Evaluation and Comparison Germplasm Resource of Two Species of Macrobrachium in China (M. hainanense and M. nipponense)
Abstract
The Macrobrachium genus thrives in freshwater and brackish environments. M. hainanense, a nocturnal freshwater prawn, is economically significant in China due to its delicious meat and rich nutrition. Its distribution is limited to regions like Zhejiang, Guangdong, Guangxi, Fujian, and Hainan due to breeding habits near estuaries and low-temperature tolerance. Despite its economic importance, there are limited studies on M. hainanense, particularly regarding its germplasm resources. This study investigates the growth traits, nutritional composition, enzyme activity, and genetic diversity and structure of seven M. nipponense populations and three M. hainanense populations to inform sustainable aquaculture and conservation efforts, comparing them with the new hybrid varieties of M. nipponense, which successfully cultivated, Because of its fast growth, high adaptability, and strong reproductive capacity was chosen as a control sample due to its well-established quality, economic value, reputation, ensuring competitive comparison. The amino acids, fatty acids, and physiological and biochemical indicators among M. hainanense populations and M. nipponense populations showed significant differentiation. M. hainanense populations showed minor differences in nutritional components except for total sugar and astaxanthin content that were significantly different, whereas M. nipponense populations showed almost no differences. Essential amino acids in M. hainanense comprised 34.42%–37.53% of the total amino acid content. Differences in glycine, isoleucine, and cystine were notable in M. hainanense, while glycine and proline differed in M. nipponense. Fatty acid components varied among the populations studied. Genetic diversity analysis revealed that the polymorphism information content (PIC) values for M. hainanense were higher than those for M. nipponense. The Changhua River (CH) and Nandu River (ND) populations of M. hainanense had the lowest nucleotide diversity, while the Oujiang River (OU) population had the highest. The OU population also exhibited the greatest genetic diversity, with the lowest inbreeding coefficient, while the CH population had the highest. There was slight genetic differentiation among M. hainanense populations, with geographical isolation and artificial selection contributing to genetic structure differences. This study is the first to examine population-wide genetic variability in M. hainanense, highlighting the need for comprehensive conservation and breeding strategies to maintain and enhance genetic variability in this species. The study concludes that both M. hainanense and M. nipponense have rich protein content and low-fat content, with varied genetic relationships and differentiation among populations.
1. Introduction
The rapid expansion of global aquaculture, driven by the growing demand for aquatic protein amid uncertainties surrounding wild fisheries, highlights the importance of crustaceans in this expanding industry [1]. China’s aquaculture industry has a rich history, playing a vital role in the country’s seafood exports; with a commanding position in the global production and export of aquatic animal products, including freshwater prawns, China holds a substantial 35% share of the total market [2].
As a member of the Palaemonidae family, the numerically dominant Macrobrachium genus, encompassing over 260 species, thrives in a range of freshwater and brackish environments, with some venturing into offshore waters [3]. It holds significant economic importance with delicious meat and rich nutrition as a larger species of freshwater prawn in China, M. hainanense is a conspicuous nocturnal shrimp [4, 5]. It is a high-quality aquatic product with great potential for development and utilization; M. hainanense, spanning regions such as China, Vietnam, and Java, underscores its adaptability to diverse environments [6]. Its distribution in China is limited to specific regions, including the southern region of the Oujiang River (OU) in Zhejiang, Guangdong, Guangxi, Fujian, and Hainan, due to its breeding habits near estuaries and limited tolerance to low temperatures [6, 7].
M. hainanense and M. nipponense share several biological and ecological characteristics, such as their omnivorous feeding habits and territorial behavior. Both species also exhibit similar reproductive traits, with females laying large numbers of eggs multiple times per year and growth being closely tied to molting. Different developmental stages exhibit distinct behaviors: larvae are phototactic and float, while adults prefer freshwater habitats and are active in well-oxygenated flowing waters, especially at night. However, there are notable morphological differences between the two species.
The rostrum of the M. hainanense extends beyond the first antennal segment, with most reaching between the first and second antennal scales and a few extending to the end of the scales. The carapace is covered with small spines, denser in males than in females, and the second pair of pereiopods are equal in size, with males being larger.
Regarding behavior, M. hainanense demonstrates a higher degree of cannibalistic behavior, particularly when food is scarce. In contrast, M. nipponense may show less territorial aggression and fewer instances of cannibalism under similar conditions. Growth is closely linked to molting, with significant increases in length and weight after each molt. Optimal growth temperatures range from 22 to 32°C, with the best growth occurring at 26−28°C. The species has strong reproductive capabilities, laying eggs 3–4 times annually with 2000–10,000 eggs per batch. M. hainanense farming in China began in the 1970s, with successful pond farming established in Lishui, Zhejiang, in 1987. Various farming methods include pond, large surface, cage, and high-density factory farming, while in M. nipponense, males tend to have unequal second pereiopods. Furthermore, M. hainanense exhibits strong sexual dimorphism, with males generally being larger and spinier.
Understanding these differences is crucial for optimizing farming practices for each species, as the optimal conditions for one may not necessarily apply to the other.
Currently, limited studies have been performed on M. hainanense, particularly regarding the exploration and utilization of its germplasm resources, representing an area with insufficient existing data. The lack of comprehensive research in these areas leaves much unknown about the intricacies of its environment and ecological interactions. It is necessary to conduct an in-depth evaluation of M. hainanense germplasm resources. We investigate the genetic diversity and structure of three populations of M. hainanense and seven M. nipponense populations, aiming to inform sustainable aquaculture practices and conservation efforts. Then, compared them—according to the convergent genetic diversity between M. hainenanse and M. nipponense —it is a species with fast growth, high adaptability, strong reproductive capacity, and widespread distribution across China; this species has been continuously bred since the late 1950s, especially in the lower reaches of the Yangtze River and Taihu Lake basin. Where, the new hybrid varieties of M. nipponense which successfully cultivated ‘Taihu No. 2’ and “Taihu No. 3”, was chosen as a control sample due to its well-established quality, economic value, reputation, ensuring competitive comparison can lead to discovering opportunities to breed new varieties with excellent comprehensive characteristics of M. hainanense.
2. Materials and Methods
2.1. Sample Collection
A total of 630 wild M. hainanense individuals were collected from two rivers in Hainan city (Nandu River “ND,” Changhua River “CH”) and “OU” in Zhejiang city. Besides, 1470 M. nipponense were collected from seven water bodies located in Zhejiang, Anhui, and Jiangsu city. Daqing, Nanxun, Xuancheng, Liyang, Nanjing, Yixing1, Yixing2 and grouped into DQ-F1, NX-F3, AH-F1, LY-F1, NJ-F1, DP-2, and DP-3, respectively (Table 1). The individuals from the main local artificially bred strain “Taihu No. 2” and “Taihu No. 3” were obtained from the Dapu scientific research station of the Freshwater Fisheries Research Center at the Chinese Academy of Fishery Sciences in Yixing. Where grouped into DP-2 (Yixing “Taihu No. 2”) and DP-3 (Yixing “Taihu No. 3”). Totally, 210 prawns individuals were sampled for each population. Muscle tissue samples were stored in 95% ethanol.
| City | Water body | Location | Sampling date | Tissue samples collected and spice | Number of samples sequenced |
|---|---|---|---|---|---|
| Hainan | Changhua River (CH) | 20.03°N, 110.33°E | April-13-2023 | 30 | 30 |
| Hainan | Nandu River (ND) | 19.40°N, 109.06°E | April-14-2023 | 30 | 29 |
| Zhejiang | Oujiang River (OU) | 28.45°N, 119.9°E | November-19-2022 | 30 | 30 |
| Zhejiang | Daqing (DQ-F1) | 30.53°N, 119.97°E | November-19-2021 | 30 | 30 |
| Zhejiang | Nanxun (NX-F3) | 30.88°N, 120.42°E | November-19-2021 | 30 | 30 |
| Anhui | Xuancheng (AH-F1) | 30.94°N, 118.76°E | November-20-2021 | 30 | 30 |
| Jiangsu | Liyang (LY-F1) | 31.42°N, 119.48°E | November-20-2021 | 30 | 30 |
| Jiangsu | Nanjing (NJ-F1) | 32.04°N, 118.77°E | November-20-2021 | 30 | 30 |
| Jiangsu | Yixing (DP-2) | 31.34°N, 119.82°E | November-19-2021 | 30 | 30 |
| Jiangsu | Yixing (DP-3) | 31.34°N, 119.82°E | November-20-2021 | 30 | 30 |
2.2. Measurement of Growth Traits (Basic Data Indicators)
The growth traits, including weight, body length, and cephalothorax length, of 30 prawns from each group were meticulously measured and recorded.
2.3. Investigation of Nutrient Components and Enzyme Activities
The routine nutritional components of prawn muscle samples were determined according to national standards, and each sample was analyzed in triplicate with three biological repetitions. Each assessment, whether for nutrient composition or enzyme activities, required 50 g of prawn muscle, equivalent to 30 individuals. Moisture was determined using GB 5009.3-2016. Ash was determined using GB 5009.4-2016. Fat was determined using GB 5009.6-2016. Protein was determined using GB 5009.5-2016. Total sugar was measured using GBT10782-2006. Astaxanthin content was determined using high-performance liquid chromatography (SC/T 3053-2019).
The amino acid content was determined by chemical derivation and GC–MS analysis, while fatty acid content was measured by gas chromatography–mass spectrometry.
A total of 36 antioxidant capacity assay kits (Jiancheng Bioengineering Institute, Nanjing, China) used three replications for each group, One kit for 25 samples. Superoxide dismutase (SOD) activity was detected using the hydroxylamine method. Catalase (CAT) activity was detected using the ammonium molybdate method. Total antioxidant capacity (T-AOC) was measured using the ABTS method. A Shimadzu UV-120 UV–visible spectrometer was used for the analyses.
2.4. Genetic Diversity Analysis
The genomics DNA extraction method by Sambrook and Russell [8] was used to extract DNA from prawn tail muscle tissues. The purity and concentration of the extracted genomic DNA were determined using an ultraviolet spectrophotometer by reading the OD values at 260 and 280 nm and calculating the ODA260/ODA280 ratio. The DNA concentration was estimated using the formula: OD260 × nucleic acid dilution factor × 0.05, and the samples were stored at −20°C.
Genome resequencing was performed by BGI (Shenzhen, China) using the MGISEQ series sequencer. The process involved fragmenting the DNA samples, selecting appropriate-sized fragments, purifying, end repairing, A-tailing, and adaptor ligation. Then, the DNA was amplified via rolling ring amplification and sequenced on an array sequencing chip. The raw data were filtered using SOAPnuke (v2.1.5) to obtain high-quality sequencing data. The raw data were deposited in the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) under Bio-project PRJNA1140876.
Reads alignment was performed to map the obtained reads to their positions on the reference genome, reflecting their location and consistency with the reference sequence. Sentieon (v201711.03) was used for reads alignment, position sorting, marker repeat alignment, and single nucleotide polymorphisms (SNPs) detection. The genetic diversity analysis was performed with Vcftools (v0.1.16) [9].
2.5. Data Processing and Analysis
The experimental data were recorded in an Excel sheet, and the average values were calculated using Excel’s average function. SPSS 25.0 software was used for analysis [10, 11], employing one-way ANOVA and independent-samples T test. Levene’s test was conducted for homogeneity of variances, and Duncan’s test was used for multiple comparisons. The data were expressed as mean ± standard deviation of the mean (X ± SDM), with significance levels set at 0.05 and 0.01.
3. Results
3.1. Biological Characteristics
The growth traits show that the average weight, body length, and carapace length of M. hainanense groups were 7.07–7.95 g, 53.89–57.30 mm, and 21.41–23.97 mm, respectively (Table 2). These traits align with national standards for M. hainanense. The average weight of the three M. hainanense groups was between 7.07 and 7.95 g. For the seven M. nipponense groups, it ranged from 2.7553 to 4.3990 g. Among M. nipponense, NX-F3 had the highest average weight, and LY-F1 had the lowest (Figure 1). The DP-3 group’s mean weight was significantly higher than LY-F1 and DQ-F1 (p < 0.05), but similar to DP-2, AH-F1, NJ-F1, and NX-F3. DP-2′s mean weight was significantly higher than LY-2 (p < 0.05), but like the other groups. NX-F3, DP-3, NJ-F1, AH-F1, and DP-2 had a significant weight advantage, while LY-F1 and DQ-F1 had smaller weights (Table 2).
| Species | Survey point | Body length (mm) | Body weight (g) | Carapace length (mm) |
|---|---|---|---|---|
| M. hainanense | CH | 57.30 ± 7.87a | 7.95 ± 3.57a | 23.97 ± 3.47a |
| ND | 53.89 ± 6.54a | 7.07 ± 3.94a | 21.41 ± 3.58a | |
| OU | 55.64 ± 5.25a | 7.50 ± 0.71a | 23.48 ± 2.60a | |
| M. nipponense | DP-2 | 54.5183 ± 4.48c,d | 3.8047 ± 1.04b,c | 31.1663 ± 7.34b,c |
| DP-3 | 56.4383 ± 5.14d | 4.1777 ± 1.14c | 30.6290 ± 4.85b,c | |
| AH-F1 | 53.1137 ± 5.36b,c | 3.8650 ± 1.40b,c | 28.9727 ± 4.81a,b | |
| LY-F1 | 49.8117 ± 4.99a | 2.7553 ± 1.03a | 27.5533 ± 3.67a | |
| NJ-F1 | 54.8810 ± 5.44c,d | 4.0683 ± 1.22c | 30.4703 ± 3.48b | |
| NX-F3 | 57.3567 ± 5.74d | 4.3990 ± 1.21c | 33.0803 ± 2.80c | |
| DQ-F1 | 50.3630 ± 7.26a,b | 3.2800 ± 1.37a,b | 28.5827 ± 4.42a,b | |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River.
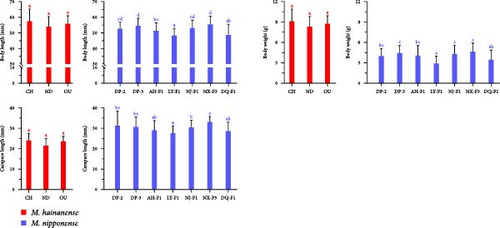
The average body length of the above seven groups was 49.8117–57.3567 cm. Among them, NX-F3 had the largest average body length, and LY-F1 had the smallest average body length. The mean body length of DP-3 was significantly higher than that of AH-F1, LY-F1, and DQ-F1 (p < 0.05), but there was no significant difference with DP-2, NJ-F1, and NX-F3. The average body length of DP-2 was significantly higher than that of LY-F1 and DQ-F1 (p < 0.05), but there was no significant difference with DP-3, AH-F1, NJ-F1, and NX-F3.
The average cephalothorax length of the above seven groups was 27.5533–33.0803 cm. Among them, NX-F3 had the largest average cephalothorax length, and LY-F1 had the smallest average cephalothorax length. The mean cephalothorax length of DP-3 was significantly higher than that of AH-F1, LY-F1, NJ-F1, and DQ-F1 (p < 0.05), but there was no significant difference between DP-2 and NX-F3. The mean cephalothorax length of DP-2 was significantly higher than that of LY-F1 (p < 0.05), but there was no significant difference with DP-3, AH-F1, NJ-F1, NX-F3, and DQ-F1.
3.2. Comparison Results of Nutrient Components
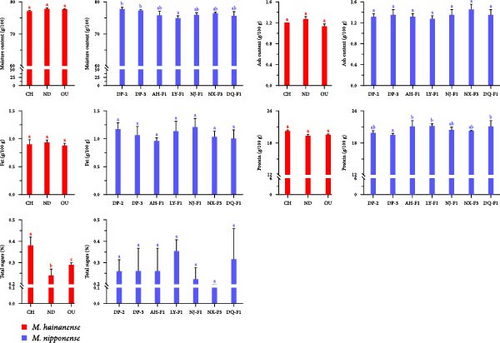
The routine nutritional composition of M. hainanense muscle showed that there was no significant difference in muscle water, ash, crude fat, and crude protein among the three groups (p > 0.05). However, there were significant differences in total sugar and astaxanthin, which were 2452.667–3807 g/100 g and 0.6183–2.8996 mg/kg, respectively (Table 3).
| Species | Groups | Moisture content (g/100) | Ash content (g/100 g) | Fat (g/100 g) | Protein (g/100 g) | Total sugars (%) | Astaxanthin (mg/kg) |
|---|---|---|---|---|---|---|---|
| M. hainanense | CH | 77.1333 ± 0.1699a | 1.200a | 0.9 ± 0.0816a | 20.1666 ± 0.2494a | 0.38 ± 0.04a | 2.8996 ± 0.0613a |
| ND | 77.8 ± 0.4082a | 1.2666 ± 0.0471a | 0.9333 ± 0.0471a | 19.3333 ± 0.2624a | 0.24 ± 0.03b | 0.6406 ± 0.0461b | |
| OU | 77.6333 ± 0.1247a | 1.1333 ± 0.0471a | 0.8666 ± 0.0471a | 19.5 ± 0.1632a | 0.29 ± 0.01c | 0.6183 ± 0.0418b | |
| M. nipponense | DP-2 | 77.7333 ± 0.6110b | 1.2667 ± 0.0577a | 1.1333 ± 0.1155a | 19.7667 ± 0.4509a,b | 0.2667 ± 0.0577a | 0.4133 ± 0.0681a |
| DP-3 | 77.2667 ± 0.2887b | 1.3000 ± 0.1000a | 1.0333 ± 0.1528a | 19.3667 ± 0.3055a | 0.2667 ± 0.1155a | 0.5633 ± 0.2754a | |
| AH-F1 | 75.8333 ± 1.2503a,b | 1.2666 ± 0.0577a | 0.9333 ± 0.0577a | 20.9333 ± 1.1240b | 0.2667 ± 0.1155a | 0.6833 ± 0.1570a | |
| LY-F1 | 74.8667 ± 0.8387a | 1.2333 ± 0.0577a | 1.1000 ± 0.1732a | 21.0667 ± 0.4041b | 0.3667 ± 0.0577a | 0.6500 ± 0.1652a | |
| NJ-F1 | 75.9333 ± 0.7572a,b | 1.3000 ± 0.1000a | 1.1667 ± 0.1528a | 20.3000 ± 0.5568a,b | 0.2333 ± 0.0577a | 0.8500 ± 0.3123a | |
| NX-F3 | 76.4000 ± 0.3606a,b | 1.4000 ± 0.1000a | 1.0000 ± 0.1000a | 20.1333 ± 0.1155a,b | 0.2000 ± 0.0000a | 0.9367 ± 0.2801a | |
| DQ-F1 | 75.6000 ± 1.3748a,b | 1.3000 ± 0.1000a | 0.9667 ± 0.1528a | 20.9333 ± 1.1240b | 0.3333 ± 0.1528a | 0.9333 ± 0.4782a | |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River.
The detection results of comparison of nutritional components (Figure 2) show that the average moisture content of the seven populations of M. nipponense was 74.8667–77.7333 g/100 g, the average ash content was 1.2333–1.4000 g/100 g, the average fat content was 0.9333–1.1667 g/100 g, the average protein content was 19.3667–21.0667 g/100 g, the average total sugar content was 0.2000%–0.3667%, and the average astaxanthin content was 0.4133–0.9367 mg/kg (Table 3). Except that the average moisture content of the LY-F1 group was significantly different from that of DP-2 (p < 0.05), there was no significant difference in the content of nutrients between DP-2 and the above groups.
3.3. The Amino Acid Composition and Content
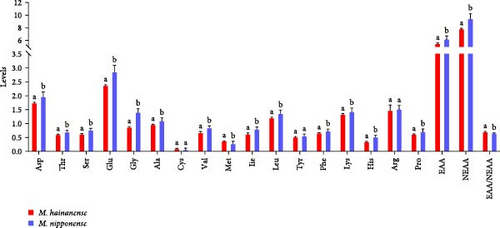
According to Table 4, the results of the amino acid content showed that 17 amino acid components were detected in all populations of M. nipponense and M. hainanense, including seven essential amino acids (Lys, Phe, Thr, Ile, Leu, Val, and Met), and eight nonessential amino acids (Asp, Ala, Glu, Gly, Ser, Cys, Pro, and Tyr), and two semi-essential amino acids (His and Arg). The average contents of Asp, Thr, Ser, Glu, Gly, Ala, and Cys were 1.8900–2.1333 g/100 g, 0.6700–1.6700 g/100 g, 0.7367–0.8000 g/100 g, 2.6967–3.0533 g/100 g, 1.2267–1.5200 g/100 g, 1.0433–1.1933 g/100 g, and.0407–0.1300 g/100 g, respectively, for M. nipponense and were 1.7066–1.7766, 0.5866–0.6366, 06400–0.8000, 2.4133–3.0533, 0.8433–0.9133, 0.9533–1.1933, and 0.0683–0.1333, respectively, for M. hainanense. The average contents of Val, Met, Lle, Leu, Tyr, Phe, and Lys were 0.8100–0.9033 g/100 g, 0.2133–0.3500 g/100 g, 0.7533–0.8600 g/100 g, 1.2800–1.4567 g/100 g, 0.5000–0.6067 g/100 g, 0.7033–0.7700 g/100 g, and 1.3400–1.6433 g/100 g, respectively, for M. nipponense. And were 0.5333–0.76, 0.3733–0.3833, 0.4866–0.7166, 1.1766–1.2366, 0.48–0.56, 0.65–0.6833, 1.2633–1.3766, respectively, for M. hainanense. The average His, Arg, and pro content were 0.4967–0.5733 g/100 g, 1.4333–1.6100 g/100 g, and 0.6100–0.8167 g/100 g, respectively, for M. nipponense, and were 0.3366–0.3633, 1.4266–1.5133, 0.6–0.63 for M. hainanense. There was no significant difference in the average amino acid content between DP-2 and the other six populations of M. nipponense. The amino acid composition and content of seafood, in general, and prawns and shrimps, in particular, are highly nutritious with good source of protein and the amino acids [12].
| Amino acids | M. hainanense | M. nipponense | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH (g/100 g) | ND (g/100 g) | OU (g/100 g) | DP-2 (g/100 g) | DP-3 (g/100 g) | AH-F1 (g/100 g) | LY-F1 (g/100 g) | NJ-F1 (g/100 g) | NX-F3 (g/100 g) | DQ-F1 (g/100 g) | |
| Asp | 1.7766 ± 0.0601 | 1.7566 ± 0.0368 | 1.7066 ± 0.0249 | 1.9000 ± 0.0608a | 1.9367 ± 0.0404a | 2.1333 ± 0.0815a | 2.0000 ± 0.2207a | 1.8900 ± 0.3551a | 1.9667 ± 0.0321a | 2.0167 ± 0.0569a |
| Thr ∗ | 0.6366 ± 0.0249 | 0.5866 ± 0.0047 | 0.6033 ± 0.0124 | 0.6833 ± 0.0379a | 0.6933 ± 0.0058a | 0.7533 ± 0.0379a | 0.7133 ± 0.0808a | 1.6700 ± 0.1300a | 0.7067 ± 0.0116a | 0.7067 ± 0.0058a |
| Ser | 0.64 ± 0.0294 | 0.6567 ± 0.0205a | 0.5867 ± 0.0125a | 0.7367 ± 0.0322a | 0.7367 ± 0.0416a | 0.8000 ± 0.0265a | 0.7867 ± 0.0862a | 0.7633 ± 0.1443a | 0.7767 ± 0.0208a | 0.7700 ± 0.0100a |
| Glu | 2.4133 ± 0.0771 | 2.3300 ± 0.0500a | 2.3800 ± 0.0374a | 2.7833 ± 0.1332a | 2.8500 ± 0.0500a | 3.0533 ± 0.1457a | 2.9133 ± 0.3927a | 2.6967 ± 0.4706a | 2.8533 ± 0.0231a | 2.9100 ± 0.0557a |
| Gly | 0.9133 ± 0.0329c | 0.8666 ± 0.0262b | 0.8433 ± 0.0188a | 1.3500 ± 0.0781a,b | 1.4733 ± 0.4041a,b | 1.4900 ± 0.0819b | 1.3733 ± 0.1060a,b | 1.2267 ± 0.2307a | 1.4467 ± 0.0208b | 1.5200 ± 0.0700b |
| Ala | 0.9533 ± 0.0339 | 0.9833 ± 0.0170a | 0.9667 ± 0.0236a | 1.0467 ± 0.0681a | 1.0700 ± 0.0200a | 1.1933 ± 0.0473a | 1.1233 ± 0.1563a | 1.0433 ± 0.2050a | 1.1067 ± 0.0351a | 1.1533 ± 0.0306a |
| Cys | 0.0683 ± 0.0155a | 0.1333 ± 0.0094b | 0.101 ± 0.0232b | 0.0900 ± 0.0243a | 0.1300 ± 0.0173a | 0.0507 ± 0.0495a | 0.0463 ± 0.0504a | 0.0567 ± 0.0119a | 0.0407 ± 0.0366a | 0.0623 ± 0.0781a |
| Val ∗ | 0.7433 ± 0.0094 | 0.5333 ± 0.0286 | 0.76 ± 0.0141 | 0.8333 ± 0.0306a | 0.8500 ± 0.0173a | 0.9033 ± 0.0379a | 0.8667 ± 0.0950a | 0.8100 ± 0.1562a | 0.8533 ± 0.0208a | 0.8533 ± 0.0208a |
| Met ∗ | 0.3733 ± 0.0094 | 0.3833 ± 0.017 | 0.38 ± 0.0081 | 0.2900 ± 0.0608a | 0.2700 ± 0.0361a | 0.3500 ± 0.0755a | 0.2500 ± 0.0819a | 0.2133 ± 0.0404a | 0.2533 ± 0.0252a | 0.3333 ± 0.1617a |
| IIe ∗ | 0.6866 ± 0.0124b | 0.4866 ± 0.0249a | 0.7166 ± 0.0169b | 0.7833 ± 0.0379a | 0.8100 ± 0.0100a | 0.8600 ± 0.0346a | 0.8167 ± 0.1002a | 0.7533 ± 0.1443a | 0.8000 ± 0.0100a | 0.8133 ± 0.0116a |
| Leu ∗ | 1.2366 ± 0.0385 | 1.1766 ± 0.0205 | 1.2266 ± 0.0169 | 1.3333 ± 0.0643a | 1.3733 ± 0.0153a | 1.4567 ± 0.0666a | 1.3900 ± 0.1752a | 1.2800 ± 0.2339a | 1.3633 ± 0.0116a | 1.3967 ± 0.0231a |
| Tyr | 0.5166 ± 0.0286 | 0.56 ± 0.0216 | 0.48 ± 0.0081 | 0.5000 ± 0.0346a | 0.5200 ± 0.0265a | 0.6067 ± 0.0351a | 0.5767 ± 0.0950a | 0.5500 ± 0.1039a | 0.5733 ± 0.0153a | 0.5833 ± 0.0503a |
| Phe ∗ | 0.6833 ± 0.0124 | 0.65 ± 0.0081 | 0.66 ± 0.0081 | 0.7567 ± 0.0252a | 0.7333 ± 0.0231a | 0.7700 ± 0.0529a | 0.7367 ± 0.0950a | 0.7033 ± 0.1447a | 0.7267 ± 0.0058a | 0.7400 ± 0.0346a |
| Lys ∗ | 1.3766 ± 0.0385 | 1.2633 ± 0.0047 | 1.3733 ± 0.0205 | 1.3967 ± 0.0666a | 1.4400 ± 0.0200a | 1.6433 ± 0.2150a | 1.4533 ± 0.1804a | 1.3400 ± 0.2512a | 1.4400 ± 0.0200a | 1.4533 ± 0.0252a |
| Hiset | 0.3633 ± 0.0047 | 0.3366 ± 0.00471 | 0.3533 ± 0.0169 | 0.4967 ± 0.0058a | 0.5033 ± 0.0208a | 0.5100 ± 0.0200a | 0.5367 ± 0.0289a | 0.5100 ± 0.1217a | 0.5267 ± 0.0153a | 0.5733 ± 0.0666a |
| Arget | 1.5133 ± 0.0262 | 1.4266 ± 0.0094 | 1.5033 ± 0.0531 | 1.4633 ± 0.0551a | 1.5200 ± 0.0200a | 1.6100 ± 0.1229a | 1.5633 ± 0.2120a | 1.4333 ± 0.2658a | 1.4933 ± 0.0116a | 1.5633 ± 0.0651a |
| Pro | 0.63 ± 0.0141 | 0.6266 ± 0.0124 | 0.6 ± 0.0141 | 0.6967 ± 0.0153a,b | 0.6100 ± 0.0100a | 0.8167 ± 0.0351b | 0.7667 ± 0.0874a,b | 0.7400 ± 0.1473a,b | 0.6767 ± 0.0306a,b | 0.7233 ± 0.0322a,b |
| EAA | 5.7363 ± 0.0207 | 5.0797 ± 0.0155 | 5.7198 ± 0.0138 | 6.0766 ± 0,2003a | 6.17 ± 0,0700a | 6.6366 ± 0,2438a | 5.77 ± 1,0912a | 6.2266 ± 0,8065a | 6.2966 ± 0,2470a` | 6.1433 ± 0,0643a |
| NEAA | 7.9117 ± 0.3523 | 7.9133 ± 0.2303 | 7.6643 ± 0.1191 | 9.1033 ± 0,4252a | 9.3266 ± 0,0709a | 10.144 ± 0,4801a | 8.9666 ± 1,6598a | 9.5863 ± 1,1869a | 9.739 ± 0,3610a | 9.4406 ± 0,1042a |
| EAA/NEAA | 0.7256 ± 0.0167 | 0.6423 ± 0.0201 | 0.7463 ± 0.0049 | 0.6675 ± 0,0102a | 0.6615 ± 0,0026a | 0.6542 ± 0.0093a | 0.6434 ± 0.004a | 64.95 ± 0.0037a | 0.6465 ± 0.0033a | 0.6507 ± 0.008a |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River.
- ∗Indication essentiel amino acid.
The total essential amino acids accounted for 34.42%−37.53% of the total amino acid content, which was 2.5700–3.2359 times that of the total nonessential amino acid content. Only Ser, Gly, Ile, and Cys had significant differences in the amino acid content among different groups of M. hainanense (p < 0.05), and there were no significant differences in the other amino acid contents (p > 0.05). The comparison of amino acids between M. hainanense and M. nipponense in Figure 3 reveals a similar distribution of values, with significant differences noted (Table 5), except for Tyr and Arg.
| Amino acids | M. hainanense | M. nipponense |
|---|---|---|
| (Asp) ∗ | 1.7467 ± 0.0185a | 1.9776 ± 0.1593b |
| (Thr) ∗ | 0.6089 ± 0.0094a | 0.7038 ± 0.0573b |
| (Ser) ∗ | 0.6278 ± 0.0131a | 0.7671 ± 0.0612b |
| (Glu) ∗ | 2.3744 ± 0.0235a | 2.8657 ± 0.2310b |
| (Gly) ∗ | 0.8744 ± 0.0140a | 1.4114 ± 0.1334b |
| (Ala) ∗ | 0.9678 ± 0.0101a | 1.1052 ± 0.1021b |
| (Cys) ∗∗ | 0.1009 ± 0.0116a | 0.0681 ± 0.0474a |
| (Val) ∗ | 0.6789 ± 0.0371a | 0.8529 ± 0.0666b |
| (Met) ∗ | 0.3789 ± 0.0046a | 0.2800 ± 0.0817b |
| (IIe) ∗ | 0.6300 ± 0.0367a | 0.8052 ± 0.0659b |
| (Leu) ∗ | 1.2133 ± 0.0133a | 1.3705 ± 0.1105b |
| (Tyr) ∗∗ | 0.5189 ± 0.0138a | 0.5586 ± 0.0620a |
| (Phe) ∗ | 0.6644 ± 0.0060a | 0.7381 ± 0.0627b |
| (Lys) ∗∗ | 1.3378 ± 0.0207a | 1.4381 ± 0.1188b |
| (His) ∗ | 0.3511 ± 0.0054a | 0.5224 ± 0.0523b |
| (Arg) ∗∗ | 1.4811 ± 0.1837a | 1.5210 ± 0.1317a |
| (Pro) ∗ | 0.6189 ± 0.0068a | 0.7186 ± 0.0852b |
| EAA ∗ | 5.5122 ± 0.1116a | 6.1886 ± 0.5116b |
| NEAA ∗ | 7.8298 ± 0.0838a | 9.4724 ± 0.7839b |
| EAA/NEAA ∗∗ | 0.7047 ± 0.0165a | 0.6534 ± 0.0099b |
- Note: Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- ∗ p Value used = 0.05.
- ∗∗ p Value used = 0.01.
3.4. Fatty Acid Content
A total of five fatty acid components were found in the muscles of for M. hainanense (Table 6). The average contents of myristic acid C14:0, pentadecanoic acid C15:0, palmitic acid C16:0, palmitoleic acid C16:1, heptadecenic acid C17:0, stearic acid C18:0, oleic acid C18:1n9c, linoleic acid C18:2n6c, α-linolenic acid C18:3n3, eicosadienoic acid C20:1, uncosanoic acid C21:0, arachidienoic acid C20:2, arachidonic acid ARA C20:4n6, erucic acid C22:1n9, eicosapentaenoic acid C20:5n3, and docosahexaenoic acid C22:6n3, were 8.2–9.2, 3.8333–4.5667, 117.8–125.5, 23.4333–24.7333, 9.7–12.633, 63.1667–74.2, 76.4–88.7, 10.8–28.1333, 13.1–16.6667, 0–4.3667, 0–4.2667, 0–4.0333, 2.3–3.8, 57.5333–65.0, 53.5–61.1667, and 14.6667–21.0667, respectively.
| Fatty acid | CH (g/100 g) | ND (g/100 g) | OU (g/100 g) |
|---|---|---|---|
| C14:0 | 8.2000 ± 0.4359a | 9.2000 ± 0.3000a | 8.4667 ± 0.4041a |
| C15:0 | 3.8333 ± 0.2309a | 4.5667 ± 0.1528a | 4.5333 ± 0.2082a |
| C16:0 | 125.5000 ± 6.8637a | 117.8000 ± 3.651a | 121.2667 ± 6.2083a |
| C16:1 | 23.4333 ± 1.2741a | 24.0667 ± 0.7506a | 24.7333 ± 1.3279a |
| C17:0 | 9.7000 ± 0.6083a | 12.6333 ± 0.6110a | 12.6333 ± 0.7234a |
| C18:0 | 74.2000 ± 4.0780a | 63.1667 ± 2.1008a | 66.7667 ± 3.4356a |
| C18:1n9c | 86.1333 ± 4.6608a | 76.4000 ± 2.5534a | 88.7000 ± 4.5967a |
| C18:2n6c | 28.1333 ± 1.4468a | 10.8000 ± 0.3000b | 12.7000 ± 0.6083b |
| C18:3n3 | 16.6667 ± 0.8327a | 13.1000 ± 0.4000a | 13.2000 ± 0.6928a |
| C20:1 | 0a | 4.3667 ± 0.1528a | 3.9333 ± 0.2082a |
| C21:0 | 0a | 4.2667 ± 0.2082a | 3.7000 ± 0.2646a |
| C20:2 | 4.0333 ± 0.2309a | 0a | 0a |
| C20:4n6 | 2.3000 ± 1.9975a | 3.4000 ± 0.1000a | 3.8000 ± 0.1732a |
| C22:1n9 | 65.0000 ± 3.6865a | 57.5333 ± 1.7502a | 60.9667 ± 3.0925a |
| C20:5n3 | 53.5000 ± 2.6851a | 58.8667 ± 1.9009a | 61.1667 ± 3.2655a |
| C22:6n3 | 14.6667 ± 0.7234a | 21.0667 ± 0.7506a | 20.9333 ± 0.9866a |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River.
The content of linoleic acid C18:2n6c in the OU and ND populations was significantly lower than that in the CH population (p < 0.05). Eicosadienoic acid C20:1 and eicosadienoic acid C21:0 were not detected in CH population. Arachidienoic acid C20:2 was only detected in the CH population. In addition, myristic acid C14:0, pentadecanoic acid C15:0, palmitic acid C16:0, palmitoleic acid C16:1, heptadecenic acid C17:0, stearic acid C18:0, oleic acid C18:1n9c, arachidonic acid ARA C20:4n6, erucic acid C22:1n9 eicosapentaenoic acid C20:5n3 and other fatty acids in the muscles of the three groups had no significant difference (p > 0.05). While 11 fatty acid components were found in the muscles of both species. The average C14:0 content of the seven populations of M. nipponense was 0.0059–0.0099 g/100 g, the average C16:0 content was 0.1300–0.1633 g/100 g, the average C16:1 content was 0.0181–0.0289 g/100 g, the average C18:0 content was 0.0532–0.0670 g/100 g, and the average C18:1n9c content was 0.1203–0.1621 g/100 g (Table 7). The average contents of C18:2n9c, C18:3n3, C20:2, C20:4n6, C22:1n9 and C22:6n3 were 0.1026–0.1540 g/100 g, 0.0067–0.0148 g/100 g, 0.0079–0.0107 g/100 g, 0.0163–0.0359 g/100 g, 0.0046–0.0084 g/100 g and 0.0286–0.0772 g/100 g, respectively. Except for the C18:0 content of NX-F3, DQ-F1, and DP-F1, and the C18:1n9c content of AH-2 and DP-2 (p < 0.05), there was no a significant difference in other fatty acid contents between DP-2 and the above groups. There was a significant difference between the two species for all the fatty acid contents except for C14:0, C16:1, C18:3n3, and SFA (Table 7).
| Fatty acid | DP-2 (g/100 g) | DP-3 (g/100 g) | AH-F1 (g/100 g) | LY-F1 (g/100 g) | NJ-F1 (g/100 g) | NX-F3 (g/100 g) | DQ-F1 (g/100 g) |
|---|---|---|---|---|---|---|---|
| C14:0 | 8.5 ± 1.706a | 5.9 ± 1.493a | 7.367 ± 2.228a | 9.867 ± 1.710a | 9.1 ± 2.955a | 9.4 ± 0.600a | 9.733 ± 1.701a |
| C16:0 | 145.967 ± 10.489a | 129.967 ± 15.431a | 133.133 ± 13.359a | 159.167 ± 12.447a | 150 ± 21.211a | 151.6 ± 2.869a | 163.3 ± 7.102a |
| C16:1 | 25.3 ± 1.908a | 19.87 ± 2.566a | 18.133 ± 2.566a | 28.933 ± 5.326a | 23.8 ± 7.213a | 20.4 ± 0.458a | 20.567 ± 4.895a |
| C18:0 | 57.1 ± 2.816a | 53.4333 ± 2.290a | 55.867 ± 2.948a | 56.4 ± 3.831a | 58.733 ± 6.062a | 65.7 ± 4.321b | 67 ± 3.161b |
| C18:1n9c | 162.067 ± 15.397b | 150.967 ± 9.144a,b | 120.267 ± 9.029a | 158.7 ± 13.164a | 135.67 ± 20.214a,b | 129.633 ± 6.239a,b | 139.3 ± 20.098a,b |
| C18:2n9c | 106.833 ± 25.985a | 102.6 ± 9.712a | 126.8 ± 17.296a | 135.8 ± 15.161a | 154.033 ± 21.628a | 106.8 ± 2.951a | 102.9 ± 58.026a |
| C18 : 3n3 | 10.2 ± 3.176a | 6.733 ± 0.896a | 14.733 ± 2.684a | 14.767 ± 1.960a | 13.033 ± 1.882a | 12.067 ± 1.942a | 8.333 ± 7.223a |
| C20:2 | 7.9 ± 1.800a | 8.1 ± 0.624a | 10.667 ± 1.150a | 9.067 ± 1.115a | 10.033 ± 1.498a | 9.067 ± 0.252a | 8.833 ± 4.539a |
| C20:4n6 | 27.133 ± 8.721 | 34.3 ± 2.100a | 26.1 ± 4.703a | 30.3 ± 6.864a | 35.933 ± 5.921a | 27.1 ± 2.960a | 16.333 ± 14.235a |
| C22:1n9 | 6.733 ± 2.454a | 8.367 ± 1.429a | 7.867 ± 2.608a | 4.633 ± 0.208a | 5.033 ± 0.971a | 4.667 ± 0.666a | 5.733 ± 1.401a |
| C22:5n3 | 79.2 ± 32.084a | 90.867 ± 6.357a | 72.7 ± 17.883a | 79.533 ± 18.475a | 77.167 ± 13.880a | 99.767 ± 10.537a | 75.967 ± 62.192a |
| C22:6n3 | 31.03 ± 14.216 | 34.067 ± 3.002 | 31.6 ± 8.619 | 33.033 ± 8.969 | 28.667 ± 4.188 | 36.167 ± 4.308 | 28.567 ± 24.748 |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
There was no significant difference (p < 0.05) in fatty acids C14:0, C16:1, C18:3n3, and SFA between the two species (Table 8).
| Fatty acid | M. hainanense | M. nipponense |
|---|---|---|
| C14:0 | 8.6222 ± 8.5524a | 0.5585 ± 2.0949a |
| C16:0 ∗ | 121.5222 ± 147.5905a | 5.9916 ± 16.0544b |
| C16:1 | 24.0778 ± 22.4286a | 1.1421 ± 4.9892a |
| C18:0 ∗ | 68.0444 ± 59.1762a | 5.6529 ± 5.8466b |
| C18:1n9c ∗∗ | 83.7444 ± 142.3714a | 6.6272 ± 18.9141b |
| C18:2n6c ∗ | 17.2111 ± 119.3952a | 8.2716 ± 29.5527b |
| C18:3n3 | 14.3222 ± 11.4095a | 1.8512 ± 4.1073a |
| C20:2 ∗∗ | 1.3444 ± 9.0952a | 2.0200 ± 1.9459b |
| C22:1n9 ∗∗ | 61.1667 ± 28.1714a | 4.1267 ± 8.7730b |
| C20:4n6 ∗∗ | 3.1667 ± 6.1476a | 1.2083 ± 1.9861b |
| C20:5n3 ∗∗ | 57.8444 ± 82.1714a | 4.1207 ± 25.9496b |
| C22:6n3 ∗∗ | 18.8889 ± 31.8762a | 3.2475 ± 10.4129b |
| SFA | 216.8111 ± 215.3190a | 9.9646 ± 22.4891a |
| MUFA ∗∗ | 113.7556 ± 170.9476a | 7.7424 ± 22.9847b |
| PUFA ∗∗ | 170.7778 ± 282.1190a | 11.3924 ± 71.4338b |
| DHA + EPA ∗∗ | 76.7333 ± 114.0476a | 7.1650 ± 36.1501b |
| n-3 ∗ | 91.0556 ± 125.4571a | 5.9697 ± 38.7132b |
- Note: Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- ∗ p Value used = 0.01.
- ∗∗ p Value used = 0.05.
3.5. Enzyme Activities
Significant differences were observed in various physiological and biochemical indicators of M. hainanense populations (p < 0.05). The T-AOC ranged from 3.4280 to 4.1997 mmol/g, the activity of SOD ranged from 553.0536 to 587.7435 U/g (p < 0.05), and the activity of CAT ranged from 4.4950 to 6.2488 U/g (p < 0.05).
The results of enzyme activities in Table 9 show that the average of SOD, CAT and T-AOC activities of the above seven populations of M. nipponense was 30.8658–44.0317 U/mgprot, 0.2040–0.2926 U/mgprot, and 0.0726–0.1377 mmol/L, respectively, and for M. hainanense was 553.0536–587.7435 U/g, 4.4950–6.2488, and 3.4280–4.1997 mmol/g, respectively. The average SOD activities of LY-F1 were the largest, and the average SOD activities of DP-2 were the lowest. The average SOD activities of DP-2 were significantly lower than that of DP-3, AH-F1, LY-F1, NJ-F1, NX-F3, and DQ-F1. In general, the SOD activities of LY-F1 and DQ-F1 were relatively high, and the other groups were relatively low. The average CAT activities of DQ-F1 were the highest, and the average CAT activities of NX-F3 were the lowest. The average CAT activities of DP-2 were significantly higher than that of NX-F3 (p < 0.05), and there was no significant difference with DP-3, AH-F1, LY-F1, NJ-F1, and DQ-F1. In general, the CAT activities of NX-F3 were significantly lower than that of the other six populations (p < 0.05), while DP-3, AH-F1, LY-F1, NJ-F1, and DQ-F1 were not significantly different from DP-2. The average T-AOC activities of NJ-F1 were the highest, and the average T-AOC activities of DQ-F1 were the lowest. The average T-AOC activities of DP-3 were significantly higher than AH-F1, LY-F1, NX-F3, and DQ-F1 (p < 0.05), significantly lower than NJ-F1 (p < 0.05) and had no significant difference with DP-2. The average T-AOC activities of DP-2 were significantly higher than that of AH-F1, NX-F3, and DQ-F1, significantly lower than that of NJ- F1, and had no significant difference with DP-3 and LY-2. The average T-AOC activities of AH- F1, LY-F1, NX-F3, and DQ-F1 were relatively low, while the average T-AOC activities of DP-2, DP-3, and NJ-F1 were relatively high.
| Species | Group | SOD (U/mgprot) | CAT (U/mgprot) | T-AOC (μmol/gprot) |
|---|---|---|---|---|
| M. hainanense | CH | 12.5682 ± 1.0623a | 0.1348 ± 0.0328b | 83.75 ± 5.0031c |
| ND | 12.7829 ± 1.4300a | 0.1027 ± 0.0326b | 95.6033 ± 5.7565c | |
| OU | 12.7236 ± 2.202a | 0.1125 ± 0.0336b | 78.4548 ± 13.1091c | |
| M. nipponense | DP-2 | 30.8658 ± 3.1897a | 0.2622 ± 0.0365b,c | 22.8351 ± 7.0152cd |
| DP-3 | 39.5784 ± 6.4762b | 0.2555 ± 0.0811b | 25.2838 ± 6.1533d,e | |
| AH-F1 | 40.0817 ± 7.3219c,d | 0.2922 ± 0.0470c | 15.4513 ± 3.9560a,b | |
| LY-F1 | 44.0317 ± 7.5383e | 0.2444 ± 0.0793b | 22.1238 ± 10.5967b,c | |
| NJ-F1 | 38.5228 ± 6.5112b,c | 0.2318 ± 0.0733a,b | 28.9334 ± 9.7919e | |
| NX-F3 | 39.3803 ± 7.4073c,d | 0.2040 ± 0.0645a | 18.3614 ± 6.5055a,b | |
| DQ-F1 | 42.7582 ± 6.2617d,e | 0.2926 ± 0.0445c | 16.9149 ± 6.2568a | |
- Note: p Value used = 0.05. Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CAT, catalase; CH, Changhua River; ND, Nandu River; OU, Oujiang River; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Significant differences in enzyme activities were observed between M. hainanense and M. nipponense (Table 10).
| Enzyme activities | M. hainanense | M. nipponense |
|---|---|---|
| T-AOC ∗ (μmol/gprot) | 85.9360 ± 7.9562a | 21.41148 ± 7.1822b |
| SOD ∗ (U/mgprot) | 12.6916 ± 1.5648a | 39.3170 ± 6.3866b |
| CAT ∗ (U/mgprot) | 0.1167 ± 0.0330a | 0.2547 ± 0.0609b |
- Note: Different letters indicate that the same item is significantly different in different groups (p < 0.05).
- Abbreviations: CAT, catalase; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
- ∗ p Value used = 0.05.
3.6. Genetic Diversity and Genetic Differentiation
In the whole genome sequencing study, 90 samples of M. hainanense generated a total of 4174.21 GB of data, with an average of 68.6 GB per sample and an average sequencing depth of 20.95x. The average number of SNPs per individual was 30,837,088. For M. nipponense, 210 samples from seven groups generated a total of 9217.79 GB of data, with an average of 43.89 GB per sample and an average sequencing depth of 18.93x. The average number of SNPs per individual was 2,159,084 (Table 11).
| Item | Sample number | Total sequencing bases | Average sequencing bases | Average depth (X) | Total SNP sites |
|---|---|---|---|---|---|
| Description of M. hainanense | 90 | 4174.21 (Gb) | 68.6 (Gb) | 20.95 | 30,837,088 |
| Description of M. nipponense | 210 | 9217.79 (Gb) | 43.89 | 18.93 | 2,159,084 |
The SNP data for M. hainanense (Table 12) shows SNP density per Kb among the three populations ranges from 1.568 to 1.810, with an overall density of 1.920. The ND population has the lowest SNP density (1.568), and the OU population has the highest (1.810). Average nucleotide diversity (π) ranges from 0.00034 to 0.00049, with the CH and ND populations having the lowest π value (0.00034) and the OU population the highest (0.00049), resulting in a total population π of 0.00042. Polymorphism information content (PIC) ranges from 0.235 to 0.269, with a total population PIC of 0.240. Observed heterozygosity (Ho) varies from 0.133 to 0.273, with a total population Ho of 0.173. The inbreeding coefficient (F) ranges from 0.116 to 0.4177, with a total population F value of 0.281. These findings suggest minimal gene flow and limit genetic exchange among the populations.
| Species | Group | Population SNP density (SNP/kb) | Population nucleotides polymorphism (π) | PIC | Observed heterozygosity (Ho) | Inbreeding coefficient (F) |
|---|---|---|---|---|---|---|
| M. hainanense | CH | 1.59826 | 0.00034 ± 0.00033 | 0.235 ± 0.106 | 0.13301 ± 0.12014 | 0.41688 ± 0.09472 |
| OU | 1.8097 | 0.00049 ± 0.00046 | 0.269 ± 0.117 | 0.27297 ± 0.16613 | 0.11635 ± 0.08383 | |
| ND | 1.56864 | 0.00034 ± 0.00032 | 0.236 ± 0.105 | 0.13624 ± 0.12141 | 0.40792 ± 0.09483 | |
| M. nipponense | AH-F1 | 6.447 | 0.00107 ± 0.000837 | 0.153 ± 0.109 | 0.099 ± 0.096 | 0.449 ± 0.128 |
| DP-2 | 5.609 | 0.000821 ± 0.00065 | 0.149 ± 0.107 | 0.068 ± 0.074 | 0.601 ± 0.085 | |
| DP-3 | 6.621 | 0.00121 ± 0.000930 | 0.161 ± 0.111 | 0.125 ± 0.115 | 0.343 ± 0.134 | |
| DQ-F1 | 6.895 | 0.00093 ± 0.00100 | 0.154 ± 0.109 | 0.109 ± 0.106 | 0.397 ± 0.179 | |
| NJ-F1 | 6.583 | 0.00119 ± 0.0010 | 0.152 ± 0.109 | 0.103 ± 0.100 | 0.422 ± 0.144 | |
| LY-F1 | 6.219 | 0.00099 ± 0.00078 | 0.151 ± 0.108 | 0.086 ± 0.080 | 0.508 0.065 | |
| NX-F3 | 6.228 | 0.00099 ± 0.00077 | 0.151 ± 0.108 | 0.086 ± 0.086 | 0.516 ± 0.147 | |
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River; PIC, polymorphism information content.
For M. nipponense, the genetic diversity analysis of seven populations showed that SNP counts ranged from 11,133,367 (DP-2) to 13,687,500 (DQ-F1). Nucleotide polymorphisms (π) varied between 0.000821 ± 0.00065 (DP-2) and 0.00121 ± 0.000930 (DP-3). The inbreeding coefficient ranged from 0.343 ± 0.134 (DP-3) to 0.601 ± 0.085 (DP-2). Ho was lowest in DP-2 at 0.068 ± 0.074 and highest in DP-3 at 0.125 ± 0.115.
3.7. Genetic Differentiation Between Populations
For M. hainanense, the pairwise Fst values among three groups ranged from 0.000 to 0.009, with the highest Fst value between CH and OU and the lowest between CH and ND (Table 13). For M. nipponense, Fst values indicating genetic differences between two groups ranged from 0.001 to 0.030, with the highest Fst value between DP-3 and DP-2 and the lowest between NJ-F1 and AH-F1 (Table 14).
| Population | CH | OU |
|---|---|---|
| OU | 0.00908 | — |
| ND | 0.00000 | 0.00853 |
- Abbreviations: CH, Changhua River; ND, Nandu River; OU, Oujiang River.
| Population | AH-F1 | DP-2 | DP-3 | DQ-F1 | NJ-F1 | LY-F1 |
|---|---|---|---|---|---|---|
| DP-2 | 0.006 | — | — | — | — | — |
| DP-3 | 0.021 | 0.030 | — | — | — | — |
| DQ-F1 | 0.004 | 0.009 | 0.019 | — | — | — |
| NJ-F1 | 0.001 | 0.006 | 0.019 | 0.002 | — | — |
| LY-F1 | 0.002 | 0.003 | 0.022 | 0.003 | 0.000 | — |
| NX-F3 | 0.002 | 0.004 | 0.021 | 0.005 | 0.002 | 0.001 |
- Note: The Fst value indicates the level of genetic differentiation between populations: a value of 0–0.05 suggests very small and insignificant genetic differentiation; a value of 0.05–0.15 indicates moderate genetic differentiation; and a value of 0.15–0.25 denotes large genetic differentiation.
3.8. Population Genetic Structure
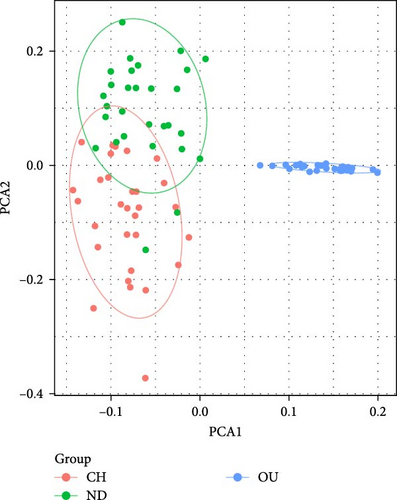
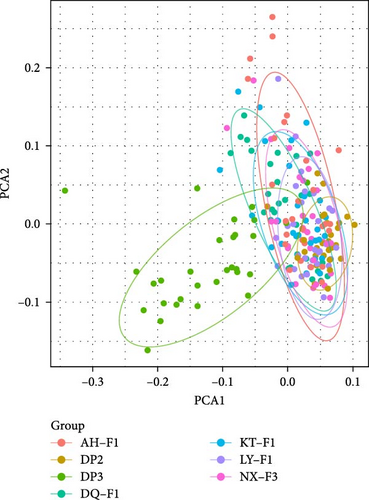
Based on whole-genome SNPs of M. hainanense and M. nipponense, principal component analysis (PCA) (Figure 4) was performed to evaluate the population genetic structure of sequenced individuals. The PCA results (Figure 4A) indicate a close genetic relationship between the CH and ND populations in M. hainanense, while the OU population is more genetically distant from both. In M. nipponense, the PCA (Figure 4B) reveals significant genetic differentiation of the DP-3 population from NJ-F1, LY-F1, DQ-F1, AH-F1, DP-2, and NX-F3.
4. Discussion
4.1. Growth Traits, Nutritional Composition, and Enzyme Activity of M. hainanense and M. nipponense
The study of growth traits in the freshwater prawn species M. hainanense and M. nipponense revealed distinct differences in their morphometric characteristics.
In the case of M. hainanense, there were no significant differences observed in weight, body length, and carapace length among the various groups analyzed. This suggests a level of uniformity in growth traits across these groups, indicating that environmental or genetic factors may not have significantly influenced their development. Conversely, the analysis of M. nipponense demonstrated significant differences in growth traits among the seven groups studied. This variability suggests that different environmental conditions or genetic backgrounds may be affecting the growth patterns of this species, highlighting the importance of considering these factors in aquaculture and conservation efforts. These findings underscore the need for further research to understand the underlying causes of growth variability in M. nipponense while recognizing the stability in growth traits within M. hainanense groups.
These variations can be attributed to genetic differences among the populations, as suggested by Thanh et al. [13]. These genetic correlations indicate that selection based on estimated breeding values can effectively improve growth traits, as demonstrated by Hung et al. [14]. High genetic correlations between body traits and carcass weight traits suggest that selection for harvest body weight can improve both growth rate and carcass weight within breeding programs. Additionally, George et al. [15] noted a linear relationship between fecundity, body weight, and body length. Similarly, the length–length and length–weight relationships in M. rosenbergii were shown to exhibit linear regression and significant power correlations [16].
To enhance the traits of M. hainanense in China, a combination of within-family and between-family selection can improve body weight, as demonstrated by Xing et al. [17], where a 20% improvement in body weight was achieved over three generations of selection in M. rosenbergii. However, in M. nipponense farming, the occurrence of small shrimp sizes due to environmental and germplasm issues often results in significant economic losses for farmers [18, 19].
Regarding nutritional components, there were almost no differences among the seven populations of M. nipponense. Minor differences in the nutritional composition of M. hainanense populations, particularly in total sugar and astaxanthin content, may be attributed to the microalgae and phytoplankton consumed by the prawns. Astaxanthin is produced by microalgae or phytoplankton within natural aquatic environments and is accumulated by crustaceans through the food chain.
The nutritional value of food is determined by the composition and quantity of amino acids [20]. Our findings indicate that both M. hainanense and M. nipponense are highly nutritious, providing an excellent source of protein and amino acids, consistent with Wang et al. [12] in their research on seafood, particularly prawns and shrimps. For humans, food with higher amino acid content offers superior nutritional value, as stated by Zhou et al. [21]. Among the various amino acids, only glycine, isoleucine, and cystine showed significant differences in content across M. hainanense populations, while Glycine and Proline varied among M. nipponense populations. These variations in amino acid content are influenced by both internal factors (e.g., species, size, and sexual maturity) and external factors [22]. The most abundant amino acids were glutamic acid, aspartic acid, lysine, leucine, and arginine, similar to findings in M. amazonicum [23].
The composition of fatty acids and proteins in fish muscle reflects dietary habits, as discussed by Valente et al. [24]. Species within Macrobrachium exhibit a range of feeding habits, from planktivorous to carnivorous [25]. When comparing marine and freshwater fish, marine species tend to have higher lipid content, while shrimp species, including M. nipponense, exhibit lower lipid content [26]. Seasonal variations also influence the lipid content and fatty acid composition in shrimp and prawn species, as demonstrated by Ayas, Ozogul, and Yazgan [27].
Li et al. [28] found that the SOD concentration in M. rosenbergii increased significantly during Aeromonas hydrophila infection, indicating SOD’s role in redox regulation and antibacterial immunity, consistent with earlier studies [28, 29]. CAT, a key antioxidant enzyme, plays a crucial role in scavenging free radicals and supporting immune function [30].
Our results show no significant differences in physiological and biochemical indicators among the M. hainanense populations (p > 0.05). However, in M. nipponense populations, SOD and CAT activities were relatively high in the LY-F1 and DQ-F1 groups, while T-AOC was lower. This suggests that M. nipponense populations activate defense mechanisms, potentially leading to inflammation and impaired antioxidant protection, whereas M. hainanense populations may not exhibit this response. Studies have shown that genetic factors significantly influence the chemical composition of farmed species under similar conditions [31]. The chemical makeup of aquatic organisms is affected by factors such as genetics, geographical origin, domestication level, food availability and quality, and seasonal changes [23].
Differences in antioxidant capacity suggest potential advantages within hybrid M. nipponense populations, as indicated by Li et al. [28]. The differentiation observed in M. nipponense populations may be attributed to several factors:
Environmental Differences: Studies have shown that environmental changes, such as hypoxia caused by high breeding density, can affect growth traits and enzyme activities [32].
Breeding Methods: Scientific and reasonable aquaculture practices can improve water quality, reducing the likelihood of hypoxia and its impact on growth traits and enzyme activities [33].
Germplasm Resources: In the farming process, local species may be mixed with M. nipponense in areas like Deqing and Liyang, leading to genetic differences and the observed variations.
The comparison between two species, M. hainanense and M. nipponense, revealed significant differences between two Macrobrachium species concerning the amino acids, fatty acids, and enzyme activity, identifying them as separate populations. However, exceptions were noted; cysteine, tyrosine, and arginine did not exhibit significant differences. Similarly, among the fatty acids, C14:0, C16:1, C18:3n3, and SFA did not reach significant differences.
4.2. Genetic Diversity and Genetic Differentiation of Populations of M. hainanense and M. nipponense
Johnston, Keats, and Sherman [34] defined population genetics as the study of genetic variation within and among populations to understand evolutionary factors. Li et al. [35] highlighted the importance of genetic diversity in domesticated species for adaptation to environmental changes. Hao et al. [36] noted that genome resequencing, which can identify over one million SNP markers, is a powerful tool for mapping functional genes and studying population genetics in aquatic animals.
The CH and ND populations have the lowest nucleotide diversity at 0.00034 each, while the OU population has the highest at 0.00049. The overall nucleotide diversity for the total population is 0.00042, like the findings for M. rosembergii [37]. Given that Macrobrachium is a common specialty food in the area, the significantly decreased nucleotide diversity (π < 0.005) may result from commercial overexploitation of wild sources. Table 14 shows that the DP-2 population has the lowest nucleotide diversity, while the DP-3 population has the highest, indicating that DP-3 has outstanding genetic advantages.
Ho measurements indicate that the CH population has the least genetic diversity (Ho = 0.13301 ± 0.12014), while the OU population has the greatest nucleotide diversity (Ho = 0.27297 ± 0.16613), showcasing its genetic advantage. Across various studies, Ho values in shrimp populations range widely from 0.05 to 1, reflecting substantial genetic variability [37–43].
Inbreeding, distinct from random mating, is measured by the inbreeding coefficient (F) and is crucial in population genetics [44]. The OU population had the lowest inbreeding coefficient (F = 0.012), indicating distant relatedness, while the CH population had the highest (F = 0.042), likely due to its origin and multiple generations. This suggests potential germplasm mixing in CH and ND populations. For example, Li et al. [20, 45] reported F values ranging from 0.0395 to 0.1069 in M. nipponense. The DP-3 population had the smallest F, indicating the least inbreeding, while the DP-2 population had the highest F, reflecting its status as an original species with extensive purification. The analysis also suggests germplasm mixing in the DQ-F1, NX-F3, AH-F1, LY-F1, and NJ-F1 populations, introduced by DP-2, due to spatial and temporal genetic diversity differences leading to varied population structures.
M. hainanense populations showed slight genetic differentiation among these populations. Comparatively, Ma, Feng, and Li [39] reported Fst values ranging from 0.03 to 0.07 in M. nipponense populations; for Cui et al. [46], the Fst varied from −0.0298 to 0.2994. While Sinonovacula. constricta populations exhibited Fst values ranging from 0.0282 to 0.1480 [39, 47]. These differences highlight varying levels of genetic connectedness or isolation among different species’ populations. Genetic and morphological parameters are important for the interpretation of the results; Chesnokov and Artemyeva [48] emphasized the significance of morphological parameters and the correlation between morphological and genetic traits, considering the genotype–environment interaction. They highlighted that these parameters may differ not only in phylogeny but also in ontogeny, underscoring the complexity of interpreting genetic parameters. However, the results showed that the Fst between the DP-3 population and the DP-2 population was the largest (Fst = 0.030). Due to geographical isolation, living environment, population bottleneck, gene flow, selection, and other factors that will affect the genetic structure of the population, any change in any of these factors may cause the genetic structure of the population to change [49].
According to a previous study, the genetic diversity of M. nipponense in China is not high, and the Fst between wild populations in different places is generally low (less than 0.05), which is consistent with this study. Unlike DP-2, whose parent group is collected from a hybrid population in Yangtze River, DP-3 was bred from wild populations in Yangtze River, Huai River, and Pearl River. Thus, compared with DP-3, DP-2 is more closely related to other wild populations from Yangtze River captured in this study, which is consistent with the results of PCA analysis.
Because DP-3 jumps out of the hybrid breeding method compared with DP-2, multigeneration selection starting from wild M. nipponense is adopted. Therefore, artificial selection is one of the important reasons for genetic differentiation between populations. The Fst between AH-F1 and NJ-F1 populations was 0.001, and Fst <0.05 produced little genetic differentiation. This indicates that AH-F1 and NJ-F1 are less hybridized.
Understanding the genetic structure, diversity, and population dynamics in M. hainanense is vital for the formulation of improved breeding programs and strategies. However, our study is the first to examine the population-wide genetic variability of M. hainanense. The varied levels of genetic diversity, inbreeding, and differentiation among populations signify the need for comprehensive conservation and breeding strategies to maintain and enhance genetic variability in this species.
5. Conclusion
M. hainanense and M. nipponense muscles have rich protein content and extremely low-fat content.
The growth traits, nutritional components, antioxidant enzyme activities, genetic diversity, and genetic differentiation were compared and analyzed. The results showed that there were minor differences in nutritional components except a significant differentiation for total sugar, astaxanthin and physiological and biochemical indicators between the groups.
In M. hainanense, the genetic relationship between the CH and ND populations is very close, while the genetic relationship between the OU population and the two is farther. In M. nipponense, the above results showed that DP-3 had good genetic diversity, and DP-2 had relatively low genetic diversity due to multigeneration purification.
Further work can focus on the collection and evaluation of M. hainanense germplasm resources, screening for superior populations, and conducting research on artificial breeding techniques and pond farming models. This will enhance the production and efficiency of M. hainanense aquaculture, promoting the formation and development of the M. hainanense farming industry.
Ethics Statement
All experimental protocols and methods were approved in October 2020 (Authorization No. 2020100701) by the Animal Care and Use Ethics Committee in the Freshwater Fisheries Research Center (Wuxi, China).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Hui Qiao: conceptualization. Zijian Gao: formal analysis. Khadraoui Djamel Eddine and Peter Daka: investigation. Yiwei Xiong, Wenyi Zhang, and Sufei Jiang: resources. Khadraoui Djamel Eddine: writing–original draft. Zijian Gao: writing–review and editing. Hongtuo Fu: supervision. All authors have read and agreed to the published version of the manuscript. Khadraoui Djamel Eddine and Zijian Gao contributed equally to this work.
Funding
This research was funded by grants from the earmarked fund for CARS-48-07, the Jiangsu Agricultural Industry Technology System, the Seed Industry Revitalization Project of Jiangsu Province (JBGS [2021] 118), and the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2023TD39).
Acknowledgments
The authors are thankful for the Jiangsu Province Platform for the Conservation and Utilization of Agricultural Germplasm.
Open Research
Data Availability Statement
The data presented in this study are available on request from the corresponding author for scientific purposes.




