Influence of Multistrain Probiotics on Growth, Hematology, Gut and Liver Morphometry, and GH and IGFs Genes Expression in Rohu (Labeo Rohita) Fry
Abstract
Probiotic is regarded as an effective feed additive to increase the growth, feeding efficiency, survivability, and overall productivity of fishes in aquaculture. The following study was intended to examine the effects of multistrain probiotics on growth, blood indices, histomorphometry of gastrointestinal tract and liver, and growth hormone (GH) and insulin-like growth factors (IGFs) genes expression in rohu Labeo rohita. Probiotic containing Bacillus subtilis, B. thuringiencis, Lactobacillus plantarum, and L. buchneri was inoculated in water and three different doses, that is, 0 mL/L (control), 0.5 mL/L (lower dose, T1), and 1.0 mL/L (higher dose, T2) were used in this study. Each treatment had three replicates and fishes were reared in aquarium for 60 days. Results revealed that substantially higher weight gain (WG) and specific growth rate (SGR) along with least apparent feed conversion ratio (AFC) were documented in probiotic treated fish which was significantly different than that of control, however, higher dose (1.0 mL/L) showed best performance. Blood–biochemical indices such as red blood cell (RBC), white blood cell (WBC), hemoglobin (Hb), and glucose (Glu) level were observed statistically higher in probiotic additive culture water. Moreover, the inoculation of multispecies probiotic positively altered the intestinal histometry. Besides, immune response features of intestine were observed significantly higher in probiotic treated fish. The nucleus shape and distances between liver tissues (DLTs) were also improved with the upward doses of probiotic application. The mRNAs levels of GH in the pituitary and IGF-1 and IGF-2 in the liver were significantly higher in probiotic treated fish. In a nutshell, this study suggested that the inclusion of multistrain probiotics showed promising improvement in growth performance and feed conversion efficiency; additionally, uplifted the intestinal structure integrity, liver morphology, and GH and IGFs genes expression in rohu.
1. Introduction
Aquaculture has been addressed as the most promising sector that provides safe animal nutrition to human and plays a significant role in tackling global increasing food security problem [1]. The intensification of aquaculture is often resulting decreased profitability due to additional expenditure on various inputs such as feed, seed, antibiotics for disease prevention, and water deterioration management [2, 3]. The application of probiotics either with feed or in water is a recent development that is being extensively applied in aquafarming [4]. It has been successfully used as nutritional supplement, an effective alternative to antibiotics for combating pathogen invasion and to check water quality deterioration [5]. Many scientific documentations have proven that probiotics have immense effects on the growth promotion and feed utilization through increased enzymatic activity, improvement of gut health, immune system building, disease prevention, and maintenance of water quality [6, 7]. Particularly, probiotics take care the health of intestine and liver by improving the morphology and their functioning that significantly alters the feed digestion, assimilation, blood parameters, and host immunity [8]. The improved liver and intestinal conditions ultimately contribute to better growth, disease resistance, and survivability [9]. Probiotic additive in water is also used as feed supplement that minimize the feed cost and its secreted enzyme maximize the utilization of feed as well [10–12]. Therefore, in brief, probiotic supplementation in aquaculture unit enhances feed utilization, growth, survival (SR), intestinal condition, and disease prevention of aquaculture species [13, 14].
Although the use of single bacterial species is common, the usages of multispecies probiotics is rather new concept and a very few attempts have been made earlier [4]. Studies have been assumed that the integration of two or more probiotic species bring more productivity over single species usage because they not only enhance growth, feed efficiency, and SR, but also boost immunity against pathogenic organisms [12, 15]. For instances, the administration of Bacillus sp. and Lactobacillus sp. either single or mixed were reported to increase weight gain (WG), specific growth rate (SGR), and SR [9, 16]; digestion, absorption, and feed efficiency [13, 17]; intestinal microbiota and morphology [18, 19]; stress tolerance and immunity [20, 21]; and hematological parameters [1, 22, 23] in many fishes and shellfishes. Growth hormone (GH) production from the pituitary regulates somatic growth, growth of organs and tissues, and metabolic processes in fish [24]. Plasma insulin-like growth factors (IGFs), which are generated from the liver in response to circulating GH, mediate the majority of these biological processes. Indeed, it has already been proposed that the GH/IGFs axis might be employed as a marker of growth performance and nutritional status in aquaculture [25]. In addition, GH may be stimulating IGFs in nearby cells and between them in order to promote growth of fishes [26, 27].
Labeo rohita (known as rohu) is one of the popular Indian major carps and it has high consumer acceptance due its great nutritional value, fabulous taste, and comparatively lower market price [28]. However, a very few studies has been conducted regarding the administration of probiotic on the culture of L. rohita; where mostly comprising single species probiotic administration such as the effect of B. subtilis on the hematological and serum parameters [29]; the effect of L. plantarum on growth and disease resistance [30], the effect of probiotics, yeast, seaweed and enzyme on the growth and SR of rohu fries [31]; and the effect of three Bacillus spp. on growth, enzyme activity, gut morphology, and disease resistance [22]. From above discussion, it has been evinced that the bacteria genus Bacillus and Lactobacillus have independent positive effects on the growth, SR, histomorphometry, and disease resistance of carp [28, 30]. However, the combined effects of genus Bacillus and Lactobacillus on fish growth indices, blood parameters, and immunity are yet to be conducted. Therefore, this study was intended to picturize the effects of multispecies probiotic (B. subtilis, B. thuringiencis, L. plantarum, and L. buchneri) on the growth performance, feed utilization, survivability, hematological indices, intestinal and liver morphometry, and GH and IGFs genes expression in L. rohita.
2. Materials and Methods
2.1. Experimental Design
One hundred eighty (180) apparently healthy rohu (L. rohita) fish fries having approximate body weight of 0.82 ± 0.01 g were purchased from Fisheries Field Complex Hatchery, Bangladesh Agricultural University (BAU) and carefully carried to Laboratory of Fish Ecophysiology, BAU, Bangladesh. Fish fries were acclimatized in the laboratory environment for 15 days before the initiation of this experiment. Nine (09) aquaria (dimension: 75 cm × 45 cm × 45 cm) filled with 100-L clean tap water, and 20 rohu fries were stocked in each aquarium. Multispecies probiotic used in this experiment containing Bacillus subtilis (5 × 109 CFU/mL) and B. thuringiencis (4 × 109 CFU/mL) were previously isolated from intestine of L. rohita, whereas Lactobacillus plantarum (5.8 × 109 CFU/mL) and L. buchneri (6.5 × 1011 CFU/mL) were isolated from yogurt. Initially, the liquid probiotics were further cultured with rice bran and molasses for 1 day and added to the aquarium water. Three different probiotic treatments, that is, 0 mL/L (control), 0.5 mL/L (lower dose, T1), and 1.0 mL/L (higher dose, T2) were inoculated in culture aquaria for a duration of 60 days (Figure 1). During the experimental period, probiotic was inoculated in alternative days whenever necessary, if floc is more than 3.5% in Imhoff cone test. Fish were fed with commercial (Quality Feed Ltd., Dhaka, Bangladesh) diet (moisture 10%, protein 40%, carbohydrate 20%, fat 8%, fiber 4%, ash 15%, calcium 2.0%, and phosphorous 1.0%) daily two times (9.00 am and 5.00 pm) considering 5% body weight of group biomass; fish ration was adjusted fortnightly based on body weight.
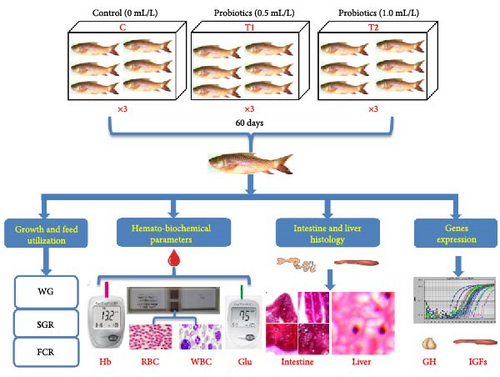
2.2. Maintenance and Ethical Consideration
Utmost care has been taken throughout the experiment to avoid cross contamination among treatments. Cleaning of feces and unused feed were done through siphoning in every alternative day to avoid ammonia poisoning. Several important water quality parameters, including temperature (28.5 ± 0.5°C), pH (7.85 ± 0.07), and NH3 (0.25 ± 0.03 mg/L), were checked once a day. Constant oxygen supply by aeration was maintained for the entire rearing period. During experiment, the guidelines of animal ethics provided by ethical and animal welfare committee of BAU, Bangladesh, were strictly followed.
2.3. SR, Feed Utilization, and Growth Parameters
- •
WG = final weight (g) − initial weight (g);
- •
SGR (%/day) = logn (final weight (g)) − logn (starting weight (g))/culture period × 100;
- •
Apparent feed conversion ratio (AFC) = dry feed fed (g)/live WG (g); and
- •
SR (%) = (number of fish harvested/number of fish stocked) × 100.
2.4. Hematometric Study
Ten fish (n = 10) from each sample were carefully handled and anesthetized using 5 mg/L clove oil containing water in order to reduce initial stress. The blood samples were then drawn by cutting the caudal peduncle and blood was pushed to collect in vials containing 20 mM EDTA (anticoagulant agent). Thereafter, red blood cells (no. of RBCs; ×106/mm3), white blood cells (no. of WBCs; ×103/mm3), hemoglobin level (Hb; g/dL), and blood glucose (Glu; mg/dL) were measured from preserved blood samples. Blood Glu and Hb concentration were determined using digital meter (EasyMate, model ET232, Bioptic Technology Inc. Taiwan) using separate strips. Samples of blood were preserved in Eppendorf tubes containing 20 mM EDTA (anticoagulant) for counting RBCs and WBCs, and subsequently counted under a light microscope using hemocytometer.
2.5. Histometry of Intestine and Liver
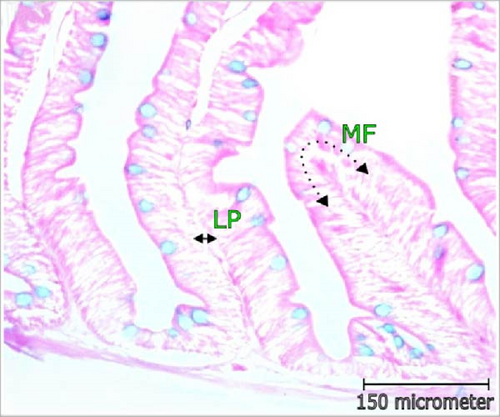
For histometric observation, five fishes (n = 5) were euthanized and ventral side incision was made. The whole intestine and liver were detached carefully by maintaining proper hygiene. Before tissue processing, fixation was performed in Bouin’s solution for 24 h, subsequently, preserved in labeled vials containing 70% alcohol and kept in refrigerator at 4°C. In order to remove water, thereafter, the tissues were dehydrated in ascending graduation of alcohol treatments and soaked in chloroform eventually. The dehydrated tissues were hardened using liquid wax and then blocks were made. The blocks were sectioned with a thickness of 5 μm in a microtome machine. Xylene treatments were followed for 5 min to eradicate wax from sections and passed through a series of descending series of alcohol treatments. Last, staining of tissue sections were performed using hematoxylin-eosin stain. Moreover, Alcian blue was also used to stain goblet cells (GCs) present in intestinal epithelium tissue. The histometric sectioning of tissues were observed under microscope (Micros, model no. MCX 100, Austria) equipped with camera (AmScope 1000). Various histomorphometric features of intestine, that is, villus area (VA; μm2), villus length (VL; μm), villus width (VW; μm), crypt depth (CD; μm), and thickness of the intestinal wall (TW; μm) and muscles (TM; μm) were determined. Other immunity indicating parameters such as mucosal fold (MF; μm), number of GCs, lamina propia width (μm), and width of enterocyte (EC; μm) were estimated. Liver cell nucleus condition and distance among tissues (μm) were also determined.
2.6. Analysis of GH, IGF-1, and IGF-2 mRNAs Expression by Real-Time PCR
The procedure of sample collection, preservation, RNA extraction, cDNA preparation, and real-time PCR are described in details by Shahjahan et al. [33]. Briefly, the pituitary and liver of sampled fish were stored at −20°C after soaked overnight in RNAlater (Ambion, Austin, TX) at 4°C. Total RNA was isolated using the SV total RNA isolation system (Promega, USA), in accordance with the provided instructions. The concentration of the extracted RNA was determined using a NanoDrop Spectrophotometer ND-1000 UV/Vis’s instrument. Following the GoScript Reverse Transcription System (Promega, USA), 500 ng total RNA were utilized to create the cDNA. In order to do real-time PCR, qTOWER3 series of real time thermal cyclers was used. The primers used to test the expression of the genes for GH, IGF-1, and IGF-2 were chosen from earlier investigations [33]. The PCR reaction mixture (10 µL) contains 1 µL of standard cDNA of sample, 0.4 µL of forward and reverse primers, and 5 µL of TE SYBR DE premix (TaKaRa, Ohtsu, Japan). Amplification was performed at 95°C for 30 s, followed by 40 cycles at 95°C for 15 s, 56°C for 15 s, and 60°C for 20 s. The ∆∆Ct technique was employed to standardize expression levels, while β-actin served as a housekeeping gene.
2.7. Statistical Analysis
Normality and homogeneity of obtained data were tested employing Levene’s and Shapiro–Wilks test in order to ensure normal distribution and less variability in data. The calculated results were presented in mean ± standard deviation (SD). One way analysis of variance (ANOVA) were calculated among treatments at 5% confidence interval (post hoc Duncan multiple range test). All other statistical analysis was performed using SPSS, ver. 22.0. Minitab statistical software version 20 was used for principal component analysis (PCA).
3. Results
3.1. Growth, SR, and Feed Utilization
The results of growth parameters, feeding efficiency, and survivability are presented in Table 1. The growth indices such as final body weight (FBW; g), WG (g), and SGR (%/day) were found comparatively better in both probiotic additives. The lower mean AFC indicated better feed utilization in probiotic treatment as well which significantly different (p < 0.05) from control. Survivability of rohu was found comparatively greatest in higher dose (1.0 mL/L), showed no notable (p > 0.05) difference from other treatments.
| Parameters | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| Initial body weight (g) | 0.83 ± 0.00a | 0.82 ± 0.00a | 0.84 ± 0.00a |
| Final body weight (FBW; g) | 2.12 ± 0.67a | 4.36 ± 1.05b | 5.76 ± 1.94b |
| Weight gain (WG; g) | 1.29 ± 0.67a | 3.56 ± 1.05b | 4.92 ± 1.94b |
| Specific growth rate (SGR; %/day) | 0.70 ± 0.22a | 1.30 ± 0.18b | 1.46 ± 0.23b |
| Apparent feed conversion ratio (AFC) | 2.50 ± 1.89b | 1.45 ± 0.42a | 1.08 ± 0.33a |
| Survival (SR; %) | 96.0a | 98.0a | 100.0a |
- Note: The superscript alphabets indicate significant (p < 0.05) variations among different treatments in a row (post hoc Duncan multiple range test).
3.2. Hematometric Study
The findings of hemato-biochemical indices of rohu reared with multispecies probiotic treatments are shown in Table 2. The highest abundance of RBCs (1.93 × 106/mm3) and WBCs (2.53 × 103/mm3) were estimated in higher dose treatment (T2). One way ANOVA showed statistical variation in probiotic treated fish from control (p < 0.05) in terms of cell count. The maximum mean level of Glu and Hb was recorded in T1 (114.6 mg/dL) and T2 (11.78 g/dL), respectively. The results of Glu and Hb remained insignificant between probiotic treatments (p > 0.05), whereas significant with control.
| Parameters | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| RBC (×106/mm3) | 0.97 ± 0.14a | 1.63 ± 0.08b | 1.93 ± 0.08b |
| WBC (×103/mm3) | 1.83 ± 0.36a | 2.30 ± 0.45b | 2.53 ± 0.31b |
| Hb (g/dL) | 9.48 ± 1.42a | 11.28 ± 2.11b | 11.78 ± 1.35b |
| Glu (mg/dL) | 85.80 ± 8.47a | 114.60 ± 5.37b | 112.00 ± 7.24b |
- Note: The superscript alphabets indicate significant (p < 0.05) variations among different treatments in a row (post hoc Duncan multiple range test).
- Abbreviations: Glu, glucose; Hb, hemoglobin; RBC, red blood cell; WBC, white blood cell.
3.3. Changes in Histomorphology of Intestine
Histomorphometric alterations of intestine observed in L. rohita are illustrated in Table 3 and Figure 2. The CD and dimensions of villi (area, length, and width) along with the TW and TM was found improved with the increasing concentration of probiotic treatment. The maximum VL (168.0 μm), width (58.75 µm), and area (12.0 mm2) were recorded in higher dose probiotic additive (1.0 mL/L). Incorporation of probiotics had positive influence (p < 0.05) on the villi’s length, width, and area over control. Cryptic depth and thickness of gut wall and muscle were also found greatest in T2; reported significant variation from control (p < 0.05).
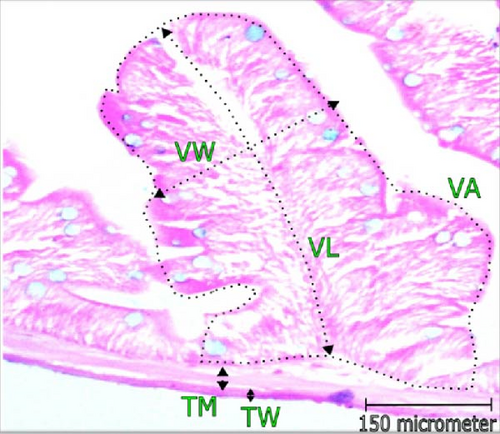
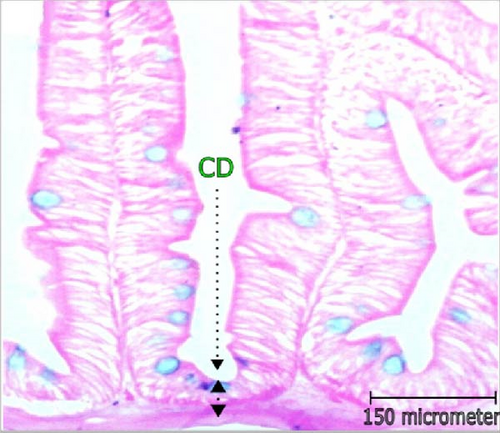
| Parameters | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| Villus length (VL; μm) | 125.25 ± 4.27a | 156.00 ± 4.32ab | 168.00 ± 5.12b |
| Villus width (VW; μm) | 38.50 ± 3.67a | 49.25 ± 2.21ab | 58.75 ± 2.75b |
| Villus area (VA; mm2) | 4.41 ± 0.22a | 9.10 ± 0.55ab | 12.00 ± 0.97b |
| Thickness of wall (TW; μm) | 5.00 ± 2.11a | 7.90 ± 0.72ab | 12.01 ± 1.32b |
| Thickness of muscle (TM; μm) | 14.25 ± 0.95a | 21.75 ± 2.22ab | 30.50 ± 1.29b |
| Crypt depth (CD; μm) | 17.02 ± 3.32a | 23.77 ± 1.87ab | 34.86 ± 2.88b |
- Note: The superscript alphabets indicate significant (p < 0.05) variations among different treatments in a row (post hoc Duncan multiple range test).
3.4. Immune Response Indicators in Intestine
The abundance of mucus producing GCs was highest in T2 which was around two times higher than control. Fattening of MF (µm), width of lamina propia (µm), and EC width (µm) increased significantly (p < 0.05) with multispecies probiotic additives and recorded greatest in 1.0 mL/L probiotic supplement. The results of intestinal GC abundance, fattening of MF (µm), EC width (µm), and width of lamina propria (LP; µm) are depicted in Table 4 and Figure 3.
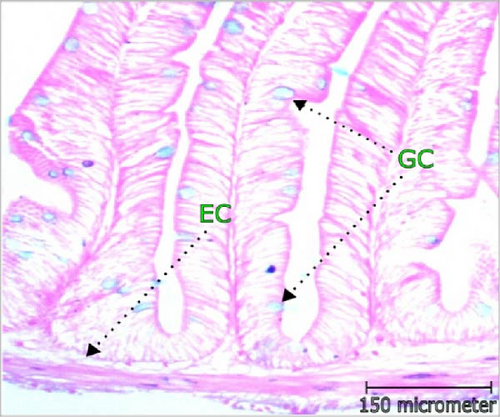
| Parameters | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| Fattening of mucosal fold (MF; μm) | 45.11 ± 1.22a | 52.70 ± 1.87ab | 58.01 ± 2.63b |
| Abundance of goblet cell (GC) | 22.34 ± 2.77a | 37.00 ± 1.40ab | 44.00 ± 4.52b |
| Width of lamina propria (LP; μm) | 16.55 ± 0.98a | 19.82 ± 1.73ab | 23.90 ± 0.57b |
| Enterocyte (EC) width (μm) | 6.94 ± 0.66a | 10.70 ± 0.43ab | 14.01 ± 1.22b |
- Note: The superscript alphabets indicate significant (p < 0.05) variations among different treatments in a row (post hoc Duncan multiple range test).
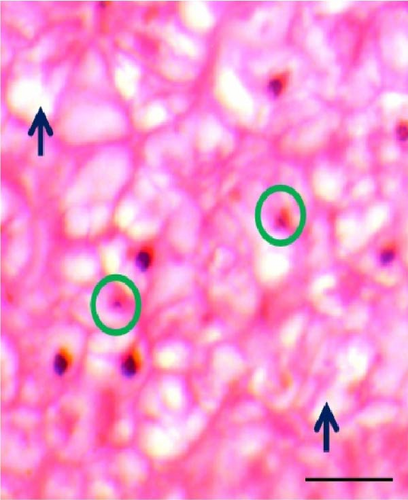
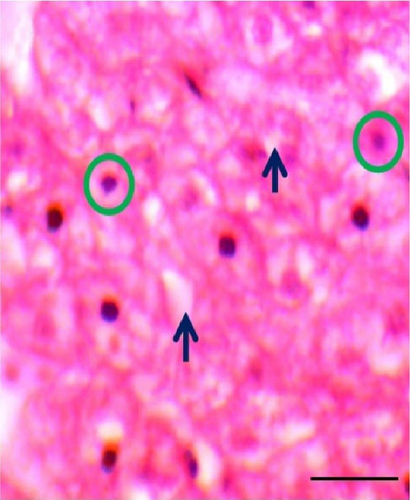
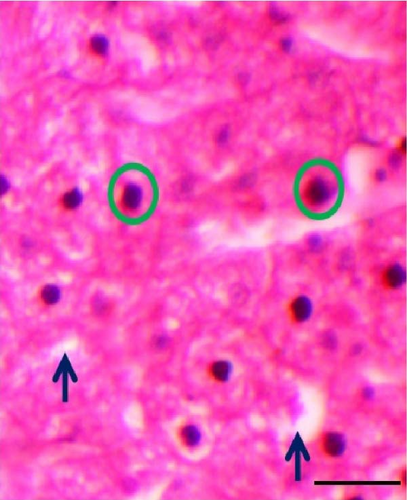
3.5. Histomorphometric Observation of Liver
The shape of liver cells found mostly regular in higher dose probiotic supplement, whereas the amount irregular shaped nucleus (ISN) remained highest (21.01 ± 1.50) in control. The distance between liver tissues (DLTs) were significantly different (p < 0.05) in multispecies probiotic administration from control. The histomorphological modifications of liver recorded in rohu (L. rohita) are exhibited in Table 5 and Figure 4.
| Parameters | Treatments | ||
|---|---|---|---|
| Control | T1 | T2 | |
| Irregular shaped nucleus (ISN) | 21.01 ± 1.50b | 16.05 ± 0.78ab | 11.00 ± 0.62a |
| Distance of liver tissue (DLT; μm) | 28.76 ± 4.09b | 19.11 ± 3.31ab | 12.44 ± 3.04a |
- Note: The superscript alphabets indicate significant (p < 0.05) variations among different treatments in a row (post hoc Duncan multiple range test).
3.6. mRNA Expressions of GH in the Pituitary
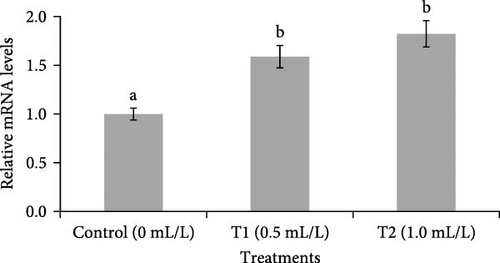
Changes in the expression of GH mRNA in the pituitary are shown in Figure 5. A significantly (p < 0.05) higher GH mRNA level was observed in the pituitary of fish reared in both concentrations of probiotics (0.5 and 1.0 mL/L), compared to control group (0 mL/L).
3.7. mRNA Expressions of IGF-1 and IGF-2 in the Liver
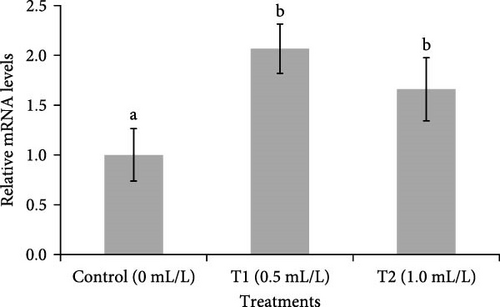
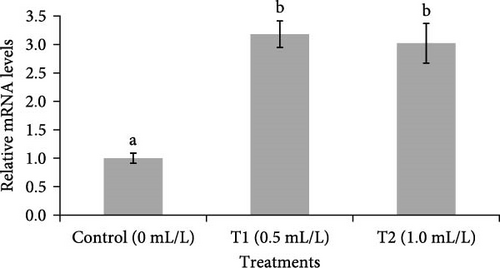
Figure 6 showed the changes in mRNA expression of IGF-1 and IGF-2 in the liver of fish. The expressions of IGF-1 and IGF-2 mRNA were significantly (p < 0.05) higher in the liver of fish reared in both concentrations of probiotics (0.5 ml/L and 1.0 ml/L), compared to control group (0 mL/L).
3.8. PCA
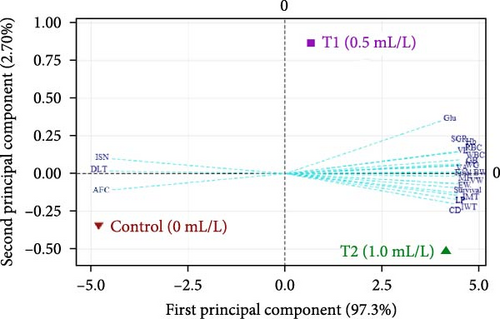
Biplot of growth, feed conversion ratio, SR, histomorphometry, immune response, and blood related indices of L. rohita fries is shown in Figure 7. The cumulative variance (100%) was contributed altogether by first principal component (97.30%) and second principal component (2.70%). The plot illustrated that the application of multispecies probiotic with different doses drawn positive changes in growth performance (FBW, WG, and SGR), SR, intestine, liver morphometry, and hematology. Whereas, comparatively poor feed utilization (AFC) and liver morphometric parameters (irregular nuclear shape, ISN, and DLTs) were distinguished control (no probiotic) from probiotic treatments.
4. Discussion
Multispecies probiotic additives in aquaculture are reported to be beneficial for the escalation of growth, feed utilization, SR, immunity, intestinal microbiota, and disease resistance of many fishes and shellfishes [34]. Among several significant genus, different species from genus Bacillus and Lactobacillus either in singular or in combination have been extensively studied for carp culture in recent times [22, 30]. It has been suggested that the probiotic additives of B. subtilis improved FBW, SGR, feed utilization, and protein conversion efficiency, whereas L. rhamnosus increased immunity factors such as serum lysozyme, phagocytic activities, and other blood complement in rainbow trout [35]. Besides, usage of different bacterial species with comparative higher dose might be more advantageous to elevate the performance of fish species [36]. Taking this into account, this research was, therefore, conducted considering two gradual doses of B. subtilis, B. thuringiencis, Lactobacillus buchneri, and L. plantarum in combination on L. rohita for duration of 60 days. The result of this experiment showed noteworthy increase in growth, feed usage efficiency, survivability, histomorphometric features, hematometric indices, and immunity.
The colonies of bacteria especially Bacillus spp. and Lactobacillus spp. are evident to release extracellular digestive hydrolytic enzymes like amylase, cellulose, protease, trypsin, lipase, phosphatase, and peptidase in the intestine of fish [37, 38]. According to Ray, Ghosh, and Ringø [39], the protease enzymes secreted by bacterial strains are capable of digesting complex protein structures present in feed and provided with additional nutrients. These extracellular enzymatic activities and nutrients supplementation of probiotics influence the fishes’ digestibility, nutrient assimilation, and metabolism [40]. Furthermore, probiotic supplement is also a source of growth promoting essential fatty acid, amino acids, and vitamins (B7, B12, and vitamin K) [5]. In the current study, the administration of probiotic contributed to the substantial increment in growth indicators. The WG (g) of multispecies probiotic treated rohu was over two times higher than the control. The least AFC value (indicates better growth) and highest survivability were recorded in the same treatment. However, several studies have reported that the inoculation of probiotics improved weight and feeding regime in carps and other fish species, such as multispecies probiotic with inoculation level of 1.0 mL/L produced better growth and feed conversion ratio (FCR) in mrigal Cirrhinus cirrhosus [1]; L. plantarum improved WG and FCR in L. rohita when fed with feed for 60 days [30]; feed additives of B. subtilis enhanced WG (g), SGR (%/day), and FCR of grass carp [41]; B. subtilis in feed with different doses escalated WG and survivability of rohu fingerling [29]; L. acidophilus with baker yeast improved SGR (%/day) and FCR of koi carp [42]. The combination of Bacillus spp. and Lactobacillus spp. enhanced the growth, feed efficiency, and survivability of Nile tilapia [15], freshwater prawn Macrobrachium rosenbergii, and white leg shrimp [43].
Different hematological and biochemical indices effectively represent the welfare condition of fishes and have immense significance in comprehending the subsequent physiological or pathological alterations [44–46]. Here, the incorporation of multispecies probiotics in water as feed supplement considerably escalated the concentration of RBCs, WBCs, Glu, and Hb that could play an important part in the immune-stimulation of L. rohita. The increase of hemato-biochemical parameters using multispecies bacteria especially from genus Bacillus and Lactobacillus has also been corroborated by many other recent scientific publications [22, 28, 47]. Probiotics are evident to secrete organic acids that improve the assimilation of iron (vital component of RBCs) in the intestine, ultimately, induce the proliferation of RBCs, WBCs (leucocyte, macrophage, lymphocyte, and granulocyte), and other blood components in fishes [29, 48]. For instance, Hossain et al. [1] interference that multispecies probiotic improved the innate immunity of mrigal (C. cirrhosus) through elevating leucocyte production which are usually engaged in toxic cell phagocytosis process. Moreover, diet formulated with B. subtilis improved nonspecific immunity of rohu [47]. Usually, the Hb level in fish is greater in young age and decreases with the progression of time and finally become steady in adult [32]. Therefore, the results of RBCs, WBCs, and Hb are comparable with the findings of rohu (L. rohita) treated with yeast and mrigal (C. cirrhosus) with multispecies probiotics [15, 28]. Glu concentration in blood is a vital source of energy that plays significant role in energy metabolism. Comparatively higher concentration of blood Glu indicates fishes might have been under environmental stress during rearing condition [49]. Procedurally hyperglycemia stimulates central nervous system with the release of cortisol and catecholamine from adrenaline gland that produces physiological stress [50]. Probiotics combat against stress and at the same time increase immunity which has been exhibited with decreased blood Glu [38].
The histomorphometric observations of intestine are the crucial features of the healthy functioning of intestine [44]. The improved dimensions of intestine obliquely indicate the augmented absorptive surface area which facilitates more efficient digestion and nutrients absorption that would have been reflected through escalated growth performance in fishes [51, 52]. It was observed in the present study that the usage of multispecies probiotics with increasing doses considerably modified the morphology and absorption surface of intestine through enhancing length, width, depth, and area of villus as well as TW and TMs of L. rohita. In compliance with present findings, previous studies reported that either single or combination of Bacillus spp. and/or Lactobacillus spp. uplifted the digestion, nutrient assimilation, and absorptive surface area of gut in many fishes including carp species [53, 54]. Moreover, dietary probiotic supplement positively alters the existing microbial community and morphology of fish intestine [41, 55]. Besides, probiotic application in fish diet is reported to increase immune response through the interaction between bacterial cells and the gastrointestinal epithelial cells [34]. The abundance of GCs is also useful indicator for the immune response of a healthy intestine [12]. The present investigation observed that the higher dose of multispecies probiotics perhaps contributing the improvement of immunity in L. rohita by bringing positive alteration in immune-stimulating parameters. Similarly, the additive of Bacillus spp. increased the abundance GCs and intestinal MF in Nile tilapia [56]. GCs secrete mucin, a glycoprotein and major component of mucus, which lubricates the intestinal tract in order to protect it from being dehydrated [1, 57]. Furthermore, GCs remove pathogenic bacteria by decolonizing them from intestinal epithelial cells [51]. Therefore, greater number of GCs stimulates immune response and ensures protection from diseases. The increased thickness of MF and EC width along with the length of villi provides more space for regulating nutrient absorption that in turn secures better growth performance with boosted immunity [58]. However, in present experiment, the integrity of regular shaped liver cell nucleus increased, and the intracellular distances reduced with increasing doses of probiotic supplementation. Study reported that L. plantarum regenerated liver cells and improved liver functioning; similarly, multispecies probiotic proved its efficiency in reducing excess accumulated fat in liver [1, 8].
The current study found that probiotics improved and controlled the physiological condition of the experimental fish based on substantial changes in GH and IGFs levels when compared to control fish. Many of the growth-promoting activities of GH are initiated by binding to GH receptors (GHRs) and activating the synthesis and release of IGFs from the liver and other locations. At the same time, bacterial integrators positively altered the expression of genes involved in muscle growth, supporting probiotics’ favorable role in overall metabolism. Probiotic-fed juvenile sea bass (Dicentrarchus labrax) displayed considerably greater IGF-1 expression compared to the control group [59]. Oreochromis niloticus fed with synbiotics had considerably greater levels of GH and IGF-1 expression. IGF-1 is vital for regulating the growth and maintenance of segregated cell functions through endocrine, paracrine, autocrine signaling [25], and encouragement of cellular propagation [60]. This research is essential to comprehending how these growth factors’ expression is regulated under the investigated treatments and will aid in defining the biological importance of these growth factors in L. rohita.
In conclusion, it can be summarized that the additives of multispecies probiotic elevated the growth performance and feed utilization through uplifting the intestinal morphology, immune response, hematometric indices, and expressions of GH and IGFs genes. It is noted that multispecies probiotic showed best performance regardless of the dose used in this study, therefore, recommended for the culture of rohu at large scale. Further research could be carried on focusing the performance of this multispecies probiotic in the disease resistance through experimental infection, specific organ development, and brood stock rearing. In addition, more species-specific research can be done to examine the enzymatic activity.
Ethics Statement
The experimental procedures were carried out by following the approved regulations of the ethical committee of the Bangladesh Agricultural University, Mymensingh (Approval Number: BAU-FoF/2022/003).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zannatul Ferdous performed the experiment and drafting the manuscript. Fouzia Fariha and Nusrat Jahan performed the experiment and collected data. Sheik Istiak Md Shahriar assisted in drafting the manuscript. Md Kabir Hossain assisted in data collection. Md Jasim Uddin edited the manuscript. Md Shahjahan conceived designed, supervised, analyzed data, and edited the manuscript. All authors have read the final version and approved the manuscript.
Funding
The study was supported by a Grant CGP TF 75-F/20 from the Krishi Gobeshona Foundation and Bangladesh Agricultural Research Council, Dhaka, Bangladesh to the corresponding author.
Acknowledgments
We sincerely thank Bangladesh Agricultural University Research System (BAURES) for overseeing the project’s finances (Project No. 2020/954/KGF). We are grateful to Central Dogma Lab, Invent Technologies Ltd., Dhaka, Bangladesh for their support to analyze genes expression of this study.
Open Research
Data Availability Statement
The corresponding author (Md Shahjahan) is bound to provide data on request that support the findings of this study.




