolic pathways that balance oxygen consumption and energy production in muscle tissues [46]. Together, these pathways coordinate cellular responses to maintain redox homeostasis and energy efficiency under stress.
In gill tissues, dietary restriction significantly influenced a wide array of biochemical parameters, including GSH, CAT, SOD, GPx, GR, GST, G6PD, ROS, and LDH. This highlights the crucial role of nutritional inputs in modulating oxidative stress and antioxidant defense systems. Swimming activity, on the other hand, specifically affected LPO, CAT, GPx, and GST levels, reflecting the physiological demands imposed by increased metabolic activity [35, 49]. The interplay between these parameters emphasizes the importance of balancing dietary and exercise regimes to optimize fish health and development.
Muscle tissues exhibited responses similar to gill tissues, with dietary restriction affecting GSH, CAT, GPx, GR, GST, ROS, and LDH levels. These findings align with the notion that dietary manipulation significantly impacts the antioxidant capacity and metabolic status of fish, influencing tissue integrity and function [50]. Notably, exercise-induced oxidative stress stimulated mitochondrial biogenesis and the production of endogenous antioxidants through pathways involving PGC-1α and AMPK activation. These mitochondrial adaptations mitigate ROS-related damage and support sustained energy production [35].
The concurrent changes in amino acid and fatty acid metabolism suggest a coordinated metabolic reprogramming that prioritizes antioxidative and energy-producing pathways to maintain homeostasis. For example, histidine and cysteine are redirected toward the synthesis of carnosine and GSH, aligning with increased demands for redox balance. Meanwhile, fatty acids such as linolenic acid are mobilized to synthesize LC-PUFAs like EPA and DHA, which play critical roles in maintaining membrane integrity and modulating inflammation [46, 47].
These metabolic adaptations highlight the integration of glycolysis, lipid oxidation, and amino acid catabolism to sustain energy production and enhance resilience to oxidative stress. This dynamic reprogramming not only supports physical performance but also preserves tissue function by reducing oxidative damage [38]. The observed effects of swimming on GSH, LPO, CAT, SOD, GST, and LDH levels further underscore the dual role of physical activity in enhancing antioxidant defenses while imposing metabolic and oxidative challenges.
Malnutrition and oxidative stress are interconnected health concepts that significantly impact fish well-being. An imbalance in nutrient intake can lead to oxidative stress, characterized by excessive ROS production and insufficient antioxidant defense. Antioxidant systems, comprising enzymes such as SOD, CAT, GR, GST, GPx, and G6PD, along with nonenzymatic molecules like GSH, play a critical role in neutralizing ROS and protecting cellular components [39]. Elevated ROS levels can lead to LPO, disrupting membrane integrity, while increased LDH activity indicates tissue damage caused by oxidative stress [51]. These parameters are valuable indicators for assessing oxidative damage and the effectiveness of antioxidant systems in fish.
The relationship between oxidative stress and aquatic organisms provides insights into how stressors impact fisheries and aquaculture systems. Insufficient nutrients and restricted swimming may exacerbate ROS production, leading to increased oxidative damage [52, 53]. Therefore, evaluating enzymatic and nonenzymatic antioxidant defenses under these conditions is essential for understanding the extent of oxidative damage and implementing effective aquaculture strategies [54].
Swimming and dietary restriction are key factors influencing the antioxidant defense systems within fish tissues. While exercise enhances antioxidant biochemical parameters and reduces LPO, excessive physical activity may lead to muscle loss, inflammation, and increased oxidative stress [55]. Elevated LDH activity, often associated with intense exercise, serves as a diagnostic marker for cellular damage. Striking a balance between physical activity and nutrition is crucial to fostering optimal growth conditions and stress resilience in fish.
The 6-week observation period may not have been sufficient to fully capture the long-term biochemical and physiological adaptations to swimming and dietary restriction. Extended studies are necessary to further elucidate the cumulative effects of these interventions on fish health and development.
5. Conclusion
This study highlights the significant impact of dietary restriction and swimming exercise on the health and growth of juvenile rainbow trout. The findings demonstrate that swimming and dietary restriction collectively induce metabolic reprogramming, redirecting amino acid and fatty acid metabolism to meet heightened energy demands and oxidative stress. For instance, increased histidine levels support muscle buffering and antioxidation through carnosine synthesis, while γ-linolenic acid enhances membrane stability and modulates inflammation, illustrating the adaptive physiological responses to combined physical and nutritional challenges.
The significant upregulation of antioxidant enzymes (e.g., SOD, CAT, and GPx) and the reduction in oxidative damage markers such as LPO indicate improved cellular resilience under these conditions. These results underscore the importance of optimizing both dietary plans and physical activity levels to enhance fish welfare and production quality. Specifically, incorporating controlled swimming and dietary strategies can maximize antioxidant defenses, promote tissue health, and improve the nutritional composition of farmed fish.
From a practical perspective, aquaculture systems should aim to replicate the natural swimming conditions of rainbow trout by providing water flow rates equivalent to at least one body length per second. This approach, combined with balanced feeding strategies, can yield substantial benefits for fish welfare and product quality. However, the 6-week duration of this study may not fully capture the long-term effects of these interventions, suggesting the need for extended studies to explore cumulative physiological adaptations. Future research should also focus on the scalability and implementation of these findings in commercial aquaculture to optimize both fish health and production outcomes.
Ethics Statement
The present experiments were carried out according to the legislation on the use of aquatic animals for experimental and other scientific purposes in Turkey with approval of Istanbul University, Animal Experiments Local Ethics Committee (Number: 2019/10 and 2019/19).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Gökhan Tunçelli: formal analysis, investigation, data curation, writing–original draft, writing–review and editing, visualization. Onur Ertik: investigation, data curation, writing–review and editing. Bertan Boran Bayrak: investigation, data curation. İdil Can Tunçelli: investigation, data curation, writing–review and editing, visualization. Özkan Özden: investigation, data curation, writing–review and editing, visualization. Refiye Yanardag: data curation, writing–review and editing. Devrim Memiş: conceptualization, methodology, validation, resources, writing–review and editing, supervision, project administration.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University with Project numbers FDK-2020-37081 and FBA-2022-38230.
Acknowledgments
We express our gratitude to Dr. Juhani Pirhonen, the second supervisor, for his advice on Gökhan Tunçelli’s draft PhD thesis.
Open Research
Data Availability Statement
Data are available from corresponding author upon reasonable request.




