Mortality Risk of Juvenile Japanese Sea Cucumber Apostichopus japonicus by the Sympatrically Occurring Hermit Crab Paguristes ortmanni
Abstract
The density of the hermit crab, Paguristes ortmanni, in the artificial reef of juvenile Apostichopus japonicus increased almost threefold from June to December 2018. Calcareous ossicles of A. japonicus were identified from the stomach contents in 28.8% of the hermit crabs (18.6% in males and 10.2% in females) in December; season hatchery-produced juveniles are frequently introduced into the wild in Hokkaido, Japan. The mortality rate of juvenile A. japonicus by P. ortmanni was estimated to be 2.5 ± 2.4 individuals day−1 based on laboratory predation experiments. Interestingly, 3% to 5% of individuals survived despite being attacked and injured in all trials, escaping on the shells of hermit crabs. Over 50% of females in the ossicle-not-detected group had shield lengths (SLs) smaller than the smallest individual in the ossicle-detected group. The average SL of the ossicle-detected group in females was significantly higher (p< 0.01) than that of the not-detected group, indicating an increased predation risk for A. japonicus juveniles when larger female P. ortmanni were present. The present study offers new insights into the predatory behavior of P. ortmanni toward A. japonicus juveniles, showing that these sympatric hermit crabs present a considerably high mortality risk to A. japonicus juveniles. It also emphasizes the importance of implementing appropriate measures to protect juveniles from predators during the release process, providing an essential viewpoint for enhancing and rebuilding the wild population of commercially important endangered A. japonicus.
1. Introduction
Sea cucumbers are marine invertebrates classified in the phylum Echinodermata, class Holothuroidea [1]. Sea cucumbers have been traditionally harvested in China for over a thousand years [2] and have been primarily consumed by East Asian populations, including Japan, for centuries [3]. China dominates the global sea cucumber trade, and the number of countries exporting sea cucumbers to China increased from 35 to 83 between 1996 and 2011 [4]. Concurrently, seven sea cucumber species traded in China are listed as “endangered” on the International Union for Conservation of Nature (IUCN) Red List [5]. Apostichopus japonicus Selenka, 1867 (known as Japanese sea cucumber), a common temperate sea cucumber species inhabiting the coast of Russia, Korea, China, Japan, and the United States [6], is one of the seven endangered sea cucumber species [7]. Of these, A. japonicus, distributed in Hokkaido, the northernmost island of Japan, is characterized by its distinctive morphology, particularly the six rows of relatively sharp papillae on the dorsal and ventral surfaces [8]. The quantity and morphology of these papillae significantly influence market value, with A. japonicus from Hokkaido commanding premium prices [8, 9].
Despite the early implementation of protective measures in Japan [10], wild populations of A. japonicus have been reported to have declined by 30% over the past 30 years [6]. Elevated fishing pressure and the emergence of systematic, large-scale poaching operations have been identified as critical concerns [3, 11], while habitat degradation due to coastal anthropogenic activities and numerous environmental stressors associated with climate change have been extensively discussed as factors contributing to the population decline of sea cucumbers [3, 12, 13]. Moreover, given that A. japonicus employs broadcast spawning as its mode of fertilization [14], insufficient numbers of matured males and females in close proximity during spawning events may significantly impair fertilization success, thereby posing a critical risk to population recovery [15]. In other words, once sea cucumber populations have declined below a certain threshold, in addition to the conventional protective measures, introducing hatchery-produced juveniles into natural habitats is the essential strategy for stock restoration [16]. In Japan, hatchery-produced A. japonicus juveniles have been widely released. For example, ~15 million juveniles (>5 mm) were released in Hokkaido in 2020 [17].
Previous studies have suggested that predators of adult sea cucumbers are relatively few [18, 19] due to their extensive antipredatory behaviors, such as producing saponins as feeding deterrents [20], contracting body wall muscle [21, 22], and controlling buoyancy and strength of attachment when detached from a substratum [23, 24] and ejection of internal organs [25]. Meanwhile, predation is often observed as the most crucial factor of early juvenile mortality for a wide range of marine benthic invertebrate species [26–28]. Some studies have reported intensive predation on juvenile sea cucumbers, with Holothuria scabra being preyed upon by fish [29] and decapod crustaceans [30–32] and A. japonicus by the kelp crab [33]. These studies emphasize the importance of expanding our knowledge on sea cucumber predators and the impact of predation, particularly on the early juvenile stages of sea cucumbers.
In this study, we collected and measured the most dominant hermit crab, Paguristes ortmanni Miyake 1978, within A. japonicus artificial intermediate reefs composed of stacked scallop shells (Figures 1a–c), deployed at a fishing port in southwestern Hokkaido, Japan, between June and December 2018. The stomach contents of P. ortmanni were subjected to microscopic examination. Additionally, laboratory experiments were conducted to estimate the mortality rate of juvenile A. japonicus (green type) commonly distributed in the Hokkaido region [34] by P. ortmanni, and predator and prey behaviors were observed.


2. Material and Methods
2.1. Field Sampling, Measurement, and Observation of Stomach Contents
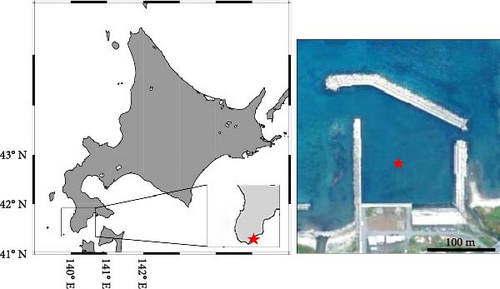
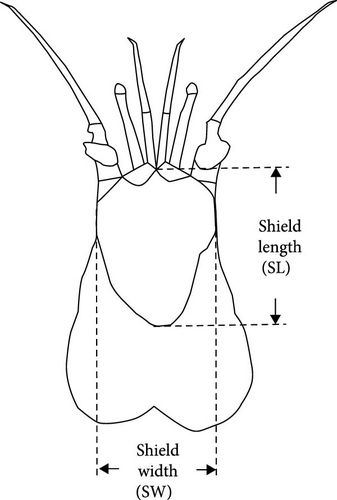
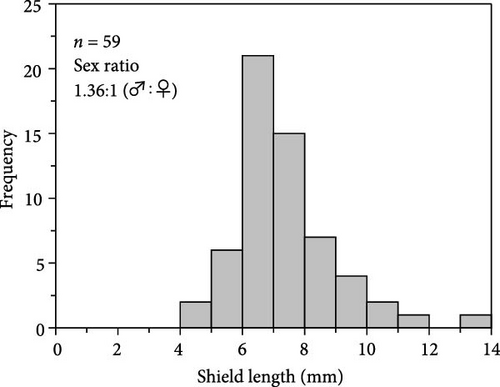
An artificial intermediate sea cucumber reef for A. japonicus, previously deployed at a depth of 4–5 m at a fishing port located in southwestern Hokkaido, Japan (41°27′44.8” N 140°14′52.7” E; Figure 2), was carefully wrapped in underwater nets and landed every 3 months from June 7 to December 6, 2018, to collect all hermit crabs inhabiting the inside of a reef. The collected hermit crabs were preserved using 70% ethanol immediately after sampling on-site and brought back to a laboratory, and P. ortmanni were identified [33, 35–37]. Body size measurements (shield length [SL] and wet weight) of all samples (n = 97) were performed using a digital vernier caliper to the nearest 0.01 mm (Figure 3a). Sex was determined from the gonopore position [38]. After these measurements, the stomach was dissected with a clean scalpel and tweezer to obtain food content from all of the P. ortmanni collected in December (n = 59), the season when hatchery-produced juveniles are often released in Hokkaido. The content was dissolved in a sodium hypochlorite solution (NaClO, 5%), and the dissolved content was microscopically observed using Axiovert 135 (Zeiss, Germany) to detect the ossicles of A. japonicus [33, 39]. The population density of P. ortmanni per unit volume was estimated based on the size of the artificial reef (0.5 m length × 0.3 m width × 0.15 m height). Measured values of SL and wet weight were tested using the Kruskal–Wallis test, followed by Dunn’s multiple comparisons test to compare differences between samplings. The ossicle detection and non-detection rates between males and females were analyzed using the chi-square test. Using Welch’s t-test, the differences in the body size index of the SL and the wet weight between the ossicle-detected and not-detected groups were also compared. The analysis was conducted after detecting outliers using the interquartile range (IQR) method and removing them from the dataset [40].
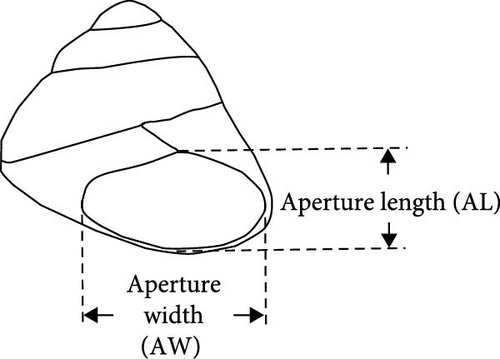
2.2. Estimation of Mortality Rate by Laboratory Experiments
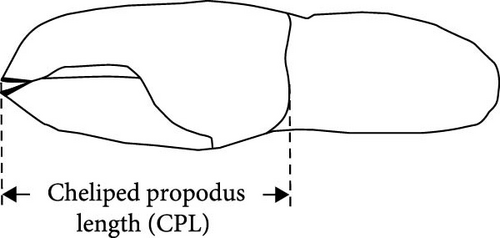
Nine P. ortmanni (two males and seven females) collected at the same sampling site described above on June 25, 2019, were gently placed into a cool, aerated plastic bucket and brought back to the laboratory alive. P. ortmanni were separately maintained in small aquaria (31 cm long × 18 cm wide) at 13 ± 1°C based on the local water temperature measured in December 2018. A 12-h light/12-h dark cycle was applied, and the depth of the seawater in the aquaria was adjusted to 3 cm, the depth at which the studied P. ortmanni were confirmed to reach the vertically attached A. japonicus. P. ortmanni were not fed for a week before the experiments. For prey preparation, 100 A. japonicus juveniles were soaked in L-menthol solution for 20–30 min to induce anesthesia and estimate the standard body length (SBL), a widely applied method for accurately measuring sea cucumbers [33, 41]. After the measurements, juveniles were separately returned to the aquarium and carefully observed for several days to check their condition before the experiments. Ten randomly selected juveniles were gently placed in each aquarium as prey. Throughout all trials, a control aquarium without P. ortmanni was maintained to confirm that any observed mortality in A. japonicus was exclusively attributable to predation rather than alternative factors such as water quality, initial health status, or other variables. The SBL and wet weight of the tested A. japonicus were 12.34 ± 1.27 mm (mean ± SD), ranging from 10.17 to 15.84 mm, and 0.03 ± 0.01 g, ranging from 0.01 to 0.07 g, respectively. The numbers of A. japonicus and predator–prey behavior were observed and noted at 1, 3, 6, and 12 h (the end of a light cycle) and 24 h (the end of a dark cycle) after the start of the experiments [33]. The experiments were repeated three times, and the studied P. ortmanni were immediately preserved in 70% ethanol to check their sex and measure their body size indices of SL, shield width (SW) at the widest part, right and left cheliped propodus length (CPL), wet weight, and shell characteristics, such as aperture length (AL) and aperture width (AW) (Figure 3a–c and Table 1). All measurements were taken with a digital vernier caliper to the nearest 0.01 mm. The A. japonicus juveniles used in this study were obtained from Hokkaido Aquaculture Promotion Corporation. By using Welch’s t-tests, the differences in mortality rates between day and night, as well as sex in the predation experiments, were assessed. Correlation analyses (Pearson’s correlation coefficient) were used to determine whether the mortality rate and each of the body size variables (SL, SW, CPL, and wet weight) and shell characteristic variables (AL and AW) were significantly correlated. Friedman tests were also applied to examine differences in the number of killed sea cucumbers in the three trials. All statistical analyses were performed using OriginPro 2020b (OriginLab Corporation, USA) and StatView statistical software (version 5.0).
| Sample ID | Sex | Wet weight (g) | Shield length (mm) | Shield width (mm) | Average cheliped propodus length (mm) | Aperture length (mm) | Aperture width (mm) |
|---|---|---|---|---|---|---|---|
| 1 | F | 0.516 | 6.56 | 5.52 | 4.26 ± 0.06 | 14.06 | 8.5 |
| 2 | F | 0.732 | 7.19 | 5.63 | 5.23 ± 0.09 | 14.59 | 7.34 |
| 3 | F | 0.398 | 5.84 | 4.47 | 4.06 ± 0.01 | 22.18 | 10.11 |
| 4 | F | 0.494 | 6.53 | 5.14 | 4.97 ± 0.03 | 13.59 | 11.10 |
| 5 | F | 0.645 | 6.38 | 5.66 | 5.05 ± 0.11 | 12.67 | 10.32 |
| 6 | F | 0.412 | 6.25 | 4.99 | 4.46 ± 0.05 | 14.29 | 8.35 |
| 7 | F | 0.560 | 6.85 | 6.10 | 5.41 ± 0.05 | 23.77 | 12.31 |
| 8 | M | 0.582 | 7.34 | 5.98 | 5.88 ± 0.07 | 24.69 | 11.60 |
| 9 | M | 0.899 | 7.92 | 6.28 | 6.67 ± 0.02 | 14.51 | 12.41 |
2.3. Ethical Statement
The present study was performed in compliance with the relevant laws, institutional guidelines, and ethical guidelines for animal experiments by the Science Council of Japan and approved by the institutional ethics review board.
3. Results
3.1. Population Dynamics and Calcareous Ossicles in the Stomach Contents
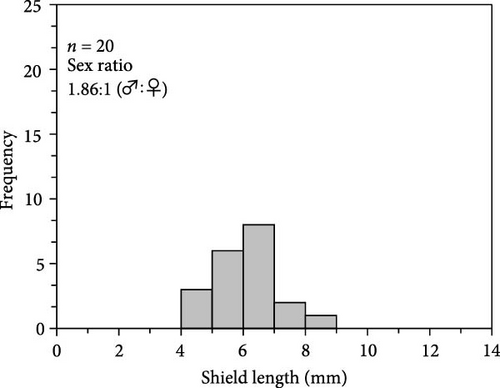
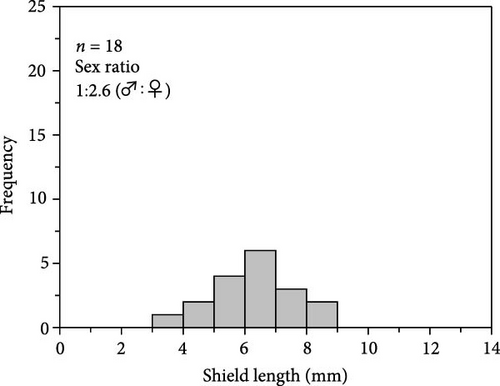
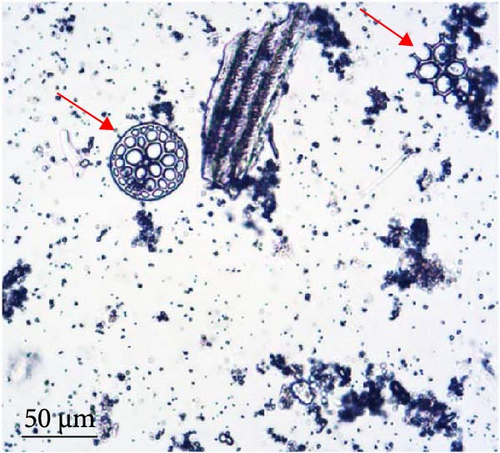
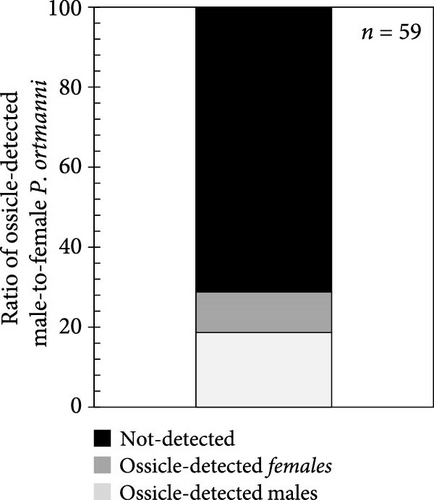
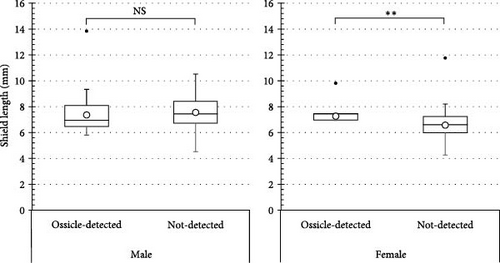
The number of individuals, sex ratio, and SL frequency distributions of P. ortmanni during the sampling period are shown in Figure 4a–c. Although the population density of P. ortmanni showed similar values in June and September 2018, their population density increased to 59 individuals (c.a. 524 individuals per m3) consisting of 34 males and 25 females in December, approximately threefold the population density in June and September. Both SL and wet weight also increased from June (6.02 ± 1.08 mm [mean ± SD] and 0.71 ± 0.32 g) to December (7.41 ± 1.62 mm and 1.34 ± 0.97 g), and both values in December were found to be significantly higher than those in June and September (p< 0.05). A representative picture of A. japonicus ossicles found in stomach contents and the ratio of ossicle-detected male-to-female P. ortmanni collected in December 2018 are shown in Figure 5a,b. Ossicles were detected in the stomach contents at a rate of 28.8% (18.6% males and 10.2% females). There were no statistically significant differences in ossicle detection rates between male and female hermit crabs (p> 0.05). The averages of the SL and wet weight of the ossicle-detected and not-detected groups were 7.85 ± 1.84 mm (mean ± SD) ranging from 5.80 to 13.83 mm, 7.23 ± 1.48 mm ranging from 4.27 to 11.76 mm, 1.69 ± 1.31 g ranging from 0.49 to 6.11 g, and 1.20 ± 0.75 g ranging from 0.17 to 4.52 g, respectively. Although the average SL and wet weight were high in the ossicle-detected groups of both males and females, a statistically significant difference was observed only when comparing the average SL among female hermit crabs (p< 0.01) (Figure 6).
3.2. Estimating the Mortality Rate and Predator–Prey Behavior
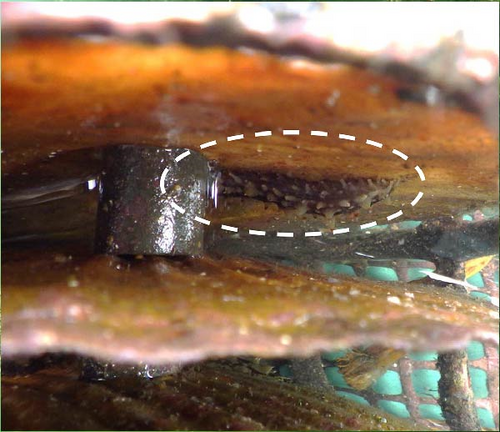
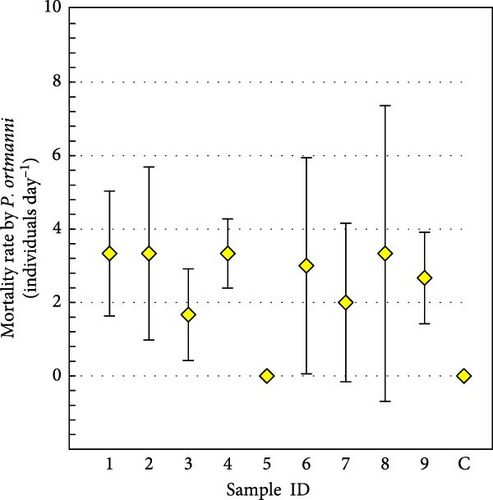
The mortality of juvenile A. japonicus was observed in eight of nine aquariums regardless of the size or sex of the tested P. ortmanni, and the estimated average mortality rate was 2.5 ± 2.4 individuals day−1 (mean ± SD) (Figure 7a). P. ortmanni preying on A. japonicus is also available as a video in the Supporting Information (Video S1). Although the male P. ortmanni with the longest AL (24.69 mm) was capable of killing a maximum of nine juvenile A. japonicus within the first 12 h (Figure 7b,c), no statistically significant differences in mortality rates between the sexes were observed in these experiments. Although the studied hermit crabs foraged for food both during the day and night, the number of A. japonicus consumed during the day was significantly higher (p< 0.001). The mortality rate did not correlate significantly with any body size variables (SL, SW, CPL, or wet weight) or shell characteristic variables (AL or AW). On average, 3% to 5% of A. japonicus survived despite being attacked and injured in every study (Figure 7d), and noteworthy predation avoidance behavior of A. japonicus directly adhering to the shell of P. ortmanni was consistently observed in these experiments (Figure 7e). After adhering to the shell, most individuals remained on the shells until the end of the experiment.




4. Discussion
Most hermit crabs are believed to feed on detritus and carrion and are classified as “omnivorous detritivores” [42, 43]. Still, some studies have demonstrated that living organisms form a substantial part of their diet [44], particularly hermit crabs belonging to the genera Dardanus and Paguristes [42, 45, 46]. In the present study, the hermit crab Paguristes ortmanni, known to overlap widely with the habitat of A. japonicus [37, 47–49], was identified as a predator of A. japonicus juveniles, as evidenced by the frequent detection of A. japonicus ossicles in their stomach contents at levels similar to those in the predatory crab P. ferox [33], along with the observed predatory behavior. Although the male P. ortmanni (ID 8) predated nine juveniles within the first 12 h in one of the experiments, the estimated average mortality rate was about one-third that of P. ferox, which is considered to be the most powerful predator of juvenile A. japonicus [33]. However, the average mortality rate was higher than that of the sea star Asterina pectinifera, which was previously considered the principal predator of A. japonicus juveniles [19]. While several other sympatrically occurring decapod crustaceans, such as P. ferox, Pagurus proximus, and Pagurus middendorffii, were observed in the same area, the density of P. ortmanni reached approximately 524 individuals per m3, almost twice of the second most dominant crab P. ferox, in December 2018. In Hokkaido, A. japonicus juveniles are reared to approximately 10–30 mm at a land-based facility and then released to the wild from fall to winter [50, 51], which coincides with the period of population increase in the predatory hermit crab shown in this study.
Throughout the study, A. japonicus exhibited two types of common antipredatory behaviors, namely, muscle contraction and evisceration, and one unique and novel predation avoidance behavior of directly adhering to the shell of P. ortmanni (Figure 7e). P. ortmanni often struggled to feed, shifting prey from one hand to the other during feeding, especially for larger juveniles, taking much longer than P. ferox [33]. Consequently, 3% to 5% of A. japonicus survived despite being attacked and injured in every trial. Releasing size is one of the key factors in improving the survival of released juveniles for a range of invertebrates, including sea cucumber [52], abalone, clam, topshell [28], and crab [53], and is often explained by a higher tolerance to environmental stressors (water temperature and salinity) and predator-induced mortality [52, 54]. It is not certain whether the predation avoidance behavior observed in this study occurs in a natural environment, but the escape behavior of A. japonicus that takes advantage of the blind spot of hermit crabs accompanied by detachment [23, 24] may be very effective in escaping predation. The feeding behavior of decapod crustaceans, such as feeding rate and prey handling, is known to be influenced not only by the size of the prey but also by the size and sex of the predator [33, 55–57]. In this study, more than half of the measured SL of the not-detected group (females) were smaller than that of the smallest individual in the ossicle-detected group, and the average SL of the ossicle-detected group in females was significantly greater than that of the not-detected group. These results indicate an elevated predation risk of A. japonicus juveniles by larger female P. ortmanni. However, in the laboratory experiments, there were no significant differences in mortality rates between sexes, and the mortality rate showed no significant correlation with any body size or shell characteristic variables. The small sample size and imbalance in the male-to-female ratio of the hermit crabs used in the experiments may account for these results. Numerous studies have also reported the influence of the developmental stage, feeding selectivity, and environmental conditions of decapod crustaceans on feeding behaviors [58, 59]. Consequently, future research from these perspectives may provide a more comprehensive understanding of relationships with predatory organisms that contribute to enhancing the population of commercially important endangered A. japonicus.
5. Conclusions
This study presents the first evidence that the sympatrically occurring hermit crab P. ortmanni is a predator of A. japonicus juveniles, emphasizing the necessity of comprehending their distribution and occurrence in regions where hatchery-produced juveniles are released. Furthermore, this research underscores the significance of implementing appropriate measures to ensure successful wild release, such as avoiding areas and periods with high predatory hermit crab density, utilizing physical enclosures to prevent predator invasion, and selecting an appropriate release size to mitigate predation risk.
Conflicts of Interest
Yuji Anaguchi was employed by Ocean Construction Co., Ltd. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflicts of interest.
Author Contributions
Nobuharu Inaba: conceptualization, investigation, analysis, writing–original draft, supervision. Takuma Matsumoto: investigation, writing–review and editing. Yuji Anaguchi: investigation, writing–review and editing. Kohei Matsuno: resources, writing–review, editing, supervision.
Funding
This research was conducted by a management expense grant from Public Works Research Institute, Japan, and did not receive any external funding.
Acknowledgments
We express sincere gratitude to the members of the Fisheries Engineering Research Team, Civil Engineering Research Institute for Cold Region, Japan, for administrative support, especially Sawako Shirai, a technical assistant. We would also like to show our great appreciation to Professor Satoshi Wada from Hokkaido University for his essential advice about hermit crab identification. Our sincere appreciation goes to Dr. Atsushi Yamaguchi from Hokkaido University for providing laboratory space during the investigation.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The original contributions presented in the study are included in the article/supporting information; further inquiries can be directed to the corresponding author.




