Fabrication of Algogenic Zinc Nanoparticles and Assessment of Their Biomimetics Attributes and Potential Antibacterial Efficacy Against Fish Pathogens
Abstract
This study investigates the fabrication of algogenic zinc nanoparticles (ZnNPs), examining their biomimetic properties and potential antibacterial effectiveness against fish pathogens. The rising incidence of antibiotic resistance in aquaculture demands innovative strategies for disease management, highlighting the importance of developing biocompatible and effective antimicrobial agents. In this context, ZnNPs were synthesized using Pediastrum boryanum microalgal extract as the reducing and capping agent. Transmission electron microscopy (TEM) revealed spherical and tetragonal particles with sizes ranging from 32.84 to 26.87 nm. Fourier transform infrared spectroscopy (FTIR) demonstrated distinctive functional groups in the algal extracts and ZnNPs. X-ray diffraction (XRD) showed pure crystallinity of the fabricated ZnNPs with an average crystalline size of 57.97 nm. The algal extract had high total phenolic (TPC) and flavonoid (TFC) contents (TPC: 53.83 and TFC: 12.44 mg QE/g, respectively). The obtained ZnNPs retained a considerable amount of TPC and TFC (7.92 and 5.31 mg/g, respectively). The microalgal extract and ZnNPs exhibited considerable antioxidant capacity in the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. In addition, they elicited antibacterial efficacy against several bacterial pathogens in fish in a minimum inhibitory concentration (MIC) assay. Brine shrimp lethality assay (BSLA) revealed higher potential cytotoxicity of biosynthesized ZnNPs at 29.28 μg/mL compared to that of the algal extracts at 51.56 μg/mL. These findings highlight the potential of P. boryanum as a green factory for the nanofabrication of metallic nanoparticles (NPs) and as a promising clinical candidate for fish medicine.
1. Introduction
Microalgae are the most primitive and organized species in the plant kingdom, measuring 3–20 µm in size. Aquatic microalgae are ubiquitous in many locations such as hot springs and glacial ice flows. According to previous reports, microalgae contain numerous bioactive compounds such as polysaccharides, vitamins, lipids, proteins, dietary fiber, antioxidants, minerals, carotenoids, and polyunsaturated fatty acids, as well as phenols, flavonoids, alkaloids, and terpenoids, which represent their reducing ability [1, 2].
Pediastrum spp. are colonial microalgae and colonial chlorophytes that are commonly found in stagnant freshwater bodies, including the Protista kingdom, Chlorophyta division (green algae), Chlorophyceae class, Sphaeropleales order, and Hydrodictyaceae family [3, 4]. The green microalga Pediastrum boryanum (P. boryanum) is rich in phenolic compounds, which provide natural antioxidant potential against reactive oxygen species (ROS); therefore, it can be used in the food and pharmaceutical industries [5, 6].
Microalgal extracts have recently been used in the field of algonanotechnology for the fabrication of metal nanoparticles (MNPs) because they are natural stabilizing agents, are poisonous, easy to handle, and can be developed at low temperatures with minimal equipment. In addition, the microalgal content of phytochemicals is efficient in metal-reducing and capping functions to generate a persistent coating on MNPs [1, 7]. In general, green synthesis of MNPs provides high biodegradability, photocatalytic and photo-oxidant capacity, low cytotoxicity, and a better ability to be eliminated from the body [8]. Owing to the drawbacks of traditional hazardous chemical methods, there is a growing switch towards producing eco-friendly green nanoparticles (NPs) using bioreagents [9, 10]. NPs synthesized using the biomimetic microalgal approach are biodegradable in nature, cost-effective, have low environmental risks, and can degrade toxic metals to less toxic forms [10–12]. Notably, the molar concentrations of algal or phytogenic extracts provide a good yield of metal precursors for a higher production of MNPs [13, 14]. Regarding their biological activity, biogenic NPs are generally more active than synthetic NPs, in addition to their ease of production, size and shape diversity, higher yield, and improved biocompatibility [15–17]. As the biosourced NPs showed promising antimicrobial capacity for utilization in fish medicine [18–20], biogenic MNPs showed similar efficacy either alone or in synergy with other natural phenolic reagents [21, 22].
Zinc (Zn) is a crucial trace element in living organisms and is involved in metabolic processes, protein assimilation, nervous cell synthesis, hematopoiesis, antioxidant defense, and immune-regulatory functions [23, 24]. MNPs have attracted worldwide attention from scientific researchers because they are active, selective, and have a long lifespan in various chemical processes [8, 25, 26]. Several studies have documented the biosynthesis of algogenic zinc NPs (ZnNPs) from different species of microalgae, including Arthrospira platensis [8], Sargassum [27], and Cystoseira crinite [12]. Biosynthesized ZnNPs show antioxidant and antimicrobial effects [12, 28]. During the green synthesis of ZnNPs, reducing and capping agents of plant, fungal, or algal materials replace harmful chemicals [16]. Interestingly, there are no safety concerns associated with the use of biological sources and/or microorganisms compared with the chemicals used for synthetic ZnNPs [17, 29].
To date, no study has scientifically validated the potential biological and phytochemical applications of P. boryanum in algonanotechnology for MNPs fabrication. Our recent study utilized the green microalga P. boryanum to synthesize selenium NPs [30]; however, we would like to extend our knowledge and demonstrate its synergistic action in mediating ZnNPs biosynthesis. Therefore, the current study was designed for the algogenic synthesis of ZnNPs from P. boryanum extract and to evaluate their phytochemical components and antioxidant capacity. Additionally, the potential antimicrobial efficacy against fish diseases was assessed based on the visual growth of several pathogenic bacterial isolates. Furthermore, a brine shrimp cytotoxicity assay was performed to determine the potential cytotoxic hazards of the modified ZnNPs compared to the algal extract.
2. Materials and Methods
2.1. Materials
Zn sulfate (ZnSO4; BDH Chemical Co., England) was used as a Zn precursor. Pediastrum boryanum algal extract powder was purchased from the National Research Center (Cairo, Egypt). Folin–ciocalteu (F-C) phenolic reagent (Fluka, Biochemica, Germany), tryptone soy agar (TSA), and brain heart infusion agar (BHA) were of analytical grade and obtained from Sigma–Aldrich.
2.2. Pediastrum boryanum Extract Preparation and Biosynthesis of ZnNPs
The chosen algae were extracted using a previously described protocol [31] with slight adjustments in line with the newly developed Bionanotechnology equipment by El-Ghamry et al. [32]. The P. boryanum algal powder (100 g) was mixed with magnetically stirred water (1 L) and heated in an extraction unit at 70°C for 2 h. After heating, the algal extract (1 L) was transferred to the synthesis unit. An aqueous ZnSO4 solution (IL, 1 mmol) was added dropwise under continuous magnetic stirring for 2 h at room temperature. The synthesis of ZnNPs was indicated by the color change of the reaction mixture to cloudy white [12, 33, 34]. The mixture of synthesized NPs was then exposed to ultraviolet (UV) light for 20 min using a reduction factor lamp (Vilber Lourmat-6. LC, France) at a wavelength of 254 nm, as previously described [28]. The collected ZnNPs were stored at −18°C for future research following filtration with Whatman no. 1 filter paper (Whatman International Ltd., Kent, UK). The algo-synthesis of ZnNPs was verified visually by the change in the algal green color to a yellowish-white color at the end of the reaction (Scheme 1).
2.3. Characterization of Algal-Synthesized ZnNPs
The stability and optical characteristics of the synthesized ZnNPs were monitored using a UV-visible (Vis) spectrometer (ATI Unicom, UV2-100, V 3.20, England) [35, 36], within the range of 240–440 nm. Fourier transform infrared (FTIR) spectroscopy (Thermo Fisher Scientific Nicolet IS10, USA) was performed using the potassium bromide (KBr) pellet method [12, 37], and the outcomes were documented in the 400–4000 cm−1 range. The powdered sample was subjected to a CuKα1-X Ray diffractometer radiation (λ = 1.5406 A°) operating at 30 kV and 10 mA with 2θ ranging from 5° to 79.93° to confirm the presence of ZnO and analyze the crystallite structure and size. The diffraction intensities were compared with the Joint Committee on Powder Diffraction Standards (JCPDS) files (SSD160_2 (1D mode)) [38]. Transmission electron microscopy (TEM) was used to analyze the surface morphological characteristics of the green-synthetized ZnNPs, such as their size and shape, using a TEM connected to a charge-coupled device (CCD) camera (JEOL TEM-2100, Tokyo, Japan) [12]. Furthermore, the net surface charge of the ZnNP samples was characterized using the zeta potential technique which provides insights into the stability and dispersion of the NPs in solution. A high zeta potential (either positive or negative) generally indicates good stability, suggesting strong repulsive forces between particles and minimizing their tendency to aggregate [39]. This characterization was performed using Malvern Zetasizer instruments (Malvern Ltd., Kassel, Germany).
2.4. Phytochemical Analysis
The total phenolic (TPC) of Bio-ZnNPs synthesized using P. boryanum extract was evaluated using the F-C method [40, 41], and the obtained data were reported as milligrams of gallic acid equivalent per gram of dry weight extract (mg GAE/g extract) [35]. The total flavonoid (TFC) of the extracted samples containing NPs was determined using a previously described protocol [42], and the results were expressed as milligram quercetin equivalent per gram dry weight (mg QE/g extract). The total tannin content was analyzed using the vanillin–hydrochloride assay [43], and were articulated as grams of tannic acid equivalents/100 g dry plant.
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
The free radical-scavenging capacity of the aqueous solution of algal extract and its biogenic ZnNPs was evaluated using the DPPH colorimetric assay with ascorbic acid as the standard method [44]. A stock solution of DPPH (1 mL in anhydrous methanol, w/v; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was mixed with serially diluted ZnNPs and algal extract samples in methanol. After shaking the mixtures, they were incubated at room temperature (~25°C) for 30 min in darkness. A spectrophotometer (Spekol 11, Analytic Jena AG, Jena, Germany) measured the absorbance at 517 nm using the control DPPH solution as a standard blank. DPPH radical capacity was then calculated using a standard equation [45]. The % DPPH remaining values were plotted against mg extract/mL using an exponential curve to identify the inhibitory concentration (IC50). IC50 represents the quantity of antioxidants required to reduce the initial concentration of the DPPH solution by 50%. The IC50 values indicated an inverse relationship with the antioxidant capacity of the tested samples [33, 46].
2.6. Antibacterial Efficacy
The minimum inhibitory concentration (MIC) assay based on the broth microdilution technique [18, 47] was employed to investigate the antibacterial action of the microalgal extract and the aqueous nanosuspension of ZnNPs against several pathogenic fish bacteria, including gram-negative isolates (Aeromonas veronii, Aeromonas sobria, Aeromonas hydrophila, Pseudomonas aeruginosa, and Edwardsiella ictaluri), and a gram-positive isolate (Streptococcus iniae). The strains tested were kindly supplied by the Division of Fish Health, University Clinic for Avian and Fish Medicine, University of Veterinary Medicine, Vienna, Austria, as detailed elsewhere, Except for P. aeruginosa ATCC 9027, which was graciously provided by the Helmy Institute for Medical Sciences at Zewail City of Science and Technology in Egypt. Briefly, the previously mentioned pathogenic bacteria isolated were streaked and incubated overnight on TSA plates at 28°C, except for P. aeruginosa ATCC 9027, which was streaked onto BHA plates at 37°C. In a 96-well plate, the tested ZnNPs and microalgal extracts were serially diluted twofold from 1024 to 2 µg/mL. Vancomycin and thymoquinone were used as positive controls and were serially diluted from 512 to 1 µg/mL. Ten microliter of adjusted bacteria at 1.5 × 108 CFU/mL by 0.5 McFarland standards, was added to each well. After incubation for 24 h at 37°C, the plates were checked for possible turbidity (bacterial growth). The assay was conducted in triplicate, and MIC values were interpreted as the highest dilution (lowest concentration) of the sample showing clear fluid with no bacterial strain turbidity. This process was repeated at least twice [48].
2.7. Brine Shrimp Lethality Assay (BSLA)
2.8. Statistical Analysis
One-way analysis of variance (ANOVA) was used to determine the differences in DPPH radical scavenging activity. Two-way ANOVA was used to determine the significance of the mortality percentages, as revealed in the BSLA within and between algal extract and ZnNPs using GraphPad statistics package version 8.4.2. (GraphPad Software, Inc., USA). The significance levels were set at p < 0.05, p < 0.01, p < 0.001, and p < 0.0001. All data are presented as mean ± SEM.
3. Results
3.1. Optical Properties of ZnNPs
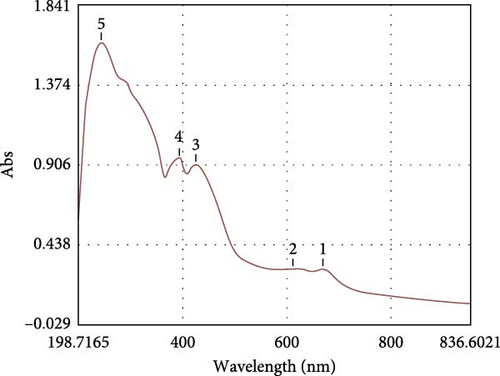
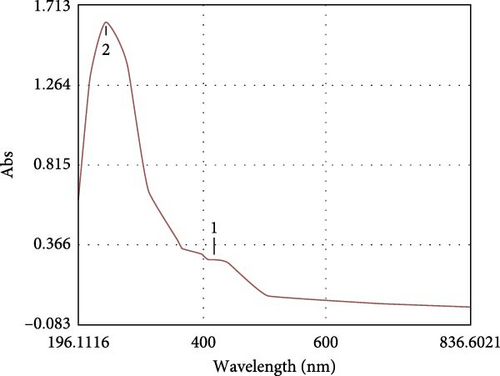
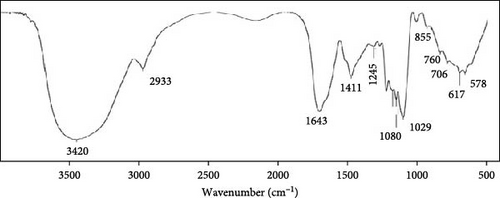
The microalgal extract displayed three higher peak absorption spectra between 200 and 800 nm. In contrast, higher emission and excitonic absorption peaks were observed for the synthesized ZnNPs between 258 and 420 nm from the microalgal extract of P. boryanum (Figure 1A, B). FTIR analysis was employed to discern the diverse chemical functional groups associated with the green synthesis of ZnNPs from microalgal plants. The peaks centered at 3569 cm−1 indicate the O─H hydroxyl groups of alcohols or phenolic compounds. The absorbance peaks observed at 2075 cm−1 corresponded to the alkane group’s C─H bond in the spectra’s stretching position. The 1619, 1402, and 1202 cm−1 peaks correspond to C═C alkenes or C═N amides, C─H bending, and C─O stretching, respectively. Similarly, the peaks at 1101, 990, 620, and 528 cm −1 indicate C─O stretching and C═C, C─Cl, and Zn─O bonds from biogenic ZnNPs (Figure 2B; Table 1). FTIR analysis of the P. boryanum extract alone was also performed (Figure 2A, Table 2).
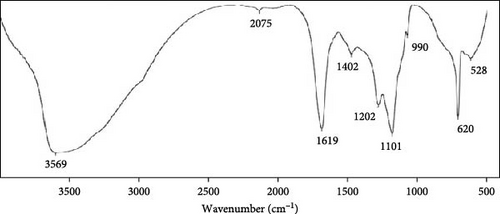
| Wavenumber (cm−1) | Characteristic groups | Appearance |
|---|---|---|
| 528 | Zn─O stretching (zinc oxide) | Strong |
| 620 | C─Cl stretching (halo compound) | Strong |
| 990 | C═C bending (alkene) | Medium |
| 1101 | C─O stretching (aliphatic ether) | Strong |
| 1202 | C─O stretching (alkyl aryl ether) | Strong |
| 1402 | C─H bending (alkane, methyl group) | Medium |
| 1619 | C═C stretching (alkene, disubstituted (cis)) or C═N or C═O (amide) | Medium |
| 2075 | C─H stretching or strong stretching N═C═S (isothiocyanate) | Medium |
| 3569 | O─H stretching (free alcohol) | Medium, sharp |
| Wavenumber (cm−1) | Characteristic groups | Appearance |
|---|---|---|
| 617 | C─I stretching (halo compound) | Strong |
| 760 | C─H bending (1,2,3-trisubstituted) | Strong |
| 855 | C─Cl stretching (halo compound) | Strong |
| 1029 | S═O stretching (sulfoxide) | Strong |
| 1080 | C─O stretching (primary alcohol) | Strong |
| 1106 | C─O stretching (aliphatic ether) | Strong |
| 1152 | C─N stretching (amine) | Medium |
| 1201 | C─O stretching (alkyl aryl ether) | Strong |
| 1245 | C─O─C stretching (ether) | Strong |
| 1411 | C─H bending (alkane, methyl group) | Medium |
| 1643 | C═C stretching (alkene, disubstituted (cis)) or stretching C═O (amide) | Weak or medium |
| 2933 | N─H stretching (amine) or weak C─H stretching | Strong, broad |
| 3420 | O─H stretching (alcohol) | Strong, broad |
3.2. Structural Characterization of the ZnNPs
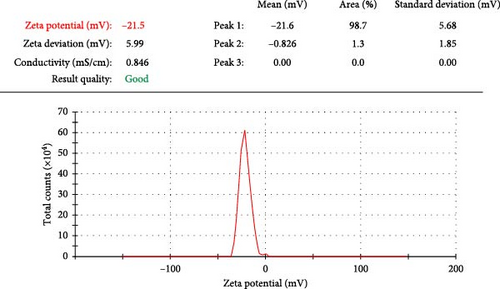
The TEM results were used to determine the morphology, size, and particle separation of the synthesized ZnNPs from the algal extract at a spatial resolution of 200 nm. The size of the synthesized ZnNPs was within the range of 32.84–26.87 nm for the synthesized ZnNPs. The predominant shapes observed were spherical rods, displaying a widely dispersed assortment of particles, including both large and small agglomerated particles of varying sizes (Figure 3). The X-ray diffraction (XRD) analysis of the ZnNPs fabricated from P. boryanum revealed characteristic peaks at 2θ angles of 33.304, 36.001, 38.883, 41.346, 55.106, 62.173, and 64.94. These peaks were assigned to the (100), (002), (101), (102), (110), (103), and (112) lattice planes, as shown in Figure 4. Using Scherrer’s formula, the crystalline size of the ZnNPs at each angle was estimated, and the average crystallite size was found to be 57.97 nm as displayed in Table 3. The P. boryanum extract-synthesized ZnNPs demonstrate relative stability in zeta potential analysis, with high zeta potential values of −21.5 mV (Figure 5).

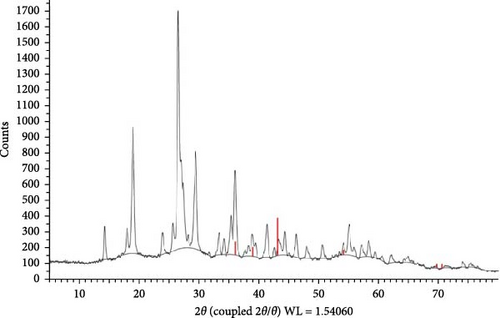
| Sample numbers | 2θ | FWHM (β) | Miller indices | Crystalline size (D; nm) |
|---|---|---|---|---|
| 1 | 33.304 | 0.298 | (100) | 29.06231 |
| 2 | 36.001 | 0.264 | (002) | 33.04695 |
| 3 | 38.883 | 0.1 | (101) | 87.99076 |
| 4 | 41.346 | 0.301 | (102) | 29.46312 |
| 5 | 43.277 | 0.1 | Unknown | 89.26423 |
| 6 | 46.254 | 0.315 | Unknown | 28.64268 |
| 7 | 47.962 | 0.1 | Unknown | 90.8126 |
| 8 | 50.621 | 0.343 | Unknown | 26.75936 |
| 9 | 55.106 | 0.341 | (110) | 27.4452 |
| 10 | 62.173 | 0.1 | (103) | 96.88787 |
| 11 | 64.94 | 0.1 | (112) | 98.3482 |
3.3. Phytochemical Analysis
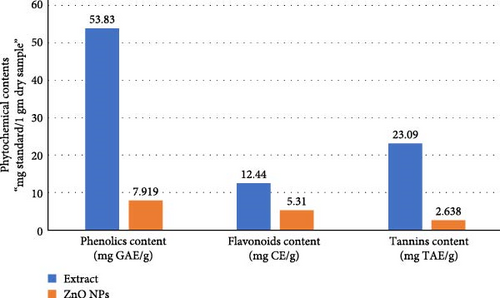
TFC was estimated in terms of catechin equivalents, TPC was measured in gallic acid equivalents per gram of dry weight of the extract, and the total tannin content was evaluated in terms of tannic acid equivalents (Figure 6). The results for the aqueous algal extract revealed the highest mean values of TFC (12.44 mg CE/g) and TPC (53.83 mg GAE/g) compared to ZnNPs. For ZnNPs, the results showed considerably lower mean values of TFC (5.31 mg CE/g) and TPC (7.919 mg GAE/g) than those of the algal extract. In addition, the highest values of tannin content were found in the algal extract (23.09 ± 1.26), compared to ZnNPs (2.638 ± 0.6; Figure 6).
3.4. Antioxidant Capacity
The antioxidant activities of P. boryanum extract, ZnNPs, and L-ascorbic acid are shown in Table 4 and Figure 7, It was observed that the algal extract exhibited the highest antioxidant activity in the DPPH assay compared to the biologically synthesized ZnNPs. The percent (%) remaining DPPH of algal extract decreased (0.54 mg mL−1–82.47%; 1.08 mg mL−1–63.85%; 2.16 mg mL−1–53.46% and 4.32 mg mL−1–17.75%) consistently with % increased the % scavenging activity of DPPH free radicals (0.54 mg mL−1–17.53%; 1.08 mg mL−1–36.15%; 2.16 mg mL−1–46.54% and 4.32 mg mL−1–82.25%) at concentration-dependently effect. Besides, % scavenging activity of DPPH free radicals of biosynthesized ZnNPs increased with an increase in the concentrations of ZnNPs (0.598 mg mL−1–8.874%; 1.195 mg mL−1–19.05%; 2.39 mg mL−1–30.95%; and 4.78 mg mL−1–42.86%). The % remaining DPPH of biosynthesized ZnNPs decreased appreciably along with increased concentrations (0.598 mg mL−1–91.13%; 1.195 mg mL−1–80.95%; 2.39 mg mL−1–69.05%; and 4.78 mg mL−1–57.14%). Nevertheless, the DPPH scavenging activity of ZnNPs was inferior to that of the algal extract and the standard antioxidant ascorbic acid (Figure 7A). In addition, the DPPH assay showed the scavenging effect of ZnNPs with a higher IC50 value of 5.844 ± 0.470 mg mL−1 than the IC50 value of L-ascorbic acid (0.022 ± 0.176 mg mL−1) and aqueous algal extract (1.883 ± 0.105 mg mL−1; Figure 7B and Table 4).
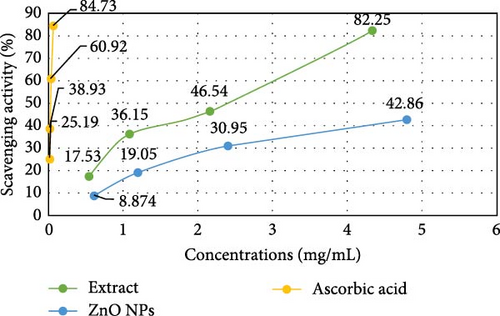
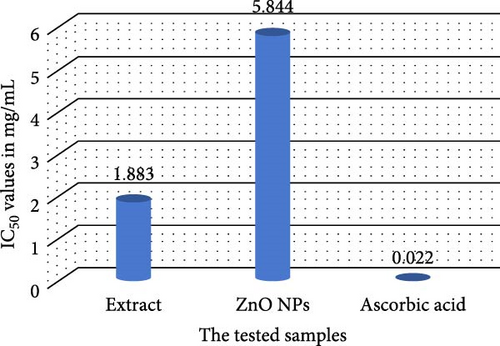
| Samples | Concentrations (mg/mL) | Remaining DPPH (%) | IC50 (mg mL−1) |
|---|---|---|---|
| Microalgal extract | 4.32 | 17.75 ± 1.33 | 1.883 ± 0.105 |
| 2.16 | 53.46 ± 2.38 | ||
| 1.08 | 63.85 ± 3.05 | ||
| 0.54 | 82.47 ± 2.01 | ||
| ZnNPs | 4.78 | 57.14 ± 2.94 | 5.844 ± 0.470 |
| 2.39 | 69.05 ± 2.00 | ||
| 1.195 | 80.95 ± 3.68 | ||
| 0.598 | 91.13 ± 1.24 | ||
| Ascorbic acid | 0.062 | 15.27 ± 0.48 | 0.022 ± 0.176 |
| 0.031 | 39.08 ± 0.19 | ||
| 0.016 | 61.07 ± 0.73 | ||
| 0.008 | 74.81 ± 1.16 | ||
- Note: Data are presented as mean ± standard deviation (SD). IC50: Concentration of the aqueous extract and synthesized nanoparticles causing 50% DPPH radical scavenging ability.
3.5. Antibacterial Efficacy
The antibacterial potential of P. boryanum crude extract and the biosynthesized ZnNPs were evaluated at lower concentrations against various bacterial microbial strains in fish using the MIC method. The MIC of ZnNPs was 1024 µg/mL for almost all bacterial isolates, excluding P. aeruginosa ATCC 9027. The MIC of the microalgal extract was 256 µg/mL for A. hydrophila and A. sobria and 128 µg/mL for A. veronii, S. iniae, and E. ictaluri. The MIC of vancomycin and thymoquinone were 8 µg/mL for all isolates, except P. aeruginosa ATCC 9027. None of the drugs effectively inhibited the growth of P. aeruginosa ATCC 9027 (Table 5).
| Pathogens | Pediastrum boryanum extract (μg/mL) | Algogenic ZnNPs (μg/mL) | |
|---|---|---|---|
| Type | Name | ||
| Gram −ve bacteria | Aeromonas sobria | 256 | 1024 |
| Aeromonas hydrophila | 1024 | ||
| Aeromonas veronii | 128 | 1024 | |
| Edwardsiella ictaluri | 1024 | ||
| Pseudomonas aeruginosa ATCC 9027 | — | — | |
| Gram +ve bacteria | Streptococcus iniae | 128 | 1024 |
- Abbreviation: MIC, minimum inhibitory concentration.
- ∗(−), MIC was not reached. The MIC of vancomycin and thymoquinone was 8 μg/mL for all tested strains.
3.6. Brine Shrimp Lethality
After 24 h of observation, shrimp nauplii exposed to the P. boryanum extract or ZnNPs showed a similar pattern, with significant concentration-dependent (p < 0.001 and p < 0.0001) mortalities (Figure 8). The mortality rates were 16%, 50%, 56%, and 100% at concentrations of 1, 10, 100, and 100 μg/mL, respectively. The mortality percentage was not significant (p > 0.05) between the concentrations of 10 and 100 μg/mL for both the algal extract and ZnNPs. Additionally, the mortality percentage significantly increased (p < 0.05) in shrimp nauplii exposed to ZnNPs compared to those exposed to the algal extract at a concentration of 10 μg/mL (Figure 8). Our findings confirmed a higher potential cytotoxicity of biosynthesized ZnNPs at 29.28 μg/mL compared to that of the algal extracts at 51.56 μg/mL (Table 6).
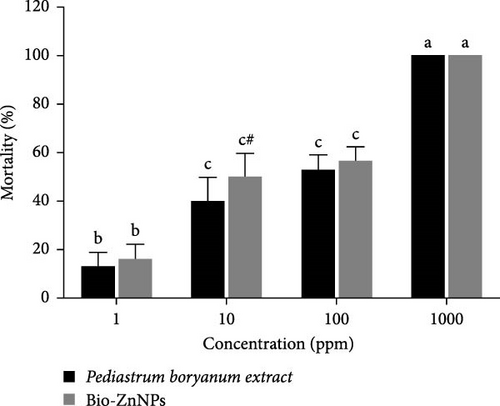
| Treatments | Concentrations (µg/mL) | Number of surviving nauplii after 24 h | Total number of survivors | Total number of dead nauplii | Percent Mortality (%) | LC50 (µg/mL) of mortality | ||
|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | ||||||
| Pediastrum boryanum extract | 1 | 8 | 9 | 9 | 26 | 4 | 13.33 | 51.56 |
| 10 | 6 | 5 | 7 | 18 | 12 | 40.00 | ||
| 100 | 5 | 4 | 5 | 14 | 16 | 53.33 | ||
| 1000 | 0 | 0 | 0 | 0 | 30 | 100 | ||
| Bio-ZnNPs | 1 | 8 | 8 | 9 | 25 | 5 | 16.66 | 29.28 |
| 10 | 5 | 4 | 6 | 15 | 15 | 50.00 | ||
| 100 | 5 | 4 | 4 | 13 | 17 | 56.66 | ||
| 1000 | 0 | 0 | 0 | 0 | 30 | 100 | ||
4. Discussion
The green synthesis of ZnNPs using algae extracts is accomplished via an unpretentious, cost-effective, and environmentally friendly method that requires minimal technical skills and equipment [51, 52]. Algal extracts act as reducing agents to reduce Zn ions (Zn2+) to form NPs and as potent stabilizing agents to prevent the agglomeration of NPs [53]. The key benefits of employing biosynthesized ZnNPs using algal extracts are their ability to remove heavy metals, organic pollutants, and pathogens from wastewater as well as catalysis, solar cells, and anticancer and antimicrobial coatings in the biomedical and cosmetic industries [1, 54, 55]. In this study, we synthesized ZnNPs using P. boryanum algal extract and determined their morphological, biological, and cytotoxic properties.
The synthesis of ZnNPs was confirmed at the nanoscale using a UV-Vis spectrophotometer to determine the absorbance or wavelength changes through surface plasmon resonance (SPR). An aqueous solution containing the algal extract and Zn2+ [1] showed a significant emission peak at 420 nm, while an excitonic absorption peak was observed at approximately 258 nm. A previous study reported a strong absorption emission band of prepared ZnNPs in an algal Cystoseira crinite extract solution at approximately 355 nm and an excitonic absorption peak at approximately 258 nm [12]. In recent publications, the presence of absorption bands between 230 and 390 nm is an indication of green-synthetized ZnNPs from microalgae [8, 56, 57].
The phytochemical constituents of the aqueous extract of P. boryanum microalgae comprise a wide range of secondary metabolites that are efficient metal-reducing and capping agents for fabricating robust coatings on MNPs [5, 58]. The study showed that the algal extract contains a higher amount of phenolics, flavonoids, and tannins based on the phytochemical analysis. Therefore, it could be used to biologically reduce and stabilize Zn metal ions on a nanoscale level. Polyphenol compounds possess an electron resonance hybrid effect, which is responsible for the green reduction of salt ions and the synthesis of their NPs in a single reactive step, subsequently maintaining these particles in a constant and non-precipitating format [33, 59, 60]. Flavonoids are a subclass of phenolic compounds that are highly stable and are utilized for the bio-reduction of Zn2+ with their conversion into NPs. They can aggregate on the surface of the fabricated NPs, depending on the number and position of free OH groups, and neutralize their charges to zero-valent molecules in the nanometer range to form very small-sized and active biological compounds along with increased surface area [35, 61, 62]. In addition, tannin content may improve the stabilization of Zn MNPs through green synthesis [63]. Concerning the prepared nano Zn particles, marked decreases were observed in the phenolic and flavonoid content compared to the algal P. boryanum extract. Similarly, lower flavonoid, phenol, and tannin contents were observed in ZnO NPs than in crude garlic extracts [35], as well as in aqueous stem extract of Ephedra aphylla plant [33]. A recent report documented that P. boryanum is a potentially rich source of phenolics, flavonoids, and tannins, which have reasonable antioxidant activity [6, 64].
The functional groups and chemical structures of P. boryanum-mediated ZnNPs fabrication were investigated using FTIR analysis. A recent study involving the FTIR spectra of ZnO NPs prepared from Spirogyra hyalina microalgal extract showed that the border peaks at 3400 cm−1 corresponded to the OH-hydroxyl group of alcohols or phenolic compounds, and the band at 1550–1600 cm−1 showed carbonyl stretching and amide absorbance peaks, whereas peaks from 1000 to 950 cm−1 corresponded to the C─N and C─H bending of aromatic and aliphatic stretching bonds of amino acids in the algal extract, which supports our findings [57]. The effective phytochemical synthesis of ZnNPs was confirmed by the characteristic Zn─O absorption band at 528 cm−1. Similarly, the absorbance peak at 500 cm−1 was attributed to Zn─O fabricated from Arthrospira platensis algal extract [8]. Our data confirmed that the bioreduction of Zn2+ and capping of NPs are mediated via the interaction of oxygen in the functional OH groups of flavonoids and other organic substances in algae [11, 65, 66]. In addition, the free carbonyl and amide residue groups in the formed Zn nanoscale indicate the binding ability to the metal after protein layer formation around the metal to prohibit agglomeration and stabilize the NPs [67], suggesting that the naturally occurring organic compounds in the algal extract played a crucial role in the synthesis and stabilization of the ZnNPs.
The particle shape and size were characterized by TEM micrography and XRD analysis. TEM offers a direct visualization of the morphology of the entire particle, including its overall size and the potential presence of amorphous regions. XRD provides information on the size of the coherent scattering domain (crystallite size). Therefore, the size measured by TEM can be larger than the crystallite size determined by XRD owing to the polycrystalline nature of the NPs. In terms of microscopic characterization of ZnNPs, TEM is a high-resolution imaging technique that is particularly useful for studying the morphological features of NPs [68]. The presence of spherical rod-shaped particles with a grain size of 24.78–32.84 nm was observed using TEM micrographs. The synthesis of ZnO NPs using Beta vulgaris plants resulted in spherical shapes with large and small particles within the range of 30 ± 3 nm [68]. Moreover, TEM image analysis demonstrated that hexagonal shapes and an average size of 42 nm of ZnO NPs were generated using an aqueous algal extract of Cystoseira crinite [12]. This emphasizes that the aqueous extract of P. boryanum can produce spherical ZnNPs. Moreover, our results are in agreement with previous reports that assayed the efficacy of metabolite content in algal cell extracts to produce spherical ZnNPs [8, 69]. XRD analysis is another important characterization tool for determining the structural properties and crystallite size of ZnNPs [48, 70]. XRD results concerning the sharp and narrow diffraction peaks indicated that the algo-synthesized ZnNPs had pure crystallinity in nature and a spherical hexagonal phase (wurtzite structure) structure with lattice parameters. However, the presence of unknown peaks in the XRD pattern suggests that the synthesis process might not be fully complete, and the results of the crystalline size and zeta potential analysis indicate that the synthesized ZnNPs have desirable properties. Therefore, the presence of these unknown peaks does not seem to have a significant impact on the overall quality of the synthesized Zn NPs.
Zeta potential analysis also showed that the ZnNPs synthesized using P. boryanum extract had a high zeta potential value of −21.5 mV, indicating relative stability [1]. This stability is important for the potential applications of these ZnNPs. ZnO nano-samples formed using rambutan (Nephelium lappaceum) peel extract, as previously reported, showed a similar particle size of approximately 50 nm in XRD analysis [71]. Nevertheless, our findings are in agreement with numerous studies that have assayed similar XRD patterns of phycosynthesized ZnO NPs [12, 38, 72, 73].
Zeta potential analysis of biosynthesized ZnNPs is a typical measurement of the surface charge on a particle and colloidal stability in suspensions [74, 75]. In our study, the zeta potential of the bio-ZnNPs was measured as −21.6 mV, which can be verified as strongly anionic, and thus confers the dispersion capacity of the algogenic ZnNPs in the aqueous solution. The zeta potential of green synthesized ZnO NPs using extracts of Myristica fragrans was evaluated as −22.1 mV, which is considered a moderately stable colloidal solution [75]. Generally, the binding affinity of microalgal extract compounds with NPs is supported by the negative surface charge, which intensifies the stability of ZnNPs and mitigates their aggregation potential [76, 77]. Additionally, there is an intimate relationship between the functional metabolites (carbohydrates, polysaccharides, etc.) of algal plants absorbed on the surface of the formed NPs and zeta potential [75, 78].
The DPPH assay is commonly utilized to evaluate the free radical scavenging activity of NPs, even at lower concentrations [79, 80], through the screening of the antioxidant potential of algal extracts and ZnNPs. Under normal conditions, DPPH represents a stable nitrogen-free radical that scavenges electrons or hydrogen radicals to generate stabilized diamagnetic compounds that can reduce the levels of other reactive nitrogen species in living cells [81, 82]. In our study, the radical scavenging activity of ZnNPs increased dose-dependently. A significant result was observed at 4.78 mg/mL; however, it was significantly lower than the algal extract and ascorbic acid. In addition, the findings of antioxidant activity were consistent with those of TFC and TPC of the algal extract and its bio-ZnNPs. The antioxidant property of the aqueous algal extract was higher than that of algal-based ZnNPs, which is linked to its higher TPC and TFC. A previous study confirmed that free phenolic compounds from P. boryanum microalga contribute to 90% of the scavenging activity in the DPPH assay [6]. A study with similar findings showed that the scavenging activity of DPPH radicals by ZnO NPs synthesized in Ceropegia candelabrum plants rose as the concentration increased from 0% to 55.43% [83]. The potent ability to scavenge free radicals has been demonstrated in ZnNPs synthesized from the leaf extracts of Phragmanthera austroarabica, Aspergillus carneus, and Curcuma longa [73, 84, 85], which could be related to the presence of potent capping and oxidizing agents on their surfaces and their tendency to lose electrons [57].
Meanwhile, the algal extract showed a lower IC50 value than that of ZnNPs, revealing significant scavenging activity, as a lower IC50 of a compound is meant to be a remarkable ROS scavenger [77]. Indeed, P. boryanum possesses the greatest DPPH radical scavenging activity (IC50: 1.883 mg/mL) and superior antioxidant potential to ZnNPs (IC50: 5.844 mg/mL), which may be due to various bioactive compounds and the higher phenolics and flavonoids present in P. boryanum microalgae. Additionally, IC50 value of Pelargonium odoratissimum leaf extract (4.56 µg mL−1) exhibited higher antioxidants than the bio synthesized ZnO NPs, which had an IC50 value of 28.11 µg mL−1 [86]. Based on these findings, oxidative stress can be inhibited by ZnNPs mediated by algal extracts by activating their phenolic and flavonoid molecules, which act as metal chelators (through electron donation) and natural scavengers of ROS [87–89]. Consequently, the ZnNPs produced through the use of P. boryanum algal extract can serve as a powerful natural antioxidant to safeguard health from oxidative reactions. It was observed that the MIC of the algal extract (128 μg/mL) had a more potent antimicrobial activity than bio-ZnNPs (1024 μg/mL) against various fish bacterial pathogenic strains; however, the higher concentration of ZnNPs displayed a significant bactericidal effect. Our results are consistent with those of previous studies [33, 90]. The MIC of ZnONPs synthesized using plant lychee extract at around 500 μg/mL notably reduced the proliferation of the following microorganisms: P. aeruginosa, Bacillus subtilis, S. aureus, and E. coli [91]. Algal Cystoseira crinite-facilitated ZnO NPs demonstrated significant antimicrobial activity against a range of pathogens including Bacillus cereus, Staphylococcus aureus, Escherichia coli, and Salmonella typhi. This activity was observed at MICs spanning from 312.5 to 1250 μg/mL [12]. As has been noted before, the improvement in the antimicrobial activity of ZnNPs can be attributed to their unique characteristics, including the ratio of specific surface area to volume, the dimensions of the particles, the concentration of the powder, and their form [48, 92]. Therefore, the inhibitory bacterial growth of ZnNPs was due to the physical interactions of the NPs with the bacterial cell membranes [33, 93]. Additionally, concentration-dependent effects can be suggested, where at lower concentrations, ZnNPs may act as antioxidants, while at higher concentrations, they can generate ROS and exhibit antibacterial activity [94]. Therefore, the increase in the generated H2O2 molecules from the surface of ZnO, with subsequent production of ROS, can penetrate the cellular membrane components and damage the bacteria [12]. Moreover, Zn2+ release, in which the interaction between the negatively charged bacterial cell membrane and positively charged Zn2+ can also disrupt the membrane permeability and integrity of the bacterial membrane [95, 96]. These contradictions in the antioxidative and antibacterial properties of ZnNPs highlight the complex and multifaceted nature of ZnNPs interactions with biological systems.
Recently, the employment of brine shrimp larvae has become a prevalent approach for evaluating the safety and toxicity of plant extracts through the investigation of their pharmacological properties [97, 98]. An increase in the concentration of the extract of Abies webbiana-mediated ZnO NPs (40–80 µg/mL) led to increased cytotoxicity against artemia salina nauplii [99]. These results are supported by similar studies on green-synthesized ZnONPs [75, 100, 101]. In the present study, the lethality concentration was directly proportional to the mortality % of ZnNPs and algal extract. Thus, the observed lethality of our compounds, P. boryanum crude extracts (51.56 μg/mL) and synthesized ZnNPs (29.28 μg/mL) to brine shrimps, indicated the presence of substantial cytotoxicity role. The current study aligns with previous research in reporting similar LC50 values for biosynthesized ZnNPs [102, 103]. The general results of the brine shrimp assay were interpreted as follows: LC50 < 1 µg/mL for highly toxic compounds, LC50 1–10 µg/mL for toxic compounds, LC50 10–30 µg/mL for moderately toxic compounds, LC50 30–100 µg/mL for mildly toxic compounds, and >100 µg/mL for nontoxic [103, 104]. Therefore, the algal extract was considered mildly toxic, whereas the Bio-Zn NPs were considered moderately toxic. NPs cytotoxicity may be linked to the substantial surface area of NPs, which facilitates their infiltration into cells where they disrupt the signaling pathways of cellular components and induce an increase in the generation of ROS [101, 105]. The cytotoxic effect of ZnNPs on shrimp larvae could be associated with anticancer activity, suggesting that ZnNPs may provide an alternative source of anticancer medications. However, this hypothesis requires further investigation.
While the current data suggest a potential conflict between the antibacterial efficacy and toxicity of ZnNPs, many explanations can be elucidated herein: (1) the antibacterial concentration (>1000 µg/mL) was determined in vitro but may differ in vivo, altering the NP behavior and efficacy; (2) brine shrimp (Artemia) is often used as a toxicity model, although their sensitivity may not apply to fish species, and fish may tolerate ZnNPs differently; (3) ZnNPs exposure and toxicity depend on how fish receive them—feed, water, or injection; and (4) the size, shape, and surface characteristics of ZnNPs affect their toxicity and antibacterial activity. Previous explanations do not necessarily preclude the use of ZnNP in fish. However, this highlights the need for careful consideration and further research to develop safe and effective applications of ZnNPs as antibacterial agents in aquaculture.
5. Conclusion
In summary, our study demonstrated that P. boryanum is a multifunctional microalga that functions as a proficient environment-friendly nano-factory to produce biogenic ZnNPs, characterized by well-defined structural attributes and substantial bioactive impacts. The use of P. boryanum extract as the reducing and capping agent enabled the plant-mediated synthesis of stable, well-dispersed ZnNPs with a well-defined morphology, as confirmed by spectroscopic and microscopic analyses. Examination of the phytochemical composition of the extract revealed that it contained higher TPC, TFC, and DPPH than Zn NPs, suggesting that these compounds are involved in NP formation. Moreover, the dual nature of ZnNPs, acting as both antioxidants at lower concentrations and exhibiting antibacterial activity at higher concentrations, highlights their complexity in biological systems. This characteristic can be harnessed strategically for therapeutic applications, and the cytotoxicity observed against brine shrimp suggests a potential link to anticancer activity. Biosynthesized ZnNPs may be viable biosafe candidates for a variety of biological activities; however, their pharmacological significance requires further investigation. This knowledge will help to optimize their use in different ecological contexts and ensure that they are integrated responsibly into environmental practices and biomedical applications. Further research is essential to explore their interactions within biological systems and to evaluate their long-term effects on both health and the ecosystem.
Ethics Statement
This study did not require the use of animal or human subjects.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Eman Zahran: conceptualization, formal analysis, validation, review, editing, and correspondence. Samia Elbahnaswy: methodology, investigation, and writing of the initial draft. Manar Elsayed, Nehal A. Saif, and Mohamed Elhadidy: antibacterial efficacy assays, contributed to the writing of the initial draft. Engy Risha, Hanan H. Abdelhafeez, Ferdaus Mohd Altaf Hossain, and Abdallah Tageldein Mansour: investigation, validation, and resources. Fatma Ahmed: reviewing and editing. All the authors have read and approved the final manuscript.
Funding
No funding was received for this research.
Acknowledgments
The authors thank the organizations and individuals who provided in-kind support for this study.
Open Research
Data Availability Statement
All data supporting the findings of this study are available within the paper.




