Salinity-Induced Gene Expressions in Pangasius nasutus (Bleeker, 1863)
Abstract
This study was designed to evaluate the molecular responses of Pangasius nasutus to salinity exposure of varying concentrations. Juveniles of P. nasutus from similar breeding history (average size of 35 ± 0.54 cm and 152 ± 1.82 g) were obtained from a known source in Terengganu. The salinity range used for this study (i.e., 0, 10, 15, and 20 ppt) was determined through a range finding test, and the juveniles of P. nasutus were exposed in triplicates to these selected salinity ranges for 2 weeks under laboratory conditions. Thereafter, muscle tissues were collected from 10 biological replicates for molecular analysis. Differential display reverse transcriptase PCR was used to identify the expressed cDNA fragments, while real-time PCR was used to determine the regulation of selected genes. The results showed that the 14 differentially expressed genes (DEGs) identified were homologous to nine known genes in GenBank. These proteins include fructose biphosphate aldolase A (ALDOA), troponin I (TnI), myosin heavy chain (MYH), myosin light chain 1a(MLC 1a), creatine kinase (CK), ATPase ii subunit 8 (ATP8) and 6 (ATP6), parvalbumin(PV), ribosomal protein L26(RPL26), and L11 (RPL11). Real-time quantitative PCR (qPCR) analysis of TnI, MYH, and PV showed that the highest expression (p < 0.05) occurred in the 20 ppt salinity treatment group, i.e., 8.79, 3.76, and 1.79 fold more induction than in the control group, respectively. However, the expression of the growth hormone (GH) gene was higher in the 10 ppt salinity treatment than in the control group (p < 0.05). This study confirmed that salinity exposure induces the expression of several genes in P. nasutus, however, future studies need to unravel the importance of these genes for fish performance.
1. Introduction
As the shortage of freshwater deepens in the coming years, inland aquaculture will have to develop adaptive strategies for production in brackish and seawater environments [1]. This includes the environment described as wasteland and areas with salty groundwater [2, 3]. Moreover, the continuous threat of inland water salinization further supports the quest to intensify efforts to produce freshwater fish in brackish water conditions [4, 5]. Habitat salinity represents a major abiotic factor that governs the activities, distribution, and metabolic processes of fishes, and other aquatic animals in the wild [6]. It is, therefore, essential to understand the organism’s ability to cope with ecological changes, especially concerning important water qualities such as salinity. Aquatic fish species are classified as either stenohaline or euryhaline depending on their tolerance to salinity [4, 7]. The differential ability of species to tolerate salinity stress has implications for thriving culture in brackish water conditions because salinity stress affects growth and survival [8].
Under captive conditions, deliberate salinity exposure or salt treatment has arguably become the most common therapy used in the aquaculture industry to treat freshwater fish diseases [9]. This is despite the unofficial approval of its use as a fish drug by the United States Food & Drug Administration [10]. Nonetheless, many fish farmers still refer to salt as the “aspirin” of aquaculture [11]. Its administration at a sublethal concentration for a short duration has been proven effective against external parasites (e.g., Costia, Epistylis, Trichodina, Chilodonella,, etc.), flukes (e.g., Dactylogyrus and Gyrodactylus), anchor worms, and fish lice [11–13]. Its prophylactic action also helps reduce the prevalence of many diseases [8, 14]. An example is the prevention of brown blood disease in channel catfish caused by the high accumulation of nitrite (NO3) in the rearing water [15]. In addition to parasiticide use, salinity treatment helps to reduce fish stress during live transportation and on-farm handling [3].
In addition, some farmers have used salt as a postharvest remedy for off-flavor problems caused by microorganisms, hence increasing the price of salt for consumers [16, 17]. Aside from the short-term application of salinity for these abovementioned purposes, prolonged use of optimized concentrations of salt has been reported to enhance the growth of several species, such as Pangasianodon hypophthalmus [7, 18, 19], Clarias spp. [1, 20], and Tilapia [21, 22]. These findings show that changes in the salinity of the aquaculture system significantly impact physiological homeostasis and routine biological processes of the organisms, as reflected in their performance characteristics. In addition to these changes, the molecular responses of freshwater fishes to salinity exposure have also been reported [23, 24]. However, determining the level of gene regulation for each aquaculture species of interest is essential because it is species-specific.
One freshwater fish species that has not been well studied in this regard is the Asian catfish Pangasius nasutus. It is a native species in Malaysia and is popularly known for its white, fine-grained, and sweet flesh [25]. Despite the slow growth rate of this species, it is three times higher than the performance previously reported for P. hypophthalmus [26]. The preference for this white-fleshed fish over P. hypophthalmus’ yellow flesh has been justified by its global demand beyond Asia to Europe and North America, which constitute its main export destinations [27]. As practiced by many fish farmers, the estuarine cage culture of fish makes the fish highly susceptible to fluctuations in environmental parameters such as salinity [9, 25]. Although salt treatment is also widely used to control ectoparasite infections and muddy flavors in P. nasutus aquaculture [13], there is a paucity of information about the effects of these practices on fish molecular characteristics.
Several genes are expressed following exposure to substantial stressors. One such genes are the ribosomal proteins L26 (RPL26) and L11 (RPL11), which catalyze protein synthesis and consist of a small 40S subunit and a large 60S subunit [28–30]. The ribosomal protein-encoding gene expression is known to be affected by various physiological conditions, hence, they play active roles during adaptation and cellular responses to stress [31]. The ATPase family is also a large family of genes encoding different enzymes that participate in the formation of ATP energy needed during exposure to stressful conditions [32, 33]. The ATPase complex utilizes proton motive force to generate ATP from ADP and Pi [34, 35]. The gene fructose bisphosphate aldolase A (ALDOA) is one of the three isozymes of fructose-1, 6-bisphosphate aldolase [36–38]. ALDOA expression is consequential under conditions that warrant environmental adaptation, hence increasing the conversion of noncarbohydrate substrates (e.g., amino acids or lipids) into glucose and glucose oxidation products to meet energetic needs [39].
Creatine kinase (CK) isoenzymes are also present in tissues with high energy demands, as they catalyze reversible phosphoryl transfer from phosphocreatine to ADP, thereby regenerating ATP from phosphocreatine during cellular work [40, 41]. Myosin is a highly conserved, ubiquitous protein found in all eukaryotic cells, where it provides motor functions for diverse movements, such as cytokinesis, phagocytosis, and muscle contraction [42–44]. TnIs are another example of central regulatory proteins of striated muscle contraction, like myosins [45]. The growth hormone (GH) gene encodes a pituitary polypeptide hormone produced and secreted by the pituitary gland in the brain [46–48]. GH acts via specific cell membrane receptors, such as the GH receptor (GHR) [49]. GH regulates muscle growth directly or indirectly by increasing the number of small-diameter fibers, protein synthesis, DNA and RNA synthesis, lipolysis, and amino acid uptake and incorporation into proteins [50, 51].
The significant sites of osmoregulation and homeostasis relevant to environmental disturbance, such as salinity variation, are the gill, liver, kidney, and intestinal tract [23]. It is, therefore, no surprise that previous studies have identified several genes and hormones from these organs following exposure to varying levels of salinity [1, 20, 21, 24, 52]. However, beyond the initial response of the fish to environmental changes, little is known about the molecular changes in the muscles that occur over a prolonged period. Therefore, this study was designed to determine the genes expressed in response to varying salinity exposure over 2 weeks in P. nasutus. To our knowledge, this study represents the first attempt at characterizing salinity-induced gene expression in P. nasutus. As an outcome of this study, future aquaculture production may be improved by adopting a salinity concentration favoring the expression genes of commercial interest.
2. Materials and Methods
2.1. Experimental Site, Fish Collection, and Range Finding Test
This study was conducted at the freshwater hatchery facility of the Faculty of Fisheries and Food Science, and laboratory analysis was performed at the Anatomy and Physiology Laboratory of the Faculty, Institute of Tropical Aquaculture Laboratory, and Institute of Marine Biotechnology all domicile at the Universiti Malaysia Terengganu. Juveniles of P. nasutus with an average size of 35 ± 0.54 cm and weight of 152 ± 1.82 g were obtained from a recognized fish dealer in Terengganu, Malaysia, and acclimatized at the freshwater hatchery for 1 month. During this period, the fish were fed twice daily at 2% body weight using Cargill Starter Pellet (Cargill, USA; 34% CP) feed. A range-finding test was performed to determine the tolerance of P. nasutus to a gradual increase in salinity. In brief, three aquarium tanks (150 L each) were each used to hold 10 P. nasutus juveniles for the test. Thereafter, the salinity of the water was gradually increased every 3 days using Instant Ocean Sea Salt (Spectrum Brands, USA) (i.e., 5 ppt or 5 g of salt per 5 L per day) until 100% of the fish died. From the challenge test, salinity concentrations of 10, 15, and 20 ppt were selected for the experiment used in this study.
2.2. Fish Exposure to Experimental Conditions and Maintenance
For the main experiment, 12 aquarium tanks were used, each with a capacity of 150 L. To maintain a constant water temperature during the study, each aquarium tank was filled with Fluval M200 Submersible Glass Aquarium Heaters (Hagen, Canada) set at 22°C. A total of 30 fish were then placed in each aquarium tank before the salinity concentration was adjusted as desired. The salinity treatments (10, 15, and 20 ppt) were prepared as described in the range finding test. After carefully weighing and adding Instant Ocean Sea Salt (Spectrum Brands, USA) to the various treatments, the salinity concentrations were confirmed using a portable salinity handheld refractometer (Milwaukee meters, Australia). A control without salt treatment (i.e., 0 ppt) was also maintained in this study. Half the water in the aquarium was changed daily using previously prepared saline water of the same concentration for each treatment. Fish behaviors were monitored frequently throughout the 14-day experimental duration. Water quality was monitored daily using the Hanna’s digital multiparameter water checker (Model HL 98126) to measure pH, dissolved oxygen, total dissolved solids, electric conductivity, and temperature. The water quality was maintained at optimum: pH = 7.01 ± 0.18; Dissolved Oxygen = 5.41 ± 0.29 mgL−1; TDS = 242 ± 0.71 mgL−1; Cond. = 553 ± 0.10 µS/cm; T°C = 22.5 ± 0.11°C. Salinity of the system however is in line with the treatment depicted.
2.3. Sample Collection, RNA Extraction, Quantification, and Purification
At the end of the study, muscle tissue samples (100 g) of 10 numbers of P. nasutus (five from each replicate) per treatment were quickly excised from the dorsoventral part of the fish using sterile scissors and submerged in liquid nitrogen to avoid RNA degradation before being stored at −80°C until use. RNA was extracted using the TRI Reagent Method (Ambion, USA) and stored at −80°C until further use [53]. The purity and quantity of the RNA were spectrophotometrically determined (using a DNA/RNA UV Spectrophotometer, Eppendorf, Germany) at 260 and 280 nm. The RNA samples were electrophoresed using a 1% agarose gel at 90 V for 85 min. The gel was then stained in ethidium bromide (10 mg/mL) for 2 minutes and destained in distilled water (dH2O) for 1 h. Thereafter, the samples were viewed under UV light and analyzed for contamination. In the event of DNA contamination, the samples were purified using the RNA purification steps recommended by the manufacturers [54]. The purified RNA products were confirmed through gel electrophoresis (1% agarose gel) and then stored at −80°C.
2.4. Gene Expression Profiling Analysis
An analysis was conducted to evaluate the differences in the gene expression of the samples from the different treatment groups. The GeneFishingTM DEG Premix Kit (SeeGene, Korea) was used for this study. The procedure included reverse transcription, PCR amplification, and analysis of the agarose gel profile. Total RNA was reverse transcribed using the anchor primer dTACP1 (5CTGTGAATGCTGCGACTACGATXXXXX (T) 18 3) (See Gene, Korea) and the reverse transcriptase enzyme MMLV (Promega, USA). Approximately 3 μg of RNA was used in the first strand cDNA synthesis in a 20 µL reaction. The first strand of cDNA obtained from this procedure was diluted with 80 μL of DNAse-free water, and the samples were stored at −20°C until further use. Thereafter, PCR was performed without a reverse transcript to confirm the lack of DNA transfer. In brief, the differentially expressed genes (DEGs) were screened using arbitrary annealing control primers (ACPs) from GeneFishing kits (SeeGene, Korea).
In the second strand cDNA synthesis, GeneFishing PCR was prepared in a 20 μL reaction volume consisting of first strand cDNA (~50 ng). The second strand cDNA synthesis was done by random PCR amplification using dTACP2 (5′CTGTGAATGCTGCGACTACGATXXXXX (T) 15 3′) and one of the 20 arbitrary ACP (Seegene, Korea) primers shown in Table 1.
| ACP | Sequence |
|---|---|
| ACP1 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGCCATCGACC3′ |
| ACP2 | 5′GTCTACCAGGCATTCGCTTCATXXXXXAGGCGATGCC3′ |
| ACP3 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCCGGAGGATG3′ |
| ACP4 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGCTGCTCGCG3′ |
| ACP5 | 5′GTCTACCAGGCATTCGCTTCATXXXXXAGTGCGCTCG3′ |
| ACP6 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGGCCACATCG3′ |
| ACP7 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCTGCGGATCG3′ |
| ACP8 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGGTCACGGAG3′ |
| ACP9 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGATGCCGCTG3′ |
| ACP10 | 5′GTCTACCAGGCATTCGCTTCATXXXXXTGGTCGTGCC3′ |
| ACP11 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCTGCAGGACC3′ |
| ACP12 | 5′GTCTACCAGGCATTCGCTTCATXXXXXACCGTGGACG3′ |
| ACP13 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGCTTCACCGC3′ |
| ACP14 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGCAAGTCGGC3′ |
| ACP15 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCCACCGTGTG3′ |
| ACP16 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGTCGACGGTG3′ |
| ACP17 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCAAGCCCACG3′ |
| ACP18 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCGGAGCATCC3′ |
| ACP19 | 5′GTCTACCAGGCATTCGCTTCATXXXXXCTCTGCGAGC3′ |
| ACP20 | 5′GTCTACCAGGCATTCGCTTCATXXXXXGACGTTGGCG3′ |
- Abbreviation: ACP, Annealing control primer.
The final product was electrophoresed using a 2% agarose gel at 90 V for 85 min. The gel was visualized under UV light, and the results were analyzed to determine the differences in the cDNA profiles between the salinity treatments. The remaining PCR products were electrophoresed at 90 V for 85 min on a 2% agarose gel and purified using a HighYield Gel/PCR DNA Fragments Extraction Kit (Yeastern Biotech, Taiwan), and the purified PCR fragments were cloned and inserted into a pTZ5R/t cloning vector using an InsTAcloneTM PCR Cloning Kit (Fermentas, USA). The procedure involves ligation, transformation, and screening for positive clones [9].
2.5. Identification of DEGs
The plasmids extracted in the earlier step were sequenced using a BigDye Terminator (version 3.1) Cycle Sequencing Kit (Applied Biosystems, USA). The sequencing procedure was performed on an AB 377 automated sequencer (Applied Biosystem, USA). The raw sequence data obtained were analyzed using several bioinformatic tools, such as CHROMAS, BLAST, and BioEdit. The sequences were identified, characterized, and compared to known sequences from GenBank using the nucleotide Basic Local Alignment Search Tool (BLAST) at the National Centre for Biotechnology Information (NCBI) website: http://www.ncbi.nlm.nih.gov/. Identifying the DNA sequenced was based on the top 20 hits with other known sequences in GenBank.
2.6. Real-Time Quantitative PCR
The sequences obtained in the earlier step were also selected for primer design, which was subsequently used for gene regulation analysis [55]. Four pairs of primers were designed for real-time qPCR using Primer Premier 5 (Premier Biosoft, USA). This study used the β-actin sequence obtained from GenBank as the housekeeping gene [56, 57]. The DEGs sequences were aligned with other known genes available in GenBank using ClustalX software to determine the conserved region within the sequences. Hence, primer pairs were designed using the conserved regions of parvalbumin (PV), TnI, and myosin heavy chain, as shown in Table 2. Similarly, the GH gene primer was designed using GenBank’s P. nasutus GH mRNA sequence (Accession No: M63713.1). All primer designs were validated via PCR amplification and confirmed via agarose gel electrophoresis. This process ensures that the amplification of each gene results in the production of a single gene-specific product. The second criterion was an amplification efficiency >90%. Amplification of PCR products was performed in a thermal cycler using forward and reverse primers designed for the target genes. PCR amplification was performed at 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 58°C for 1 min, and 72°C for 2 min and a final extension at 72°C for 10 min.
| Primer name | Primer sequence | Temperature |
|---|---|---|
| PaPV forward | 5′GTT AAG GCA TAA AAT GCT TCT GAC C3′ | 56.5°C |
| PaPV reverse | 5′CAG TAA GCA AAA TTC ATA TTT AAG ACA GG3′ | 56.5°C |
| PaTnI forward | 5′CCA AAG TAA AAA AAG GAG AAA AAG AGA3′ | 55°C |
| PaTnI reverse | 5′CTC CTT GAC CTC CTT CTT CAC TTG T3′ | 55°C |
| PaMYH forward | 5′CTG TGA ATG CTG CGA CTA CGA T3′ | 59.4°C |
| PaMYH reverse | 5′GCT GAG AGT CAA GAG CAG AGA AGT T3′ | 59.4°C |
| PaGH forward | 5′ATG GCT AGA GTG TTG GTG GTG CT3′ | 61.5°C |
| PaGH reverse | 5′CTA CAG GGT GCA GTT GGA ATC C3′ | 61.5°C |
| β-actin forward | 5′TGA ACC CCA AAG CCA ACA GG3′ | 56.5°C |
| β-actin reverse | 5′ATC ACG GGA GTC CAT AAC AAT ACC AG3′’ | 56.5°C |
The amplified products were visualized in a 2% agarose gel to determine the ability of the designed primers to amplify the cDNA products. A reproducibility test for the primer was performed. In brief, the amplified PCR product was first purified, cloned, and inserted into a pTZ5R/T vector according to the manufacturer’s protocols. This includes methods such as cloning, ligation, and plasmid extraction. The positive clone containing the fragment of interest was sequenced via BLAST and compared with other known gene sequences in GenBank. This confirmed that the primers designed earlier successfully amplified the gene of interest.
2.7. Real-Time qPCR Analysis
RNA samples collected from each salinity treatment group described earlier were subjected to real-time PCR analysis. The RNA was amplified using the primers for PV, TnI, myosin heavy chain, GH, and β-actin, as described in Table 2. The amplification results were analyzed using appropriate software, and the gene regulations were determined. Real-time quantitative PCR was performed with an MJ Mini Personal Thermocycler (Bio-Rad, USA) using the SensiMixTM SYBR NOROX OneStep Kit (Bioline, USA). Ten biological replicates were performed for each treatment (0, 10, 15, and 20 ppt). The cycling conditions were as follows: reverse transcription at 42°C for 10 min; polymerase activation at 95°C for 10 min; 40 cycles of denaturation for 15 s at 95°C, annealing for 15 s at 61.5°C, and extension for 15 s at 72°C for 40 cycles.
The melting curve was generated by increasing the temperature from 55°C by 0.5°C every 10 s until 95°C was reached to check for primer dimers. The β-actin gene was used as the normalization gene. Gene expression was analyzed using Bio-Rad CFX Manager (version 1.6) according to the Livak method [58]. The real-time quantitative PCR products were then electrophoresed on a 2% agarose gel at 90 V for 85 min. The data of the biological replicates were expressed as relative to β-actin and used to normalize any difference in reverse transcriptase efficiency. The threshold cycle (Ct) value was determined using CFX96 real-time PCR software version 1.6 (Bio-Rad, USA). The fold difference for each immune-related gene relative to that of β-actin was calculated using the 2−ΔΔCt method described by Clegg et al. [59] and Sinnasamy et al. [60].
2.8. Statistical Analysis
The gene expression data were analyzed using Minitab 14 computer software. Descriptive statistics were calculated, followed by a test for normality and homogeneity of variance before analysis of variance (ANOVA). When significant differences occurred (p ≤ 0.05), the data were separated using Fisher’s least significant difference (LSD) or the Kruskal‒Wallis nonparametric test (i.e., when normality and homogeneity of the data were insignificant). The results are presented in bar charts generated using Microsoft Excel software.
2.9. Ethics approval/declarations
All applicable international, national, and institutional guidelines for the care and use of animals were followed by the authors as approved by the University Malaysia Terengganu Animal Care Committee (UMT/JKEPHT/201538).
3. Results
3.1. The Differentially Expressed cDNAs in the Muscle Tissue of P. nasutus Exposed to Different Salinity
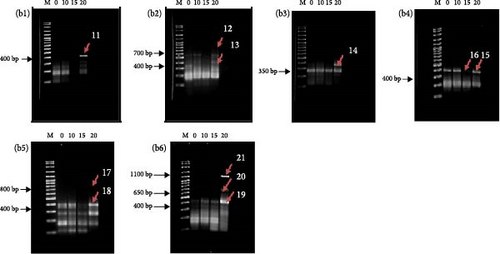
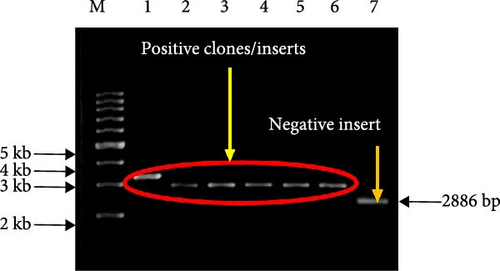
The results of the differential display agarose gel profile analysis are presented in Figure 1. The The agarose gel profiles analysis showed that there were differences in the cDNA bands in each treatment. For each set of arbitrary primers, the differential display patterns of mRNA from the muscle of P. nasutus in the different salinity treatments were, for the most part, similar. Approximately 21 fragments were detected as altered genes during the experiment with varying salinity concentrations (Figure 1(a,b)). Most of the cDNA bands were shown to be highly expressed in the 20 ppt salinity treatment group compared to the other groups. The treatment with the highest number of bands was 20 ppt (18 cDNA), while only 2 cDNAs and 1 cDNA were expressed in the 10 and 15 ppt treatments, respectively. The expression patterns of the 21 cDNA fragments in this study are summarized in Table 3. The differentially expressed cDNA fragments identified with sizes ranging from 200 to 1100 bp were purified and successfully cloned. The appearance of white colonies indicated successful cloning, while blue colonies represented unsuccessful inserts. The negative insert, extracted from the blue colony bacteria cultures, was also shown to have a plasmid size of ~2886 bp, the size of the pTZ5R/T vector (Figure 2).

| Clone name | Expression patterns | |||
|---|---|---|---|---|
| 0 ppt | 10 ppt | 15 ppt | 20 ppt | |
| DEG 1 | — | — | — | ↑ |
| DEG 2 | — | — | — | ↑ |
| DEG 3 | — | ↓ | — | — |
| DEG 4 | — | — | — | ↑ |
| DEG 5 | — | — | — | ↑ |
| DEG 6 | — | — | — | ↑ |
| DEG 7 | — | — | — | ↑ |
| DEG 8 | — | ↑ | — | — |
| DEG 9 | — | — | — | ↑ |
| DEG 10 | — | — | — | ↑ |
| DEG 11 | — | — | — | ↑ |
| DEG 12 | — | — | — | ↑ |
| DEG 13 | — | — | — | ↑ |
| DEG 14 | — | — | — | ↑ |
| DEG 15 | — | — | — | ↑ |
| DEG 16 | — | — | ↓ | — |
| DEG 17 | — | — | — | ↑ |
| DEG 18 | — | — | — | ↑ |
| DEG 19 | — | — | — | ↑ |
| DEG 20 | — | — | — | ↑ |
| DEG 21 | — | — | — | ↑ |
- Abbreviation: DEG, differentially expressed gene.
3.2. Identification of DEGs in the Muscle Tissue of P. nasutus Exposed to Different Salinity
The sequences obtained from 21 DEG clones were analyzed using BLASTN. Of all the DEG clones sequenced, 16, 17, and 19 DEG contained too much noise and hence were excluded from the BLAST analysis. Four of the 18 DEG clones analyzed did not match any known sequences in the GenBank database. However, the remaining 14 DEG clones matched sequences in GenBank, as shown in Table 4. A total of 13 clones were obtained from 20 ppt salinity treatment, while only one (DEG 8 clone) was obtained from 10 ppt treatment.
| Clone name | Insert size (bp) | Sequence homology with other fish | Database match (Acc. No) | E-value | Max ident. |
|---|---|---|---|---|---|
| DEG 1 | 458 | Oreochromis mossambicus parvalbumin mRNA, complete cds | DQ124253.1 | 1e16 | 91% |
| DEG 2 | 461 | Ictalurus punctatus clone CBFA30848 parvalbumin 2 (PRV2) mRNA, complete cds | NM_001201036.1 | 7e25 | 95% |
| DEG 3 | 343 | Unknown | — | — | — |
| DEG 4 | 579 | Hypophthalmichthys molitrix MYH scfast1 mRNA for myosin heavy chain fast skeletal type 1, complete cds | AB303685.1 | 1e72 | 83% |
| DEG 5 | 431 | Ictalurus furcatus clone CBZC23884 fructose bisphosphate aldolase A (ALDOA) mRNA | GU588017.1 | 2e132 | 86% |
| DEG 6 | 630 | Danio rerio troponin I, skeletal, fast 2a.3, mRNA | NM_205575.2 | 2e128 | 86% |
| DEG 7 | 387 | H. molitrix MYH scfast1 mRNA for myosin heavy chain fast skeletal type 1, complete cds | AB303685.1 | 2e75 | 83% |
| DEG 8 | 403 | Cyprinus carpio mRNA for myosin heavy chain | D89992.1 | 6e64 | 87% |
| DEG 9 | 461 | I. punctatus creatine kinase mRNA, complete cds | AF227793.1 | 2e1 20 | 83% |
| DEG 10 | 472 | I. punctatus creatine kinase mRNA, complete cds | AF227793.1 | 3e1 19 | 82% |
| DEG 11 | 187 | P. hypophthalmus ATPase subunit 8 (ATP8) and ATPase subunit 6 (ATP6) genes, partial cds; mitochondrial | EF446971.1 | 4e36 | 100% |
| DEG 12 | 172 | I. punctatus ribosomal protein L26 mRNA, complete cds | NM_001200285.1 | 2e33 | 96% |
| DEG 13 | 135 | Unknown | — | — | — |
| DEG 14 | 354 | Unknown | — | — | — |
| DEG 15 | 389 | I. furcatus clone CBFH3219 parvalbumin beta (PRVB) mRNA | GU587844.1 | 2e157 | 97% |
| DEG 18 | 353 | Unknown | — | — | — |
| DEG 20 | 617 | I. furcatus clone CBZF14977 60S ribosomal protein L11 (RL11) mRNA, complete cds | GU588218.1 | 0.0 | 94% |
| DEG 21 | 1090 | C. carpio mRNA for fast skeletal myosin light chain 1a, complete cds | D85139.1 | 5e133 | 79% |
BLASTn analysis revealed that the DEG 12 clone matched the I. punctatus ribosomal protein L26 (RPL26) mRNA. Moreover, DEG 20 was similar to the I. furcatus clone CBZF14977 60S ribosomal protein L11 (RPL11) mRNA. DEG11 was highly correlated with the partial sequence of the P. hypophthalmus ATPase subunit 8 (ATP8)and ATPase subunit 6 (ATP6) genes. Moreover, BLASTN results showed that the expression of the DEG 5 clone matched that of the I. furcatus clone CBZC23884 ALDOA mRNA. DEG9 and 10 clones were both found to match the I. punctatus CK mRNA. The BLASTN results revealed several DEGs matching myosins, including DEG4 and DEG7, which were homologous to H. molitrix MYH scfast1 mRNA for myosin heavy chain fast skeletal type 1. At the same time, DEG8 was homologous to C. carpio mRNA for myosin heavy chain. Similarly, DEG 21 was matched with C. carpio mRNA for fast skeletal myosin light chain 1a (MLC 1a). Additionally, the DEG6 clone matched the D. rerio troponin I, skeletal, fast 2a.3, mRNA sequence. However, the DEG 1 clone was homologous to O. mossambicus PV mRNA, while DEG 2 and DEG 15 were homologous to I. furcatus PV.
3.3. Regulation of DEGs in the Muscle Tissue of P. nasutus Exposed to Different Salinity
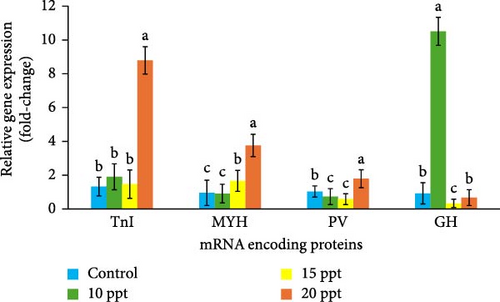
The quantification of gene expression by qPCR is shown in Figure 3. Exposure of P. nasutus to salinity upregulated TnI mRNA by 1.90, 1.47, and 8.79-fold in the 10, 15, and 20 ppt treatments, respectively (p < 0.05), which was greater than that in the control (1.32-fold). Similarly, the mRNA expression of the MYH gene was upregulated in response to 15 ppt (1.66-fold) and 20 ppt (3.76-fold) salinity treatment (p < 0.05) but downregulated in response to 10 ppt treatment (0.91-fold), similar to the control (0.95-fold) (p < 0.05). PV mRNA expression was greatest in the 20 ppt salinity treatment (1.79) but was downregulated in the 10 ppt (0.73-fold) and 15 ppt (0.58-fold) salinity treatments compared to that in the control (1.03-fold) (p < 0.05). However, the 10 ppt salinity treatment significantly enhanced the expression of the GH gene mRNA (10.51-fold) compared with that in the control (0.92-fold) (p < 0.05). In contrast, the expression of the genes in the 15 ppt and 20 ppt treatment groups was downregulated 0.33- and 0.67-fold, respectively (p < 0.05).
4. Discussion
Exposure of freshwater fishes to saline water is expected to trigger corresponding physiological changes. The induced gene expressions following exposure of P. nasutus to varying salinities is what this study was intended to determine. Our finding showed that several genes were expressed in the muscle, including both RPL26 and RPL11 mRNAs (i.e., DEG 12 and DEG 20, respectively). It has been reported that RPL26 is upregulated during anoxia in Littorina littorea and may play a role in stabilizing ribosomal subunits or reinforcing their association with each other during periods of metabolic depression, such as a lack of oxygen [61]. The findings of Lewis and Keller [62] also showed the expression of RPL26 following the exposure of the fathead minnow Pimephales promelas to higher levels of copper. Similarly, a study by MartínezGuitarte, Planelló, and Morcill [63] revealed that RPL11 and RPL13 transcript after exposure to stressors such as heat shock and acute cadmium treatment. The present study showed that RPL26 and RPL11 were expressed when P. nasutus were exposed to 20 ppt treatment. This could mean that the highest salinity exposure in this study stressed the fish, like anoxic conditions, copper toxicity, heat shock, and acute cadmium treatment previously mentioned in other studies.
The DDRTPCR results revealed that ATPase 8/6 was upregulated in the highest treatment (20 ppt), suggesting that this expression in muscle tissue may be related to the energy urgency required to maintain homeostasis. Previously, ATPase expression was observed in Pacific oysters (Crassostrea gigas) in response to pesticide exposure [32]. A similar notion was reiterated with stressors such as hydrocarbon exposure [64] and parasitic infection [65], demonstrating that any source of stress causing increased energy production would result in the expression of these genes. Moreover, the BLASTN result of the DEG 5 clone revealed the gene ALDOA”. It is found predominantly in muscle tissue and is more efficient at facilitating glycolysis than other isoforms, especially in stressful conditions [24, 66]. Perhaps its expression in P. nasutus exposed to 20 ppt treatment was increased as energy production became necessary under the stressful salinity conditions of the experimental treatment.
In addition, DEG9 and 10 matched the I. punctatus CK mRNA. Usually, CK isoenzymes are present in tissues with high energy demands [40, 41]. Moreover, CK expression has also been reported to be related to the Na+K+ATPase (NKA), which provides a driving force for water and ion transport through gulatory epithelia such as gills [35, 66]. Hence, in the study by Weng et al. [40], O. mossambicus acute responses to saltwater treatment (25 ppt) included the upregulation of CK and NKA activity in the gills. Due to the correlation of the regulation observed, the authors concluded that CK may have been upregulated to provide energy for NKA for osmoregulation purposes during acute salinity challenges [40]. This may be the same for fish in the 20 ppt treatment with upregulated CK. Interestingly, the 20 ppt treatment resulted in the expression of CK together with ALDOA and ATPase in the present study. This may be evidence of the need for extra energy in P. nasutus to tolerate salinity changes, invoking a hyperosmoregulatory process in response. However, further studies are needed to determine the relationships between the expression of CK, ALDOA, and ATPase genes and the energy budget for salinity adaptation and acclimatization in P. nasutus.
The expression of genes associated with contractile proteins was also affected by salinity exposure, as shown in the present study. The BLASTN results revealed several DEGs matching myosins (i.e., DEG 4, DEG 6, DEG 7, DEG 8, and DEG 21) and TnI in this study. The expression of these genes is often associated with fish swimming patterns [44, 45]. In general, fish swimming speed increases under stressful conditions compared to during rest [42, 67]. Therefore, since stressful conditions of higher salinity caused P. nasutus to swim faster in the 20 ppt treatment, it is no surprise that the expression of myosins and TnI was greater. However, while most of the DEG clones were upregulated at 20 ppt, DEG8 was more highly expressed after 10 ppt treatment. The cause of this is unknown and could be elucidated in future studies. While there is a paucity of information about the effects of salinity on TnI expression, Lewis and Keller [62] reported a 2.898-fold greater expression of TnI mRNA in plants exposed to 200 µg/L copper than in control. The results from the cDNA microarray in Krasnov et al. [68] study also showed that TnI expression increased after 1 day of stress treatment. This is in line with the observation of our study, where the TnI gene was increased with salinity stress.
Interestingly, PV, a gene well-known for muscle relaxation [69, 70], was also expressed in the P. nasutus muscle tissue in this study (DEG 1; DEG 2; DEG 15). According to some authors, the expression pattern of PV, together with TnI and myosins, reflects the progressive development of muscular tissues and is considered necessary for swimming activity [62, 71, 72]. These studies showed that contractile proteins (myosins and TnI) and PV are essential in responding to environmental stressors to help organisms reach challenging habitats. In contrast to our findings, Kirk [73] reported that PV expression was downregulated in medaka Oryzias latipes after exposure to hypoxic stress. A decrease in PV expression and the subsequent reduction of muscle contraction could be an energy-saving strategy for organisms facing lowered oxygen conditions [73–75]. Therefore, depending on the organism’s energy needs and the stressor’s intensity, the expression of the PV may differ for different stressors and stressor intensities. This may explain why the PV was downregulated at 10 and 15 ppt but upregulated significantly at 20 ppt compared to the control in the present study. Hence, the greater expression of PV at the highest salinity exposure (20 ppt) may have been predicated on the need to promote higher muscular contraction genes (i.e., TnI and MYH) induced by salinity stress.
In the present study, real-time qPCR showed that GH mRNA was also differentially expressed in P. nasutus muscle tissue exposed to different salinities. GH expression was most significant following moderate salinity exposure at 10 ppt and downregulated at 15 and 20 ppt compared to the control. Similar outcomes have also been reported in other studies involving stenohaline fishes. Northern blot analysis has shown a significant increase in pituitary GH mRNA in the channel catfish I. punctatus after 8 ppt saline water treatment [23]. Additionally, the plasma GH concentration in channel catfish increased following exposure to brackish water [76]. Similarly, the plasma GH levels in naked carp increase after being transferred from river water to lake water with moderately high salinity [77]. These findings showed that the expression of the GH gene in stenohaline fishes was induced by lower or moderate salinity exposure. Environmental salinity is well known to influence the growth of many stenohaline fish species, in which moderately high osmotic conditions have been shown to improve their growth [21, 78]. Perhaps the upregulation of the GH gene at 10 ppt is due to the lower energy budget needed for osmoregulation under less stressful salinity conditions, thus providing more energy for growth and development. This finding aligns with Robinson et al. [50] and Nakano et al. [79] studies, where they reported that acute physiological stress downregulates the mRNA expression of growth-related genes in Atlantic salmon and Coho salmon, respectively.
5. Conclusion
In the present study, salinity stress of different magnitudes induced the expression of several genes in P. nasutus. The differential display reverse transcriptase PCR approach identified 14 DEGs in the P. nasutus muscle tissue. Among the genes were ALDOA,TnI, MYH, MLC1a, CK, ATP8 and ATP6, PV, (RPL26), and RPL11. The insight gained from the study was that TnI, MYH, and PV were upregulated at the highest exposure (20 ppt). At the same time, the growth-related genes were notably more highly expressed at the 10ppt exposure. Because of the role of these genes in regulating the physiological functions of fish, future studies could determine the manifestation of the effects of different salinity exposures on fish performance. This approach will clarify each gene’s function and relationship with one another and the physical responses translated into performance. Identifying this as a new culture strategy could improve P. nasutus’s growth and culture for better productivity.
Ethics Statement
All applicable international, national, and institutional guidelines for the care and use of animals were followed by the authors as approved by the University Malaysia Terengganu Animal Care Committee (UMT/JKEPHT/201538).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Danish Daniel Abdullah Muhd, Asma Ariffin Nur, and Sheriff Md. Shahreza, conceptualized and designed the study. Mohamad Ali Nabilah performed the experiments and collected the needed data. Moreover, Victor Tosin Okomoda wrote the manuscript draft with Danish Daniel Abdullah Muhd’s assistance in preparing the text, figures, and tables. All the authors reviewed the manuscript and approved it for submission.
Funding
No funding was received for this research.
Acknowledgments
The authors would like to thank the Faculty of Fisheries and Food Science Hatchery staff and those of the Anatomy and Physiology Laboratory, Institute of Tropical Aquaculture Laboratory, and Institute of Marine Biotechnology. We also thank the anonymous reviewers who helped fine-tune this research for publication in this journal.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




