Zooplankton in Aquaculture: A Perspective on Nutrition and Cost-Effectiveness
Abstract
Successful aquaculture relies on effective feeding strategies for target species, from the larval stage through to sexually mature adults, in both marine and freshwater environments. Aquatic organisms, particularly larvae, instinctively prefer live feeds like algae and zooplankton; larvae are entirely dependent on live feeds due to their undeveloped digestive systems and lack of essential digestive enzymes. Additionally, fishes and crustaceans require essential nutrients, vitamins, and minerals for sexual maturity and giving birth to healthy offspring. Zooplankton plays an important role at larval feeding stages in cost-effective aquaculture. Rotifers, cladocerans, copepods, and Artemia are commonly applied in larval rearing. However, naturally live feeds are deficient in valuable nutrients. Consequently, live feeds are improved with essential vitamins, minerals, and amino acids through bio-enrichments and algal diets for better performance. Aquaculturists primarily prefer zooplankton as live prey of larvae in fish and crustacean hatcheries because larvae requires essential digestive enzymes. They always give priority to artificial feeds with minimum live feeds to maximize profit by lowering the cost. Remarkable case studies are discussed on cost-effectiveness of zooplankton in sustainable aquaculture in this manuscript. Therefore, zooplankton as live feed could be a better choice for their nutritional value, palatability, natural availability, and cost-effectiveness. The notable opportunities, challenges, and advanced researches in live feed culture are also described in this current study. Artificial feed probably is a key stroke or booster tool for rapid profit, but live feeds such as zooplankton ensure the nutritional quality for sustainable aquaculture and satisfactory profit.
1. Introduction
The live feed is one of the crucial concerns in freshwater and marine aquaculture. The growing aquaculture establishments are already facing a lot of problems such as poor nutrition, massive larval mortalities, and sexually immature adults, which are related to their diet. Notably, high mortality is observed during the posthatching and yolk absorption period due to their sensitivity to foods. Due to tinny and toothless mouth, larvae are unable to consume oversized and hard natural or artificial feeds [1–3]. The larval stages of aquaculture species are required sufficient carbohydrates, proteins, lipids, vitamins, and minerals associated with enough digestive enzymes, even broodstock management is also impossible without these ingredients [4–7]. At first 2 weeks, larvae of fishes and crustaceans are dependent on their own yolk sac or external microalgal diet; afterwards, they start to demand live zooplankton such as rotifers, copepods, and cladocerans [8, 9]. In newborn larvae, the true stomach is absent; moreover, protein digestion requires maximum place in epithelial cells of the hindgut [9, 10]). Thus, live feeds are always preferable to larvae for their digestive characteristics than inert upgraded formulated feeds [4, 11–13] (Figure 1). Larvae always prefer live prey based on their mouth gap and taste. Naturally, larvae are active feeders who like to feed on live prey, especially the movements (swimming, whelming, and jerking) of prey influence their feeding responses and frequencies [14]. Additionally, larvae always prefer to chase and consume attractively moving zooplankton rather than immovable algae or inert artificial pellet feeds [15]. Moreover, larvae requires easily digestible feeds with essential digestive enzymes due to their immature digestive track, and this unique requirement have fulfilled only by live zooplankton [16]. Despite stress-free digestibility, larvae always require obligatory carbohydrates, proteins, lipids, vitamins, and minerals for their smooth growth and healthy development. Therefore, zooplanktons are oriented with high content of water (more than 80%) and have a lean exoskeleton which is more convincing to larvae than formulated dry and hard inert feeds [9, 17].
Zooplanktons acquire their essential nutrients basically by feeding on algae and bacteria [18, 19]. So, it is obvious to mention that aquatic algae are the fundamental nutrient sources of these secondary grazers [20, 21]. The essential fatty acids are chemically known as linoleic acid and linolenic acid. Most importantly, these linoleic and linolenic acids are exclusively formed by algae. So, all essential fatty acids are either required by zooplankton directly from algae or by transforming from linolenic acid. Thus, zooplankton contributes vital nutrition to the higher trophic level [22, 23]. In aquaculture, the additional usage of zooplankton as live prey is introduced with rotifers (Brachionus sp.) and brine shrimp (Artemia sp.) for decapods and larvae of finfish from the 1960s [15]. Still, rotifers and brine shrimp are used worldwide for their rapid reproduction and nutritional value in aquaculture. Besides these two species, other zooplankton like cladocerans and copepods are also proved their nutritional contribution in aquaculture [4, 24, 25]. Freshwater cladoceran like Daphnia and Moina are widely used in larval feeding for their better growth and immunity as a potential substitute for Artemia [26, 27]. Moreover, several copepod species are commercially applied as live feeds in marine aquaculture and are found richer in essential proteins and fatty acids than other live feeds (e.g., rotifers, brine shrimp, and cladocerans) [5, 28]. However, inadequate production of copepods during mass culture is a crucial concern in sustainable aquaculture [29, 30]. The selectivity of zooplankton as live feed is dependent on some criteria such as cost-effectiveness, strain availability, culturing processes, appropriate nutrition, and digestibility to the cultivable fish and crustacean “target species” [9, 24, 29].
Zooplanktons are excellent grazers on algal communities. Thus, zooplankton regulate the algal density and diversity. Filter grazers such as rotifers, Artemia, Daphnia, and Moina can graze until the clear water phase. Additionally, copepods are capable of superior grazing on algae especially diatoms. Therefore, Artemia is used as biological control of harmful dinoflagellates (bioencapsulated Artemia). Consequently, zooplankton grazing has a crucial role to control algal bloom in aquaculture ponds. Moreover, zooplankton can also indicate water quality that is a bonus of providing zooplankton in aquaculture [4, 8, 31, 32]. The maintenance of water quality in favor of sustainable aquaculture production is also challenging. The organic waste and leftover artificial feeds are identified as a serious threat to water quality. Only usages of artificial feeds are leading to the surplus of inorganic nutrients (nitrogen and phosphorus) in the water, which induce oxygen depletion and toxicity in both concrete and earthen aquatic reservoirs. Additionally, live feed usages are more ecofriendly than artificial feeds [4, 29].
Rotifers are found more preferable to the fishes and crustaceans for their smaller size than other zooplankton groups. Moreover, rotifers have are comparatively convenient culture method. Fish larval hatcheries are capable to adapt a large scale culture of rotifer at a time. Additionally, the tolerance level of rotifers to organic pollution is comparatively higher than copepods and cladocerans. In contrast, copepods are more sensitive to survive and breed in captive environments; and cladocerans are found most sensitive to aquatic pollution, but rotifers are always preferable to induce breeding and culture in large volumes. Moreover, copepods and cladocerans are enriched nutritionally than rotifers, but their breeding and culture are complicated and sensitive.
Zooplanktons have roles as hosts or vectors of parasites (mosquito larvae and bacteria) [33]. The bacterial load of rotifers impacts on survival of fish and crustacean larvae [34–36]. The most popular live feed, Artemia, is reported as a potential vector of harmful bacterial strain of Vibrio sp. which is caused serious threats in shrimp or prawn hatcheries [19]. In contrast, bacterial load especially Vibrio sp. is found lower in copepod than Artemia. The application of newly harvested copepods from the wild environment has a risk of introducing parasites to disease-prone larvae of fishes in hatcheries [30, 37]. Moreover, zooplanktons are found contaminated by pesticides [38], heavy metals [39], and microplastics [40]. So, it is strongly advisable for aquaculture hatcheries and farms to avoid zooplankton collection from polluted environment.
A remarkable number of aquaculture literature have stated the importance of fatty acid-enriched diets that are necessary for better growth of many freshwaters, coastal, and marine fishes, crustaceans, and mollusks ([29] and references therein). The journey from the developmental stage to juvenile with planktonic larval phase in between is very critical due to regular changing of feeding habits and dramatic metamorphosis [41, 42]. During this journey, a special diet with enough nutrients is obligatory in larval (e.g., fish and crustacean) hatcheries. Moreover, brood fishes and crustaceans also require continuous nutritious feeding; otherwise, the brood stock could be collapsed [43]. The nutritional content of live or artificial feed influences feed conversion, fecundity, growth, molting, egg hatchability, tolerance to osmotic stress, and as well as the survival of broods [44].
The upgrowing aquaculture industries are remarkably dependent on artificial feeds due to market availability and cost-effectiveness. However, artificial feeds have several limitations such as difficulties in controlling nutritional composition, storage hassles, feeding frequency, and organic pollution due to leftover feeds. In contrast, live feed management could be executed with a few simple steps like culture or export of live feed, no storage stresses, and no issues of leftover and pollution. Though live prey feeding also has some critical challenges such as a mismatch between cultural volume and nutrition (e.g., rotifers: large volume-low nutrition versus copepods and cladocerans: low density-high nutrition), commercially nonviable culture methods, not available as artificial feeds in market and inadequate live feed literacy [9, 25, 29].
Artificial feeds are available in markets than live feeds. Moreover, farmers are not convinced enough to use live feeds for larval rearing. The entire concept of live feeds (e.g., rotifers, copepods, cladocerans, and brine shrimps) in commercial fisheries is discussed in Table 1. Bio-enrichments of zooplankton are need to be focused for their better performance in hatcheries. So, it is high time to focus on cost-effective bioenrichments, short time culture with maximum density, and introducing locally available species in larval feeding through induced breeding of zooplankton. The modified and advanced cofeeding regimes of live and inert feeds could be brought sustainable success to aquaculture through laboratory-based researches and experiments.
| Opportunities and challenges | Rotifera | Copepoda | Cladocera | Brine shrimp |
|---|---|---|---|---|
| Opportunities |
|
|
|
|
| Challenges |
|
|
|
|
The live or dried zooplankton meals are observed profitable and cost-effective than other fish meals in eco-friendly aquaculture. Comparatively, more nutrient oriented zooplankton meals performed with remarkable profit index than other fish diets. The left over artificial feeds are found as aquatic pollution mediators; on the other hand, live feed meals are found fast consumable feeds by larvae and fishes. Thus, the live feeds marked as economically stable diets than other inert feed items [4, 8]. The current review focused on several concerns: (i) The nutritional qualities and disputes of well-practiced live feeds (e.g., rotifera, cladocera, copepods, and Artemia), (ii) insights on fish and crustacean larval essential nutrition requirement, (iii) significance of nutritional enrichment such as algal diets and bio encapsulation in live feed culture, and (iv) discussion on several opportunities, challenges, and cost-effectiveness in live feed culture.
2. Preference for Zooplankton in Aquaculture
Several physiological disorders might be appeared in juveniles due to the lack of essential PUFA and HUFA in their early diets. Specifically, metabolic disorders and skeletal abnormalities are observed in young fishes because of insufficient linolenic, linoleic, arachidonic acid (ARA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) in their larval feeding [55]. Algae and zooplankton both are well known as earlier life feeds to finfish and crustacean larvae [21, 25, 29]. Additionally, a sufficient amount of free amino acids in zooplankton (e.g., copepods) supports a sophisticated digestive system of larvae during their first feeding by improving protein consumption and consequently, their growth performance [56, 57]. Moreover, regulation of acid–base balance, support colloidal system, and development of skeleton is controlled by several essential minerals. These minerals also influence other enzymes and hormones for better performance [58]. Artificial inert feeds are mostly used in freshwater larval hatcheries, but in marine larvae culture, live feeds are used as obligatory supplies [8, 12].
Zooplanktons have a particular important role in larval hatcheries for their edibility, nutritional values, and quick digestibility to the larvae. Despite many modified starter inert artificial feeds for larvae, appropriate larval growth still depends on live feeds such as rotifers, artemia, copepods, and cladocerans [9, 25, 29]. The preference of zooplankton depends on several parameters such as media of culture (fresh or marine water), type of fishes, and crustaceans (omnivorous or carnivorous), subsequently, their life stages (larvae, fingerling, or adult), mouth gaps, foraging and feeding strategies, and area of living (water column or benthic) [4, 25, 29]. The nutritional requirement, digestion capability, and assimilation are significantly varying from early larval stages to adult forms. The larval digestive tracks are immature and very sensitive to exogenous feeds due to insufficient digestive enzymes such as proteinases, amylases, peptidases, lipases, and other precursors that develop digestion [16]. In the early larval period, artificial feeds with complex properties of carbohydrates, proteins, lipids, and other minerals caused poor digestion because of the absence of essential digestive enzymes, hormones, and other important natural growth factors that are not supplied [29, 55, 59]. The zooplankton as live feed with their instinct nutrients, vitamins, minerals, and essential digestive enzymes overcome the critical situations in aquaculture like poor digestion, slow growth, sudden larval mortalities, and subsequently weak fingerlings. Zooplanktons are always preferable to fishes due to their active and attractive swimming or any other movements when surface-dwelling inert feeds having color and flavor for the attraction [9, 25]. Moreover, the watery, spontaneous edible, and quickly digestible zooplankton helps larvae in their growth, but 60%–70% dry matter-oriented inert artificial feeds are not attractive to them [9].
Rotifers are always preferable to aquaculture as suitable live feed for their small sizes, high reproductive performances, larger culture densities, prodigious tolerances to high temperature, easy prey to predators for slow movement, and reasonable feeding for their filter-feeding behavior [19]. Among all nutritional contents—HUFA, DHA, EPA, and ARA are more important to observe in zooplankton for larval feeding [4, 29].
Naturally, freshwater cladoceran and marine copepods are enriched with PUFA in their lipid bilayer membrane to act as antifreeze supports to live at low temperatures [23]. These in-built amino acids, fatty acids, and lipids are marque copepods and cladocerans as superior live feeds in larval growing hatcheries. Moreover, copepods are found with a higher level of highly unsaturated fatty acids and have a better balance between phospholipid and triglyceride than rotifers and cladocerans [9, 17]. So, copepods are playing an obligatory role in marine aquaculture [19]. Copepods are also found as containers of sufficient digestive enzymes [16]. These enzymes are obligatory as exoenzymes in the gut for better digestion of growing larvae. Nevertheless, the induced reproduction and insufficient batch production of copepods are still recognized as obstacles in their commercial application.
Among cladocerans, two groups (e.g., Daphnia and Moina) are commonly applied in aquaculture hatcheries as larval feeds [4, 60–62]. In nature, these two freshwater cladocerans are very much available and tolerant to the harsh environment; especially, the Moina already proved their extraordinary tolerances to low oxygen and high ammonia inclined environments [24]. Furthermore, cladocerans are naturally very efficient filter feeders on wide-ranging bacteria and algae. This exclusive filter-feeding behavior of cladocerans supports them to sustain in extremely polluted aquatic environments which are treated by huge amounts of organic and inorganic nutrients from agricultural or industrial wastes, poultry manures, cattle dung, and other chemical fertilizers. Moreover, Daphnia and Moina are also recognized as potential sources of essential digestive enzymes to the larvae such as amylase, cellulase, peptidases, proteases, proteinases, dipases, and lipases [63, 64].
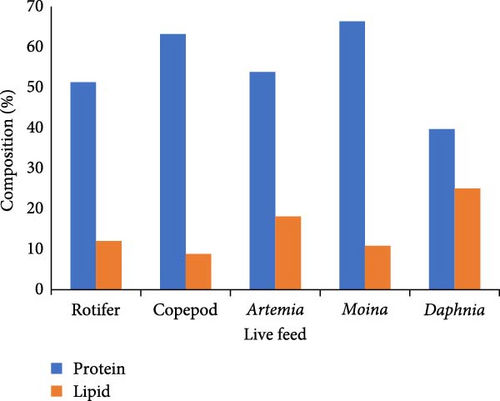
Thus, cladocerans have enough capabilities to take over the brine shrimp (e.g., Artemia) as potential competitors with enough nutritional qualities, opportune culture, and cost-effectiveness in live feeding and subsequently, reduce the dependency and crucial exploitation on Artemia [29]. The composition of protein and lipid of rotifer, copepod, Artemia, Moina, and Daphnia is shown in Figure 2 for a better understanding of the differences among them in point of nutritional value [9, 67]. The concentrations of different lipids and vitamins in copepods, nauplii, rotifer, and Artemia are described in Table 2. Hence, the solitary usage of rotifers and brine shrimp as live feeds is might be replaced by promising cladoceran (e.g., Daphnia and Monia). Additionally, the appropriate application of low-dense but highly nutritious copepods in larval rearing can ensure the nutritional strength of prospective aquaculture [69]. In a case study of gray mullet by providing Daphnia magna as live feed, it was found as a worthy substitute of fish meals on the basis of satisfactory utilization, histological grade, and cost-effectiveness [4].
| Parameters | Copepod | Nauplii | Rotifers | Artemia (01 day) | References |
|---|---|---|---|---|---|
| Neutral lipids (% of total lipid) | ~40 | ~40 | ~60 | ~80 | [68] (Table adapted from [19]) |
| Polar lipids (% of total lipid) | ~60 | ~60 | ~40 | ~10 | |
| EPA (% of total lipid) | ~15 | ~15 | ~5 | ~5 | |
| DHA (% of total lipid) | ~30 | ~40 | ~10 | ~10 | |
| Astaxanthin (µg/g DW) | ~700 | ~200 | ~20 | Very low | |
| Canthaxanthin (µg/g DW) | Very low | Very low | Very low | ~700 | |
| Vitamin C (µg/g DW) | ~600 | ~300 | ~200 | ~600 | |
| Vitamin E (µg/g DW) | ~100 | ~100 | ~600 | ~600 |
- Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
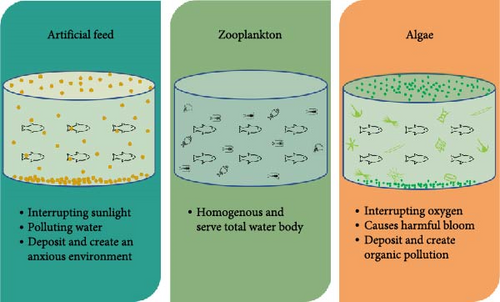
For many years, artificially formulated feeds are improving from time to time to meet the challenges in larval feeding and better growth as a completely suitable substitute for live feeds [12, 13]. After many modifications and nutritional improvements such as highly buoyant, HUFA/PUFA content, capsulation of multiple vitamins, minerals, drugs, probiotics, and antibiotics, the inert formulated feeds are still not completely comparable to live feeds. The inert immovable feeds are either dominating in surface layer might be for a long period or sink quickly and deposited in the bottom. Additionally, leftover artificial feeds are absorbed in the water column or deposited in bottom sediment consequently deteriorating water quality or needing siphoning to clear the bottom of concreted culture tanks. In contrast, the usages of zooplankton as live feeds have no these types of pollution hassles; moreover, they are homogeneously distributed in the whole aquatic body so larvae or adult fishes and crustaceans are very comfortable to feed on them at any time and any environmental conditions. Several experiments are already done and still continuing on the cofeeding strategy, associated with live and inert feeds together (Figure 3) [9, 67, 70].
A number of cofeeding cases are shown success with a minimum level of live feed; but in most of the cases, larvae are exclusively required live feeds at their early stages and inert feeds are entirely rejected. However, the addition of nutritious artificial feeds could be improved nutritional composition with the supply of live feeds which stimulates feeding behaviors of target species in cofeeding regimes [9, 25, 71].
In a case study, dried zooplankton biomass meals (ZBMs) were found more successful and cost-effective than several fish meals in rearing larvae of gray mullet (Mugil cephalus). Satisfactory growth performance, ZBM feed consumption, larval gut grade, and profit capacity were observed during larval rearing. Furthermore, entire fish diet cost reduced from 13.99 to 11.42 LE (Egyptian Pound)/kg with the enhancement in quantity of ZBM in fish diets [4]. Another study sketched the highest profit index with 100% replacement of fish meal with cladoceran (D. magna) meal [72]. Another remarkable case study on amphipod meal (APM) for gray mullet larval rearing showed significant weight gain, survival performance, and economically profitable. This dried and powdered APM could be a convenient substitution at range of 50%–75% of soyabean meal, yellow corn, rice bran, and fish meal in gray mullet fry rearing. Moreover, this renewable APMs were found cost-effective by remarkable decreased of economic conservation rate (ECR) and eco-friendly in inland aquaculture [8].
3. Exclusive Nourishment of Zooplankton
For many decades, certain species of zooplankton are applied in aquaculture for larval nourishment. Hence, there are many difficulties in live feeding in hatcheries and fish farms. Most notably, there is a significant difference between the wild and captive performance of zooplankton. In captive culture reservoirs, many aspects of zooplankton draws attention such as their spontaneous reproducing activities, adequate hatchlings, nutritional content, survival to several pollutants, and level of preference. For better utilization of zooplankton, there are several nutritional improvement techniques that are applied called bio-enhancement and bio-encapsulation [29]. Direct nutritional enrichment or traditional boosting is called bio-enhancement (e.g., rotifer and brine shrimp), and usage of several culture methods for altering essential nutrients are referred to as bio-encapsulation (e.g., copepods and cladoceran). In both bio-enrichment and encapsulation, different essential microalgae, yeast, fungi, oil emulsions, vitamins, minerals, and commercial diets even antibiotic drugs are used to prepare live feeds for boosting finfish and crustacean larvae.
Several aquafeed additives are used in green water technique, coculturing techniques, and integrated multitrophic aquaculture (IMTA) which support live feed production [21]. A nutritious marine cyclopoid copepod Oithona nana have experimentally found with highest significant population growth rate at 20 ppt salinity by several trials for better aquaculture practice with suitable live feeds [17]. Bio-enrichment and encapsulation are crucial processes and are associated with some concerns such as changes in acceptability, digestibility, and nutritional content of live feeds after encapsulation, morphological and physiological capability, and fitness to receive and grip enrichment or encapsulation. Therefore, these enrichment and encapsulation processes should be economically viable to aquaculture industries; otherwise, this innovative concept could not be applied practically. The different groups of zooplankton require diverse nourishment methods like rotifers and brine shrimp (e.g., Artemia) requisite oil emulsions; in contrast to cladocerans (Daphnia and Moina), those were found very responsive to bio-encapsulation in green algal or bacterial solutions ([73] and references therein). In contrast, encapsulations of copepods are not required necessarily due to their instinctive high nutritional (DHA and EPA) contents but are still found lack of ARA and canthaxanthin (antioxidant) in their biochemical composition [25]. Thus, encapsulations of essential vitamins and minerals are needed to apply to copepods through a nutritious algal diet. Moreover, the determination and management of appropriate algal composition for enrichment are crucial due to their selective feeding as picky palatability [21, 29].
3.1. Cladocerans
Daphnia is recognized as a model organism in the aquatic trophic level for its dual roles as exclusive grazing on algae and providing essential nutrients to higher trophic levels. Daphnia ideally could be used for bio-enrichment with soya bean [74], potassium [75], n−3 HUFA [76], canola oil [77], and commercial vitamin and mineral enriched viterna [78] and succeed in practical applications [29, 50, 51]. Moina is locally available and affordable to hatcheries with enough densities in all seasons as an inexpensive substitute for Artemia. They are inadequate in all essential nutrients obligatory for crustacean larval development [79] but ready for achieving bio-enrichments through emulsified lipids [80, 81], algal (e.g., Chlorella vulgaris) diet [82], organic wastes [83], calcium and iron (6%–9%), and vitamin B [84]. The lipids from the diet are found to influence the reproduction rates and population growth of Moina [52, 85].
3.2. Copepods
Copepods are naturally very rich in obligatory fatty acids such as DHA, EPA, and ARA; and their free amino acids and proteins (44%–52%) are found higher than bio-enriched brine shrimp (e.g., Artemia) [5, 86]. Additionally, copepods are exclusive sources of several antioxidants like astaxanthin, ascorbic acids, and vitamin E [28]. The content of phospholipids in copepods is very high compared with brine shrimp Artemia [68]. A case study on rice bran and corn starch feeding on O. nana showed remarkable growth and reproduction performance. In that experiment, rice bran provided highest amount of MUFA and PUFA and ensured significant female O. nana fecundity. However, corn starches are recommended as most supportive supplementary diet for cyclopoida and copepod population growth [17]. The larvae cultures in aquaculture are found to benefit from the high content of proteins, carotenoids, vitamins, and minerals such as manganese and copper (Cu) in copepods [87]. However, the mass density in copepod cultivation at an economically viable level is still progressing with potential challenges (e.g., induced breeding, selective feeding, and limited hatchlings) and probably cost exorbitant. Therefore, some beneficial bio-enhancements are applied to copepod mass culture, mostly by algal diet. For example, chlorophyte (e.g., Tetraselmis chui) is offered to the clinoid copepod (e.g., Pseudodiaptomus annandalei) to be adapted for nutritional enhancement [88]. In addition, a mixed algal diet (C. muelleri, I. galbana, and T. suecica) are also applied in P. annandalei culture to produce enriched hatchlings as live feeds for larvae of puffer (e.g., Sphoeroides annulatus) and red snapper (e.g., Lutjanus guttatus) fishes [89]. Thus, copepods are found with exclusive biochemical nutrition and the capability to stimulate larval feeding and appetites after manipulated diets [90–94]. Moreover, population size and egg production of copepods are promoted by diet quantity and quality (concentration of protein and lipid) [95–97]. Particularly, lipid composition and PUFA/HUFA/DHA/EPA/ARA content or their ratio got more emphasis [98] than vitamin enrichments, which are also essential for better growth and healthy survival [29].
3.3. Rotifers
Rotifers are observed deficient in essential minerals such as iodine, Cu, zinc, and especially selenium (Se) and manganese. Furthermore, Se and manganese are found remarkably lower in rotifers than copepods [99, 100]. At present, highly nutritious (HUFA/PUFA-rich) microalgae are used in the diet of rotifers to enrich their nutritional quality through applying of “green water technique” with Nannocholoropsis gaditana (green algae) [101] and Chlorella, Tetraselmis suecica, Chaetoceros, I. galbana, or N. oculata [102]. Furthermore, a number of microalgae such as Chlorella, Kirchneriella contorta, Ankistrodesmus convoluus, Phacus pyrum, and Scenedesmus costato-granulatus are used for freshwater rotifer (B. calyciflorus and B. rubens) culture [103]. Among microalgae, Nannocholoropsis gaditana is found as the most effective dietary component in rotifer enrichments [104]. Therefore, both short and long-term enrichment procedures are applied to enrich rotifers such as n−3 HUFA (marine oil emulsion) mixed through or short of Saccharomyces cerevisiae (baker’s yeast) at least up to 24 h for short-term enrichment [105]; in contrast, the long-term enrichment rotifers are supported by this baker’s yeast with n−3 HUFA emulsified essential lipids [106, 107].
3.4. Artemia
Artemia is one kind of filter feeder shrimp exclusively found in the Great Salt Lake, known as a very popular live feed in aquaculture due to its convenient availability as dormant cysts with limited generation periods and fast maturity which can be hatched rapidly at any quantity [108–110]. The production of artemia cysts has progressively escalated every year for supporting marine aquaculture [111]. Artemia could grow quickly with simple supplies of available bacteria and microalgae for instance [112]. Many essential enzymes have no alternatives in the larval growth of fishes but cannot self-synthesize in them, these crucial nutrients could be supplied by Artemia [113]. Artemia spp. are found lacking in EPA, DHA, and essential fatty acids, but sufficient in highly unsaturated fatty acids which support initial larval growth [114]. Artemia can be enriched with highly unsaturated fatty acids through many processes as nutritious algal supply or oil emulsions [29]. Artemia should be used with a satisfactory performance by selecting well-performed strains, driving on decapsulated cysts, and possibly using the newly hatched nauplii (original). The usage of marine oil–based emulsions is a traditional bio-enrichment approach to Artemia [114, 115]. Additionally, some highly PUFA-oriented microalgae like as Chlorella salina, Chaetoceros calcitrans, Nannochloropsis salina, and S. cerevisiae are exclusively used in Artemia diets for better enrichment [116].
4. Essential Nutrients in Nourishment
Silica has a role in the zooplankton’s biochemical composition and metabolism. Zooplanktons automatically acquire silica by preferring diatom for their diet which has silica-made frustules. Silica enriched larger diatoms have harder frustules than juveniles. So, the groups of zooplankton are comfortable in feeding on juvenile diatom because of ease to crack thinner shells. The ingestion rate of copepods is might be reduced due to feeding on silica enriched diatoms because digestion and evacuation of these types of diatom is probably time consumable [117].
Se influences the fertility and immune system in animals [118]. Moreover, as an important trace substance, Se accelerates thyroid hormone production in fish. This essential element is transmitted to zooplankton by algal diet and subsequently to larval fish [119]. Se-enriched yeast diet on rotifers showed higher survival rates through several trials of rotifer’s enrichment processes [99, 100]. Furthermore, the same diet was also applied in copepods for Se-enrichment and found nutritionally supportive for fish larvae in marine aquaculture [25]. Se-enriched Artemia was found with satisfactory performance on fish growth [120].
At an acceptable limit, Cu is either essential trace metal to immunity, enzyme production, and metabolic activities in fish or toxic at excess level [121, 122]. Most crustacean species require hemocyanin for carrying oxygen in their blood [123] and Cu fundamentally supports the production of hemocyanin molecules [124]. Moreover, Cu has crucial roles in hematopoiesis and Cu-oriented enzymes such as cytochrome oxidase, ferroxidase, lysyl oxidase, superoxide dismutase, and tyrosinase [123]. Cu has rapidly attached ability to the surface of microalgal groups basically consisting of prominent nutrient elements such as polysaccharides, proteins, and lipids which have the quality to impair metal molecules. The application of Cu-enriched algae in rotifer feeding was already found successful enrichment procedure [124]. Scientists also believed that the same enrichment procedure could be possibly applied in copepod feeding for supplying essential Cu to fishes and crustaceans in aquaculture.
Moreover, copepods are identified with very high iodine contents than rotifers and Artemia; this iodine content prevents the malpigmentation and other crises in metamorphosis in larvae (e.g., flatfish) [125] due to its pioneer role for thyroid hormone [9]. Rotifer enrichment with calcium is used in lordosis treatment [126]. Moreover, the lipid level could be changed in rotifers (e.g., B. pilcatillis) through Se and zinc enrichment [127, 128]. Interestingly, when thymol iodide (trace element), a potential source of iodine is used in rotifer enrichment then the iodine content in rotifer reaches up to copepod’s iodine content and might represent themselves as a competitive substitute of copepods in marine larval feeding [129].
The bio-originated pigment as carotenoids has several roles in the living body as encounter photo-oxidation of cholesterol and PUFA, removing free radicals, promoting hepatopancreatic function, and improving immunity towards severe fungal disease [130]. Moreover, a xanthophyll type carotenoid as astaxanthin is widely used in pinkish coloration in the flesh of crayfishes, lobsters, shrimp, and renowned salmonoids [131, 132]. The availability of foods may control the pigment conservation in zooplankton (e.g., freshwater cladoceran) [133]. Moreover, astaxanthin production are observed highest during the night and other antioxidant activities and astaxanthin utilization for photoprotection are detected highest during the day in zooplankton [134]. Additionally, an essential role of astaxanthin in metabolic activity and egg production in zooplankton (e.g., copepod) is also found [135].
The enrichments of vitamins A, C, and E have been modified and enrolled since 1990 [136, 137]. The presence of vitamin C (ascorbic acids) in zooplankton depends !on diversity and enrichment procedures of enrichment. Microalgal incubation with vitamin C has a potential role in vitamin C increasing in rotifers [138] and copepods [25]. Moreover, EFA and vitamin C enrichment in artemia improves digestive enzyme activities and high stress resistance of fish larvae in their earlier stages [139, 140]. Another potential antioxidant is vitamin E that reduce oxygen radicals and breaking the radical chain reaction in the biological membrane. Thus, vitamin E protects flora and fauna from lipid peroxidation. Therefore, copepod feeding with vitamin E-enriched algae for improving larvae production and blood quality in the hatchery is already reported [141].
5. Advanced Research
The molecular evaluation of zooplankton is required for a better understanding of biochemical and nutritional composition. The most useable live feed, Brachionus plicatilis has been alienated into genetically nine diverse stocks through molecular techniques [142] for better growth rate, feed conservation, and higher nutrition supply in aquaculture [143, 144]. Scientists found one bacterial mlrA gene in the gut of copepods which profoundly support in toxin-producing cyanobacteria digestion [135]. As well as microcystin (toxin) tolerant recombinant clone microorganism in D. magna empowers them against Microcystis deepened diet. This type of special gene or reformed microbiota could be inserted possibly in other preferable zooplankton species for surviving during harmful algal bloom in aquaculture [145]. Moreover, bio-enriched microalgae for zooplankton rearing is one of the most achievement in blue biotechnology [20].
In advanced research, scientists have tried preservation of adult or egg for further utilization to avoid culture hassles. Rotifers are found almost in natural quality after restoration from preservation at 4°C for some days. Another way to ensure the continuous supply of rotifers to the hatcheries is the production of rotifer’s larvae from resting eggs; it is also useful to avoid continual rotifer culture even in off-seasons. Scientists also proved that dormant eggs of rotifers could be put in storage at 5°C for many years [146]. Furthermore, amictic eggs (parthenogenesis) of rotifers are experimented by cryopreservation at −196°C in liquid nitrogen with dimethyl sulfoxide (DMSO) as cryoprotectant; but cryopreservation of huge amount of rotifers for direct use as larval feeds is not viable [46]. This cold storage technique is also applied to store diapause eggs of copepods. Experimentally subcutaneous copepod (A. tonsa) eggs are stored for 11 months [5] and 6 months [147] at approximately 4°C in anoxic cool conditions. The EPA and DHA levels were found lower; but higher ARA was found in copepod nauplii which hatched from cold preserved eggs [148]. However, further in-depth researches and trials on cold preserved zooplankton eggs are necessary for successful hatchings and healthy larvae in aquaculture.
6. Conclusion
We tried to emphasize the importance of zooplankton as live prey/feed in profitable and healthy aquaculture. The solitary usage of artificial feeds might be sustained with their availability and instant impacts on short-termed profits in aquaculture industries, but their nutritional values and solitary application to larval feeding would be questionable forever. In contrast, the groups of zooplankton have enough potentiality in aquaculture due to their nutritional values, palatability, larval acceptability, and eco-friendly behaviors. It is true that introduce and practice of zooplankton in aquaculture have to face a lot of ecological and economic challenges, but overcoming these challenges might be ensured sustainable and cost-effective aquaculture.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There is no funding for this manuscript.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were generated or analyzed in this study.




