A Case Study Documenting Growth and Welfare of Atlantic Salmon Reared to Market Size in a Novel Commercial Hybrid Flow-Through System in Norway: A Comparison With Fish Performance in Traditional Sea Sites
Abstract
Production of harvest-size Atlantic salmon in land-based hybrid flow-through systems (HFS) has recently emerged as an alternative to overcome sustainability challenges linked to both the production of postsmolts in recirculation aquaculture systems (RAS) and the grow-out phase at sea. In the present case study, we assessed growth, stress and welfare, key performance indicators (KPIs), and harvest results in a commercial seawater HFS facility, where Atlantic salmon were reared from smolt (~92 g) to market size (~5 kg) between June 2023 and May/June 2024. This was compared with the performance of salmon transferred from the HFS to open net pens at 910 g (to Site A) and 1900 g (to Site B). Salmon in HFS displayed better growth (over 80% higher body weight in May 2024), welfare and stress indicators (lower incidence of skin ulcers, fin damage, and lower plasma cortisol), better KPIs of growth, feeding efficiency and mortality, and better harvest results than in traditional sea Sites A and B. This positive effect could be linked to the absence of severe disturbances and stressful operations in HFS (i.e., well-boat transport or de-lousings) and to the provision of a high-quality rearing environment with large amounts of new fresh seawater, but isolated from external stressors like parasites or pathogens. HFS, thus, appears as a plausible alternative to produce fast-growing harvest-size salmon with high welfare standards, which may raise the interest of more salmon producers.
1. Introduction
Over more than a decade, Atlantic salmon aquaculture in Norway has been facing important challenges that have limited its scope for growth, and thus, the integration of innovative technologies is not only an opportunity, but rather a necessity for the sustainability of the sector [1, 2]. Traditionally, the salmon production strategy comprises two phases: an early land-based phase to produce smolts (up to 100 g), taking place in freshwater flow-through systems, and a subsequent grow-out phase in open net pens located in coastal areas, where salmon is raised up to harvest size (~5 kg) [3, 4]. However, this strategy presents challenges such as high mortalities of smolts after seawater transfer, severe sea lice infestations and disease outbreaks, occurrence of escapees into the wild, and organic pollution in the fjords [5–9]. As a result, Norwegian authorities have imposed strict limitations for new grow-out licenses in the sea, leading to the stagnation of production [1, 2, 10].
To address these sustainability issues, improve public perception, and increase production and profitability in the sector, the salmon industry has sought alternative production systems. One of the strategies most widely adopted is producing larger smolts or “postsmolts” of up to 1 kg in land-based recirculation aquaculture systems (RAS), instead of the traditional smolts of 100 g produced in flow-through systems [1, 11, 12]. This alternative entails extending the land-based phase in RAS under highly controlled and intensive conditions, which results in deploying larger fish in the sea and consequently reducing the period spent in pens or cages. That longer land-based period in a highly controlled and intensive rearing environment allows farmers to work with higher stocking densities and increase growth rates [1], as well as to transfer larger and more robust fish to sea [12]. In addition, the shortened period in sea pens helps reduce the huge economic impact of sea lice and disease [3, 13] and the chances for escapees among others. All considered, the strategy of producing postsmolts in RAS aims to improve fish welfare conditions, optimize the use of sea water licenses, and increase profitability of the sector [1, 11].
RAS are closed land-based facilities with tanks where fresh or sea water is continuously recirculated up to 98%–99% [4, 14]. To maintain high water quality in RAS, external and fish waste products such as particles and ammonia must be removed [15–17]. This removal is carried out, respectively, by using drum filters for particles, and biofilters with bacteria that convert ammonia to nitrite and nitrate [18]. The small amount of new water is mixed with recirculated water and subjected to mechanical filtration and UV irradiation or ozone injection to remove pathogens. Afterwards, water quality is optimized by removing CO2 with gas strippers and injecting O2 [19]. The high percentage of recirculation in RAS helps to maintain a closed environment virtually free from pathogens and parasites and a high control over water quality parameters and temperature [4, 10]. As a result, farmers can work with higher stocking densities and achieve high growth rates [1]. Unfortunately, the technological and operational requirements of RAS, as well as the energy costs and prior investments, are significantly higher than for flow-through systems [1, 11]. In addition, despite transferring larger postsmolts from RAS to sea, issues like high mortalities, severe sea lice infestation, or diseases remain as a challenge after seawater transfer [12]. Moreover, recent research has shown that postsmolts from RAS can in fact face more difficulties adapting to seawater than salmon produced in flow-through, displaying poorer osmoregulatory ability and reduced physiological robustness at sea [20]. These challenges have been linked to the artificially intense and constant conditions experienced in RAS facilities and can result in lower growth and higher mortality in sea pens [20]. All considered, some producers do not perceive RAS today as an optimal production system for postsmolts or market-size Atlantic salmon, and therefore, continue searching alternatives that operate at a lower rearing intensity.
Hybrid flow-through systems (HFS) have recently emerged as an alternative to overcome challenges associated to the production in RAS facilities, while maintaining their benefits. HFS are land-based systems that, like RAS, isolate fish from the external environment, using mechanical and UV filtration to eliminate parasites and pathogens. Consequently, HFS, like RAS, are useful to eliminate a severe issue in salmon farming like sea lice and to minimize disease incidence. In HFS, new water continuously enters the system, operating for periods strictly as a flow-through system or recirculating between 30% and 70% of the water instead of 99% as RAS (A. D. Løland, Bue Salmon AS, personal communication). This continuous supply of new clean seawater in HFS helps maintain higher water quality and reduce energy costs. HFS also allow for CO2 removal and O2 injection to ensure optimal water conditions for the fish. However, unlike RAS, HFS do not require a biofilter to remove ammonia, thus minimizing biological complexity, operational challenges, and energy costs. In addition, HFS like RAS allow increased water velocities, which can be beneficial for growth and welfare [12, 21]. All this considered, HFS appears as a promising alternative to maintain the benefits of land-based production linked to RAS (control and optimization of rearing environment, no parasites, and reduced disease incidence), while reducing biological complexity and operational costs.
Given these potential benefits, an increasing number of Norwegian companies are showing interest on producing postsmolts and market-size salmon in HFS. Examples of companies currently operating HFS are, among others, Bue Salmon AS (Bulandet, Vestland, Norway), Salmon Evolution AS (Indre Harøy, Møre og Romsdal, Norway), Hjelvik Matfisk AS (Vågstranda, Møre og Romsdal, Norway), Hofseth Aqua AS (Tafjord, Møre og Romsdal, Norway), or Steinvik Fiskefarm AS (Svelgen, Vestland, Norway). To the best of our knowledge, some of these companies (Hjelvik Matfisk AS, Hofseth Aqua AS, and Steinvik Fiskefarm AS) use HFS to produce postsmolts below 1 kg, which are later sold to other companies deploying them in open net pens. Others (Bue Salmon AS and Salmon Evolution AS) produce salmon in their HFS from smolt to harvest size. In the case of Bue Salmon AS, part of the production is sold at different sizes (i.e., 1 or 2 kg) to external companies, which deploy the fish in open net pens until harvest. This strategy allows all parties to maximize their production capacity.
However, despite this rising interest in HFS, the number of companies operating these facilities today is minor, and they have been running this system for a short period. Consequently, optimal operation parameters and fish performance and welfare in HFS are largely undocumented in academic reports. In this paper, we present a large-scale case study carried out with Bue Salmon AS, where we aimed to document for the first time fish performance, stress, and welfare in a commercial group reared in HFS to market size. Smolts (~92 g) were raised to harvest size (~5 kg) in HFS between June 2023 and May 2024. As fish grew, part of the production was sold to external companies in western Norway, at 910 g (to Site A) and at 1900 g (to Site B). These companies deployed the fish in traditional sea pens until harvest size. Fish growth, welfare, and key performance indicators (KPIs) in the three companies were assessed to compare what production strategy might be preferable.
2. Materials and Methods
2.1. Ethic Statement
The ethical guidelines of the journal have been adhered to. The study was approved by the Animal Welfare representative at the Department of Biological Sciences (University of Bergen, Norway) as an aquaculture study; up to 1 kg size, the fish were reared under standard commercial aquaculture conditions. Additionally, Bue Salmon AS holds a formal approval from the Norwegian Food Safety Authority to produce salmon above 1 kg in their HFS system (NFSA casefile 2022/062676). Samplings were performed in accordance with guidelines established by the Norwegian Animal Research Authority (NARA).
2.2. Study Design
This case study documents salmon growth, stress, and welfare in Atlantic salmon reared in a commercial HFS facility from smolt (~92 g) to market size (~5 kg) between June 2023 and May and June 2024. Part of the group was transferred to open net pens at sea when their mean body weight was 910 g (to Site A) and 1900 g (to Site B) and reared until harvest size. Performance and welfare indicators as well as harvest results in the three sites were compared to determine the optimal production strategy.
2.2.1. Rearing Facilities at the Three Commercial Sites
Three commercial sites were involved in this study, a new commercial HFS facility owned by Bue Salmon AS (Bulandet, Vestland, Norway) and two traditional sea sites located in Western Norway that use open net pens (Site A and Site B).
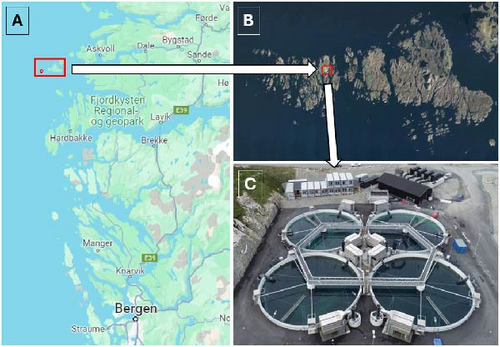
The HFS farm is located at Bulandet archipelago within Vestland county in Norway (Figure 1A,B). This is a pilot facility that commenced to produce salmon commercially in May 2022. It comprises four concrete tanks of 24 m diameter and 8 m depth (Figure 1C). The farm uses four drum filters and six UV filters in the water intake to remove particles, parasites, and pathogens. In addition, each tank is equipped with two degassers to stripe CO2 out of the water when necessary. To regulate O2 in the tanks, the facility has the capacity to inject this gas into the water until reaching adequate concentrations. The tanks are outdoors, only covered with protective nets, and thus, they are exposed to natural photoperiod, although additional constant light is used. This additional constant light is provided in each tank by four Philips fresh water fishlights (250 W) placed 2 m above the ground and at an equal distance from each other. These lights are always on and are only dimmed down during the summer when daylength is very long. The water intake is collected north of the facility from 35 m depth, aiming to avoid parasites like sea lice and to obtain a temperature gain during winter. The water is not heated, and thus, the temperature follows the seasonal pattern.
Sites A and B are traditional sea sites located in western Norway. Site A deployed the salmon from the HFS facility in two open net pens of 65 m diameter and 27 m depth, while Site B used only one open net pen of 40 m diameter and 20 m depth. Site A used additional constant light from 1 January 2024 (six 600 W Ahlsell lights per cage until mid-February, when they were replaced by two 2500 W Biomarine lights per cage). Site B did not use additional constant light in the study pen, but they did in the neighbor pens from mid-January, which entails the possibility of some light leakage.
2.2.2. Fish Stock
On 9 and 10 of June 2023, Bue Salmon AS received 547,860 Atlantic salmon smolts (92.7 ± 28.0 g) that were distributed among the four tanks in their HFS facility (Tank A: 135,717 fish; Tank B: 135,718 fish; Tank C: 137,500 fish; Tank D: 138,925 fish). These smolts were obtained from Alsaker Fjordbruk AS (Onarheim, Vestland, Norway). The fish (of Salmobreed Salmoselect strain) hatched in the Alsaker Fjon Bruk site (Sveio, Vestland, Norway) in two different batches (on 11 April 2022 the “Salar” batch, and 2 June 2022 the “Fjon” batch). The batches started feeding on 19 May and 8 August 2022 respectively. They both remained at the Fjon Bruk facility until 25 October 2022, when the “Fjon” batch was transferred to the Alsaker Salar Bruk facility (Rossland, Vestland, Norway) at a mean weight of 48 g, while the other stayed at the Alsaker Fjon Bruk site (Table 1). Both Alsaker Fjordbruk sites are flow-through facilities. All fish were vaccinated with ALPHA JECT micro 6, ALPHA JECT Moritella and Clynav. The two batches received a winter signal (see Table 1 for specific dates and photoperiod regimes) to induce smoltification and were fed salt-feed Biomar Intro Tuning +40–50 mg to support smoltification. Both batches were reared under a natural temperature profile (Table 1). Upon arrival to the HFS facility, the Salar batch was divided into Tanks A and B, while the Fjon batch was divided into Tanks C and D.
| Batch | Variable | 2022 | 2023 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May (†) | June | July | August (††) | September | October | November | December | January | February | March | April | May | June | ||
| Salar | T (°C) | 7.0 | 7.1 | 10.4 | 13.0 | 13.6 | 12.0 | 9.0 | 4.6 | 3.9 | 3.8 | 4.7 | 13.3 | 13.6 | 12.9 |
| Light | Natural | Natural | Natural | 24:0 | 24:0 | 24:0 | 24:0 | 24:0 | 13:11 | 13:11 | 13:11 | 13:11 | 24:0 | 24:0 | |
| Fjon | T (°C) | — | — | — | 13 | 13.6 | 9.4 | 7.9 | 3.9 | 3.4 | 3.1 | 3.2 | 5.4 | 10.8 | 13.4 |
| Light | — | — | — | 24:0 | 24:0 | 24:0 | 12:12 | 12:12 | 12:12 | 12:12 | 12:12 | 12:12 | Natural | Natural | |
- Note: Temperatures displayed are monthly means. The light regimes show hours of light:hours of darkness (L:D). The “†” in May is to indicate that the “Salar” batch started feeding in this month, while “††” in August indicates that the “Fjon” batch started feeding that month (both in the Alsaker Fjon Bruk facility). Transfer of the “Fjon” batch from the Alsaker Fjon Bruk to the Alsaker Salar Bruk site took place in October 2022.
2.2.3. Establishment and Rearing of Study Groups
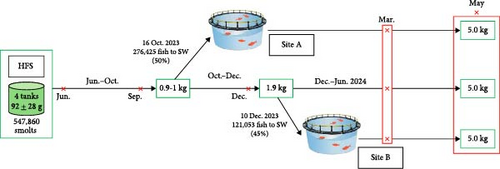
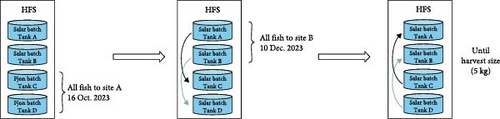
A diagram of the study design is shown in Figure 2A. Between early June 2023 and mid-October 2023, all fish were reared outdoors in the HFS facility in seawater under ambient temperature and natural photoperiod, although also providing additional artificial light (with four 250 W Philips freshwater fishlights that were continuously switched on). On 16 October 2023, the Fjon batch (276,425 fish from Tanks C and D) was transferred in a well-boat to Site A when they had a mean size of 910 g. Upon arrival, the fish were equally distributed among two open net pens of 65 m diameter and 27 m depth and were reared there until harvest size. The batch remaining in the HFS (Salar batch, in Tanks A and B) was equally split in the two empty tanks, C and D (Figure 2B). This fish remained in the HFS facility until 10 December 2023. On this date, fish from Tanks A and B (121,053 individuals, mean weight 1900 g) were transferred to Site B in a well-boat (Figure 2A). A crowding incident occurred at the HFS facility during this transfer to Site B, inducing high stress and loss of scales in the fish. Upon arrival in Site B, all fish were released in one open net pen of 40 m diameter and 20 m depth, where they were reared until harvest size. The remaining fish at the HFS facility were equally divided from Tanks C and D respectively into Tanks A and B and reared until harvest size (Figure 2A,B).
Feed supplied over time in the three sites is displayed in Table 2. At each site, feeding was optimized according to the biomass contained in each rearing unit, the water temperature, and the feeding behavior displayed. Feed was provided continuously using automatic feeders.
| Month | HFS | Site A | Site B | |||
|---|---|---|---|---|---|---|
| Feed | Supplier | Feed | Supplier | Feed | Supplier | |
| Jun. 2023 | Intro 200 HH 50 mg Q | Biomar | — | — | — | — |
| Jul. 2023 | Intro 100 HH 50 mg Q | Biomar | — | — | — | — |
| Aug. 2023 | Intro 200 HH 50 mg Q | Biomar | — | — | — | — |
| Sep. 2023 | Power 500 50 mg | Biomar | — | — | — | — |
| Oct. 2023 | Assist Skin 500 50 mg | Biomar | SG 500 70 A | Skretting | — | — |
| Nov. 2023 | Assist Skin 500 50 mg/Power 1000 50 mg | Biomar | SG S 1000 70 A | Skretting | — | — |
| Dec. 2023 | Power 1000 50 mg | Biomar | Protec SG V 1000 70 A | Skretting | SG 500 50 mg Q (6 mm) | Biomar |
| Jan. 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | Assist Skin SG 1000 V 50 mg Q (9 mm) | Biomar |
| Feb. 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | Assist Skin SG 1000 V 50 mg Q (9 mm) | Biomar |
| Mar. 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | Power 2500 50 mg Q (9 mm) | Biomar |
| Apr. 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | Power 2500 50 mg Q (9 mm) | Biomar |
| May 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | SG 1000 50 mg Q (9 mm) | Biomar |
| Jun. 2024 | Rapid HFT 1000 70A/Rapid HFT Dermic 1000 70 A | EWOS | SG V 1000 70 A | Skretting | SG 1000 50 mg Q (9 mm) | Biomar |
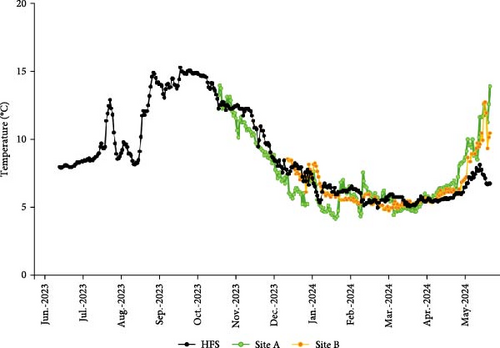
Water temperatures were monitored daily in the three sites and values over time are shown in Figure 3. Mean oxygen saturation in HFS was 92.4% ± 6.8%, in Site A 96.5% ± 10.8%, and in Site B the mean was 84% with values ranging between 73% and 103%. Mean salinity in HFS was 33.8 ± 0.9 ppt and mean pH was 7.4 ± 0.3; mean salinity in Site A was 28.1 ± 3.9 ppt and pH was not reported. None of these two parameters were measured in Site B.
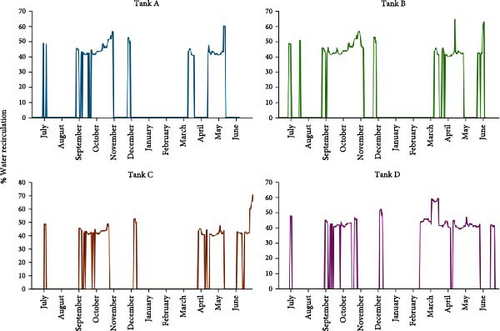
The HFS facility operated with variable percentages of water recirculation, depending on the water quality requirements. Figure 4 displays the percent of water recirculation during the study period in the four tanks. In general, the facility operated with 40%–50% recirculation during a short period in early July, between late August and late October, during a short period from late November to early December, and from March to May. The rest of the time, the HFS facility operated strictly as a flow-through system (0% water recirculation).
2.3. Samplings
Five fish samplings were carried out in the study, the first three only in the HFS facility and the last two in the three study sites (indicated with red “x” in Figure 2A). Sampling dates and number of fish collected from each site and rearing unit are displayed in Table 3. In HFS, except in the first sampling (40 fish sampled), 60 fish were collected in each sampling (15 per tank). In Sites A and B, 40 individuals were collected per sampling (Table 3). A total of 440 fish were sampled during the study and used for morphometric, somatic indexes, and external welfare indicator analyses. A subsample of 282 fish, including a random representative sample from each site and sampling, were used for plasma cortisol analyses (Table 3).
| Sampling | Date | Site | N of fish | N per rearing unit | N for cortisol |
|---|---|---|---|---|---|
| 1 | 21 June 2023 | HFS | 40 | 10 | 40 |
| 2 | 14 September 2023 | HFS | 60 | 15 | 38 |
| 3 | 27 November 2023 | HFS | 60 | 15 | 30 |
| 4 | 15 March 2024 | Site B | 40 | 40 | 27 |
| 12 March 2024 | Site A | 40 | 20 | 25 | |
| 19 March 2024 | HFS | 60 | 15 | 34 | |
| 5 | 15 May 2024 | Site A | 40 | 20 | 25 |
| 22 May 2024 | HFS | 60 | 15 | 36 | |
| 23 May 2024 | Site B | 40 | 40 | 27 | |
- Note: Except for the first sampling, 60 fish were collected from the HFS facility in each sampling (15 per tank). In Sites A and B, 40 individuals were collected per sampling. In total, 440 individuals were sampled during the study.
In each sampling, fish were collected with nets by staff at the sites and were immediately euthanized with an overdose of benzocaine (50 mg/L) in a bath (Benzoak vet. 20%, ACDPharma AS, Norway). Blood was collected from the caudal vein using heparinized syringes and centrifuged for 4 min at 5000 rpm. Plasma was immediately frozen on dry ice and stored at –80°C until cortisol analysis. Fork length and body weight were measured to the nearest 0.1 cm and 0.1 g respectively. All fish were externally inspected and scored for welfare indicators such as dorsal and caudal fin damage, presence of skin ulcers, or eye damage. This scoring assigned a number between 0 and 3, according to the severity of the welfare issue, following the systems published by Noble et al. [22]. Fish were then dissected and organs like liver, heart, and gonads were extracted and weighed to the nearest 0.001 g in order to calculate the respective somatic indexes. All individuals (n = 440) were used for morphometric and welfare assessments, while a subsample of n = 282 individuals (that included a relevant number per tank or cage in each sampling) were used for plasma cortisol analysis.
2.4. Morphometric Calculations and Data Acquisition
- •
Condition factor: K = 100 × Wbody/L3, where Wbody is the body weight (g) and L is the fork length (cm).
- •
Hepatosomatic index (HSI): HSI (%) = 100 × Wliver/Wbody, where Wliver is the weight of the liver (g) and Wbody is the body weight (g).
- •
Cardiosomatic index (CSI): CSI (%) = 100 × Wheart/Wbody, where Wheart is the weight of the heart (g) and Wbody is the body weight (g).
- •
Gonadosomatic index (GSI): GSI (%) = 100 × Wgonads/Wbody, where Wgonads is the weight of the gonads (g) and Wbody is the body weight (g).
Production data from each site were provided by the companies. This included number of fish per rearing unit, mean weight per month, biomass over time, stocking densities, feed conversion ratio (FCR), specific growth rate (SGR), mortality, number and description of operations like de-lousings, final harvest reports from the processing plant, and environmental parameters.
- •
Superior: Highest quality product, defined by skin without damages and defects, no wounds or deformities, no strange pigmentation in flesh, normal shape, and no sexual maturation.
- •
Ordinary: Salmon with certain limited faults, that is, scale loss, some minor shallow skin damage, and minor melanin deposits in the fillet, but otherwise similar to Superior in the rest of traits.
- •
Production: Salmon with skin ulcers or wounds, severe scale loss, deformities, handing deffects, wrong pigmentation in fillets, sexually maturing.
2.5. Plasma Cortisol Analyses
Cortisol concentration was analyzed in 282 plasma samples (see N per sampling and group in Table 3) using a commercial competitive enzyme-linked immunosorbent assay (ELISA) kit (Demeditec Diagnostics GmbH, DEH3388). Assays were run in the 96-well plates provided, using calibrated standards and quality controls also provided with the kit. Cortisol samples were measured in triplicate using 10 μL of plasma per well. In the plate, cortisol from the sample competes for binding to the coating anticortisol antibody with a known concentration of horseradish peroxidase-labeled cortisol. Adding 3,3,5,5-tetramethylbenzidine (TMB) causes a color change in the wells, which is determined at 450 nm in a Tecan Spark multimode microplate reader and compared to standards. Final cortisol concentration is calculated with a four-parameters Marquardt logistic regression using an extrapolation factor of 1.
2.6. Statistical Analyses
Before fitting statistical models, distribution of the response variables was checked with Shapiro–Wilks tests and histograms. Linear models (LMs) were fitted between morphometric parameters (responses) and “Site,” “Time,” and their interaction as independent variables. For plasma cortisol, we used instead a generalized LM (GLM) with a “Gamma” family and an “inverse” link, as fitting a parametric model was inappropriate due to the extremely skewed distribution of this variable. After LMs, residual plots were used to assess assumptions like normality (q–q plots), linearity (residuals vs. fitted), homogeneity of variance (scale–location plots), and the existence of influential outliers (residuals vs. leverage plots with Cook’s distance). Homogeneity of variance was also assessed with Levene’s test. When the models did not meet the parametric assumptions, the response variable was log-, square-root- or inverse-transformed, the model repeated, and the assumptions assessed again. Tukey HSD post hoc tests were used to reveal significant differences in the response variable between sites at given samplings and within sites over time. Line plots of response variables over time display mean ± standard error. Significant differences in proportion of fish with skin ulcers, dorsal, and fin damage, between sites in samplings 4 and 5, were assessed with Fisher’s exact test for count data. A significance level α = 0.05 was used. All statistical analyses were performed in R and Rstudio, using the packages “car” [24], “ggplot2” [25], “Rmisc” [26], and “emmeans” [27].
3. Results
3.1. Body Weight and Condition Factor
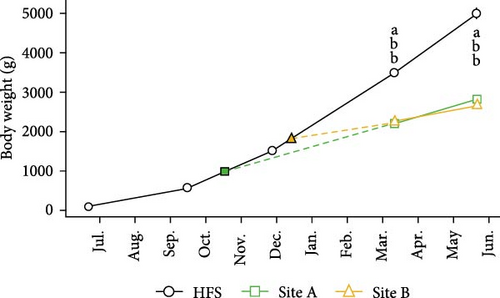
Body weight (Figure 5A) was significantly dependent on Site, Time, and their interaction (all p < 0.001). In March and May (samplings 4 and 5), mean body weight was significantly larger in HFS than in the sea Sites A and B (all p < 0.001). In fact, in sampling 5, mean weight was over 80% higher in HFS (4991.8 g) than in the Sites A (2817.4 g) and B (2664.7 g). However, it must be noted that mean body weight in Site B was probably higher, as prior to our sampling 5 the fish had been sorted to harvest the largest individuals (43,250 fish that were harvested on 14 and 15 May 2024 with a mean weight of 3.4 kg).
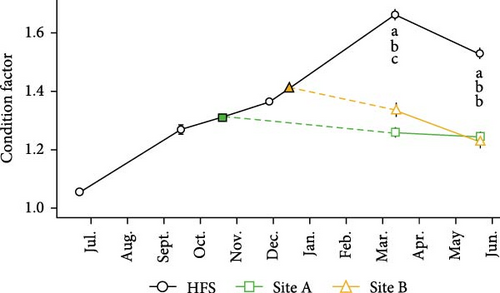
Condition factor (Figure 5B) was also dependent upon Site, Time, and their interaction (all p < 0.001). In March and May (samplings 4 and 5), condition factor was significantly higher in HFS than in Sites A and B (all p < 0.001). In addition, in sampling 4, condition factor was higher in Site B than Site A (p < 0.01).
3.2. Somatic Indexes
HSI (Figure 6A) was significantly dependent on Site (p < 0.001), Time (p < 0.01), and their interaction (p < 0.001). HSI significantly decreased in HFS through the fall (p < 0.01) until December and increased since then until March (p < 0.001). Between March and May, HSI remained similar in HFS fish, but significantly decreased in Sites A and B (both p < 0.001). In March, HSI was significantly greater in Site B than in HFS and Site A (both p < 0.001) and greater also in HFS than in Site A (both p < 0.001). In May, HSI was significantly lower in Site A than in the other two sites (both p < 0.001).

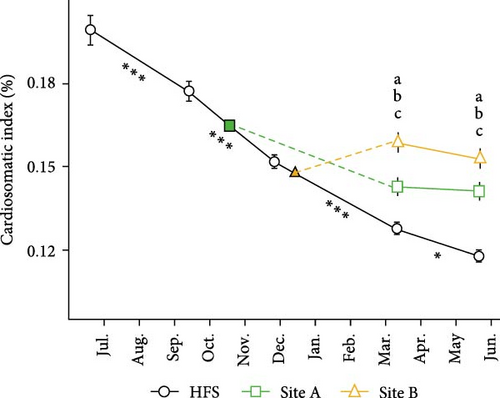
CSI (Figure 6B) was significantly dependent on Site, Time, and their interaction (all p < 0.001). CSI significantly decreased in HFS over time between all samplings (all p < 0.05), but remained stable in Sites A and B. In March, CSI was significantly higher in Site B than in Site A and HFS (p < 0.01 and <0.001, respectively) and it was also higher in Site A than in HFS (p < 0.001). Something similar was observed in May, when CSI was higher in Site B than Site A and HFS (p < 0.05 and <0.001 respectively) and higher in Site A than in HFS (p < 0.001).
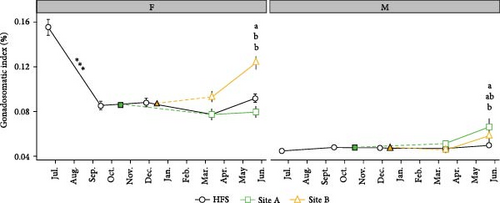
GSI for each sex (Figure 6C) was dependent upon Site and the interaction Site × Time (both p < 0.001). In females (F, left), GSI decreased significantly from July to September in HFS (p < 0.001). Female GSI was significantly higher in May in Site B than in Site A (p < 0.001) and HFS (p < 0.05). In males, GSI was significantly higher also in May in Site A than in HFS (p < 0.01).
3.3. Farm Operations and KPIs in the Three Sites
A summary including the farming operations carried out in each site, the stocking density on samplings 4 and 5, and KPIs up to sampling 5 in the three study sites is presented in Table 4. Fish in Sites A and B were subjected to well-boat transfers from the HFS, while fish remaining in HFS only experienced rapid internal transfers between tanks within this facility (Figure 2B). As mentioned, a crowding incident occurred during the transfer to Site B on 10 December 2023, eliciting high stress on the fish and causing them to lose scales. Similarly, de-lousings took place only in Sites A (mid-February 2023 with Salmosan and mid-July 2023 with FLS Caligus delouser) and B (mid-May 2023, Thermolicer at 30°C), but not in HFS.
| Site | Farming operations | Stocking density (kg/m3) | KPIs up to sampling 5 | ||||
|---|---|---|---|---|---|---|---|
| Site transfer | Delousings | On S4 | On S5 | SGR (%) | FCR | Mortality (%) | |
| HFS | 0 | 0 | 35.5 | 42.6 | 0.78 | 1.15 | 0.8 |
| Site A | 1 | 2 | 2.3 | 4.5 | 0.52 | 1.24 | 2.9 |
| Site B | 1 | 1 | 10.1 | 12.4 | 0.21 | 1.38 | 12.2 |
Stocking densities in samplings 4 and 5 were always the highest in HFS (ranging from 35.5 to 42.6 kg/m3) and the lowest in Site A (2.3–4.5 kg/m3), being in general notably higher in HFS than in the two marine sites (Table 4). Density in HFS and Site B in sampling 5 was calculated using biomass data from late April instead of from May; this aimed to avoid underestimating the density values in the two sites, as harvesting had commenced before we last sampled them in May.
The KPIs (SGR, FCR, and mortality) were the best in HFS and the worse in Site B, while results from Site A were in between but closer to HFS than to Site B (Table 4). The SGR presented in both HFS and Site A was calculated using mean fish weight at transfer to Site A (16 October 2023) as initial weight, and then, using the number of days until their corresponding date of sampling 5 (Table 3). SGR for Site B was instead calculated using the initial mean weight of the fish at transfer to this site on 10 December 2023 and the number of days until this group was sampled in sampling 5. However, it is worth considering that, as mentioned earlier, the largest fish in Site B (43,250 fish with a mean weight of 3.4 kg) had been harvested before we last sampled Site B. Without this harvest, we may expect a higher final mean weight on sampling 5 (~3 kg instead of 2.66 kg), and therefore, SGR would be close to 0.30% instead of the 0.21% displayed in Table 4. FCR in HFS and Site B was also calculated using data collected right until before the harvest started (this is, feed used and biomass gain until late April). Finally, mortality in HFS and Site A was calculated for the period 16 October 2023–23 May 2024, while in Site B, it was calculated for the period 10 December 2023–23 May 2024.
3.4. External and Physiological Welfare Indicators
The incidence of deformities in the study was negligible, with only three out of the 440 fish sampled (all of them collected in HFS in samplings 2, 3, and 4) displaying deformities (spine, jaw, and opercular deformities, respectively). Growth-stunted or emaciated smolts were collected only in sampling 1, shortly after they had been transferred to the HFS facility (7 out of 40 individuals, presenting very thin appearance with low condition factor). Eye injuries were not detected in any site during the study.
Presence of skin ulcers, dorsal, and caudal fin damage in the three sites in samplings 4 and 5 is presented in Table 5. All scores equal or greater than 2 were considered “severe” and were used to calculate the proportion of fish most severely affected by a welfare issue. In the first three samplings together (in HFS), only 25 out of the total 160 sampled fish had skin ulcers, of which nine were collected in the second sampling (mid-September, only two severe ulcers) and 16 in the third sampling (late November, six severe ulcers).
| Site | Sampling 4—mid-March 2024 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin ulcers | Dorsal fin damage | Caudal fin damage | ||||||||||||
| n | % | n severe | % severe | n | % | n severe | % severe | n | % | n severe | % severe | |||
| Site A (n = 40) | 9 | 23a | 4 | 10a | 25 | 63 | 5 | 13 | 1 | 3a | 0 | 0a | ||
| Site B (n = 40) | 22 | 55b | 13 | 33b | 30 | 75 | 9 | 23 | 26 | 65b | 7 | 18b | ||
| HFS (n = 60) | 12 | 20a | 4 | 7a | 31 | 52 | 8 | 13 | 7 | 18a | 0 | 0a | ||
| Sampling 5—mid-May 2024 | ||||||||||||||
| Skin ulcers | Dorsal fin damage | Caudal fin damage | ||||||||||||
| n | % | n severe | % severe | n | % | n severe | % severe | n | % | n severe | % severe | |||
| Site A (n = 40) | 15 | 38b | 10 | 25b | 28 | 70 | 6 | 15a | 6 | 15a | 0 | 0a | ||
| Site B (n = 40) | 30 | 75c | 15 | 38b | 33 | 83 | 21 | 53b | 33 | 83b | 4 | 10b | ||
| HFS (n = 60) | 4 | 7a | 1 | 2a | 38 | 63 | 10 | 17a | 4 | 7a | 0 | 0a | ||
- Note: Significant differences (p < 0.05) between sites in proportion of total and severe welfare issues in each sampling are displayed with different letters “a,” “b,” and “c.” If letters are not displayed, proportions in the three sites are statistically similar. These statistical differences were assessed with Fisher’s exact tests for count data.
In sampling 4, fish in Site B displayed significantly higher incidence of all types of skin ulcers and of caudal fin damage, both mild and severe, than fish in Site A and HFS (Table 5). Differences in proportion of dorsal fin damage between sites were not significant, although this issue tended to be more present also in Site B. In contrast, incidence of welfare issues in Site A and HFS were in general statistically similar, but with the tendency to be less present in HFS.
In sampling 5, proportions of all welfare problems and of all severities were also higher in Site B than in the other two sites; in addition, Site A had a higher proportion of all types of skin ulcers than HFS. However, although the incidence of skin ulcers was high in Site B, the majority of those ulcers (19 out of 30 or 12 out of 15, if considering only the severe) were clearly healing. Differences in incidence of fin damage between Site A and HFS in sampling 5 were not significant, but the proportion tended to be lower in HFS than in Site A.
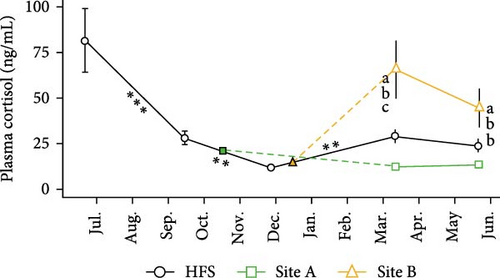
Plasma cortisol (Figure 7) was dependent upon Site and Time (both p < 0.001). Mean cortisol levels were the highest in HFS in June 2023 right after the smolt transfer. Cortisol decreased significantly from June to September (p < 0.001) and further to December (p < 0.01) when the levels were the lowest, to start increasing afterwards until March (p < 0.01). No changes over time were observed within Sites A and B. Site B displayed the highest mean plasma cortisol in both samplings, compared to Site A (both p < 0.001) and HFS (both p < 0.05). In addition, plasma cortisol was higher in HFS than in Site A in March (p < 0.01).
3.5. Harvest Reports
Harvest commenced first in HFS, on 29 of April 2024 (Table 6 and Figure 8). Since then, harvest in HFS took place in eight batches, with each of the four tanks being harvested in two different batches (the last on 17 June 2024). Harvest in Site A and B was delayed in respect to HFS, commencing and finishing on 6 August–26 August (Site A) and 14 May–12 July (Site B) (Table 6 and Figure 8). Despite starting and finishing harvest earlier, the HFS fish had the highest mean weight (4.7 kg), while the lowest was reported in Site B (3.3 kg). In addition, HFS reported the highest proportion of “Superior” fish (94.2%) and the lowest proportion of “Production” (4.5%), while the opposite occurred in Site B (68.4% “Superior” and 21.5% “Production”). Harvest results in Site A were similar to HFS in terms of mean weight and, roughly, in percentage of fish in each quality category (though slightly better in HFS); however, start of harvesting in Site A was delayed 3 months compared to HFS to reach the same mean weight (Figure 8).

| Site | Harvest | Amount | Quality (%) | ||||
|---|---|---|---|---|---|---|---|
| Period | Batches | N fish | Mean weight (kg) | Superior | Ordinary | Production | |
| HFS | 29 April–17 June | 8 | 133,890 | 4.7 | 94.2 | 1.3 | 4.5 |
| Site A | 6–26 August | 8 | 238,146 | 4.7 | 92.5 | — | 7.5 |
| Site B | 14 May–12 July | 5 | 113,350 | 3.3 | 68.4 | 10.1 | 21.5 |
- Note: The batches indicate the number of harvest groups in which the fish from each site was divided at harvest. Harvest took 49 days in HFS, 20 days in Site A, and 59 days in Site B. The three quality categories are ordered from maximum quality (“Superior”) to minimum (“Production”), and they determine the price of the salmon.
4. Discussion
The present study compared for the first time fish growth, stress, and welfare, as well as various KPIs and harvest results, in salmon reared commercially from smolt to harvest size in HFS versus in salmon reared in open net pens at sea. Results evidenced significantly faster growth, better welfare and stress indicators, and consequently better KPIs and harvest results in HFS than in two traditional sea sites, which had obtained the salmon from the HFS at 0.9 and 1.9 kg. Our findings evidence a very positive effect of HFS on salmon performance that can be genuinely attributed to this land-based rearing system. This effect is probably linked to both the reduced disturbances and stressful operations during the production period in HFS and to the provision of a high-quality rearing environment with large amounts of new fresh seawater, but highly isolated from external challenges like parasites or pathogens. Such a combination allowed for the maximization of growth and welfare indicators. These aspects are discussed in the following lines.
4.1. Production of Market-Size Salmon in HFS was Better Than in Sites A and B
Indeed, fish growth, welfare and stress indicators, farming KPIs (SGR, mortality, and FCR) and harvest results were the best in HFS, better than in salmon that had been transferred to traditional sea sites from the HFS at 0.9 and 1.9 kg. This points at HFS as a rearing system that can provide increased growth rates and improved fish welfare when producing market-size salmon, leading to outstanding harvest results. Based on this, HFS could emerge as a realistic alternative to solve long-term salmon aquaculture issues such as mortalities at sea, high incidence of sea lice, or disease outbreaks among others, while at the same time maximize growth and fish welfare. It must be however noted that welfare and stress indicators were roughly similar in HFS and Site A (although with the tendency to be slightly better in HFS); this would demonstrate that fish welfare and stress need not always be necessarily better in HFS than in sea pens in the long term. However, due to the continuous exposure to the natural environment, sea cage rearing does necessarily entail increased welfare risks, sea lice infestations, and higher probability of disease [1]. This is in addition to the reciprocal risks that sea cage farming presents for the environment, which can include escapees cross-breeding with wild samon, sea lice and disease transfer to native populations, and organic pollution due to excess feed and feces among others [28, 29]. These issues may or not be realized in each specific fish batch, but this depends on many external and nonpredictable circumstances. As a result, producers using traditional sea pens will always have to account for increased risks and higher degree of uncertainty for their production and for the environment, which they may only minimize by reducing the period the fish spends at sea (i.e., to 5–6 months). In contrast, in HFS, this degree of uncertainty is minimized as in other closed containment systems due to its isolation from the external environment [4, 30], and therefore, the probability of better fish welfare and growth performance is higher and more predictable there than in sea pens.
Despite the mentioned similarity in welfare and stress parameters in HFS and Site A, KPIs and especially growth were noticeably better in HFS; salmon here attained in late May a mean body weight of 4991.8 g, over 80% higher than in Site A (2817.4 g). Such weight gain in HFS was realized in roughly 7 months (from mid-October 2023, when fish was 0.9 kg, to late May). This means that, in such period, salmon in HFS gained 4 kg in body mass, while salmon in Site A gained 1.9 kg on average. In addition, although harvest results were roughly similar in HFS and Site A in terms of percentage of fish within the different harvest qualities, salmon in Site A required around three extra months during the summer to attain the same harvest size that salmon in HFS had attained in May (4.7 kg). These three extra months at sea represent a large risk for producers due to the longer exposure to the environmental stressors, something that, as previously mentioned, farmers currently aim to minimize (G. M. Knutsen, Bremnes Seashore AS, personal communication). All considered, our findings evidence a clear difference in growth rate in favor of HFS, similar or even slightly better fish welfare conditions and harvest results, and a reduced degree of risk or uncertainty. Reports from another company using HFS like Salmon Evolution AS indicate high consistency in the performance of this rearing system, with proportions of “Superior” salmon at harvest between 90% and 96% in four consecutive quaterly reports [31]. This number and the 94% “Superior” reported by Bue Salmon AS in the present study are well above the average 85% superior achieved by the traditional salmon industry [23], and thus, they both show the reliability and consistency of HFS performance. If this consistency continues in the future, the interest of more salmon producers on this type of production system will increase.
While as mentioned, fish in HFS and Site A showed some positive similarities, salmon in Site B performed significantly worse in almost every parameter assessed. Compared to HFS, salmon in Site B displayed reduced growth, significantly worse welfare indicators, elevated signs of stress, and poorer KPIs like FCR or mortality, as well as clearly poorer harvest results. This poorer performance can to a high extent be associated to the crowding incident occurred in the HFS when the fish was about to be transported to Site B. This crowding episode caused mucus and scale loss in many individuals and must have elicited increased levels of stress [32, 33]. Previous research has shown that skin areas where mucus and scales are lost are more vulnerable to oportunistic and especific bacterial infections [34, 35]. The vulnerability of affected skin areas will increase if the fish is highly stressed, as probably occurred in this case due to the crowding, the subsequent well-boat transport, and the release in a new and exposed environment. This occurs because high stress can depress the fish immune system, thus, impacting their skin barrier function to cope with environmental pathogens [36, 37]. In addition, this incident occurred in mid-December at around 6°C, ideal conditions for development of bacteria like Moritella viscosa, which are a main responsible for causing “vintersår” or winter ulcers in the salmon skin [34, 38, 39]. This unfortunate set of conditions resulted in the significantly higher proportion of fish affected by winter ulcers and fin damage in Site B in the last two samplings. The poorer welfare indicators were consistent with the increased levels of plasma cortisol measured in this group, indicating that individuals in Site B were experiencing more long-term stress than those in HFS and Site A, which generally displayed baseline cortisol levels typical of low stress [36, 40]. This elevated stress and the high presence of skin ulcers surely contributed to the higher mortality, delayed growth, and poorer harvest results of Site B, with 21% of this fish sold as “Production.”
The occurrence of this unfortunate episode during the transfer to Site B and the severity of its consequences reveal the risks that farming operations involving fish manipulations and movements imply for the final performance of a commercial group. As this case illustrates, unexpected incidents can sometimes occur, having severe direct consequences that may include high mortalities or physical trauma in fish, stress, and disease [22]. In addition to this, farming operations and stressors from the environment are likely to also elicit subtle but continous impacts more difficult to measure in the short term, but that may have an equally serious impact on the group’s performance in the long term (i.e., subchronic stress and subsequent growth delays, induced impacts on immune system, possible disease outbreaks, etc.) [22, 41]. The cummulative long-term effects due to the disturbances and continuous exposure to stressors must have been behind the similar growth delay observed in fish in Site A and B compared to salmon in HFS. Indeed, salmon sampled in both Sites A and B displayed similar growth performance and both were equally delayed in growth compared to HFS at the end of the study period in May, this even despite the fact that Site B was displaying significantly worse welfare and stress indicators. This suggests that long-term cummulative effects taking place only in sea pens or linked to that production strategy, something that was not present in HFS, led to the observed similar delay in growth in Sites A and B. Afterwards, the different harvest size between Sites A and B (4.7 vs. 3.3 kg, respectively) was partly explained by the longer time that fish Site A spent at sea in summer until harvest (which took place in August), compared to fish in Site B (harvested between mid-May and mid-July).
4.2. What Factors Led to Better Growth in HFS Compared to Sites A and B?
Before entering a deeper discussion on what may have caused the growth delay in the sea sites, we must first discard the contribution of factors well-known to influence growth like water temperature, feeding regimes, or stocking densities. Temperature determines the scope for growth in ecthoterm organisms like Atlantic salmon, with elevated temperatures within the tolerance range of the species generally inducing faster growth rates and increased appetite [42]. In our case, the temperature profiles in the three sites were most of the time very similar and even tended to be lower in HFS during late April and May. As such, the better growth performance in HFS cannot be linked with differences in temperature. Similarly, it seems unlikely that differences in the feeds supplied or in the rations provided would explain the large differences in growth in favor of HFS. The three sites used well-established and tested commercial feeds, at rations that were optimized for their biomass and temperatures. Thus, it is unlikely that differences in feed composition of those feeds or in the amounts of feed provided were responsible for the large differences in growth observed. We cannot relate these differences with feed utilization either. FCR was the lowest in HFS, evidencing that feed was used the most efficiently in this site thus requiring the lowest amount among the three sites to produce 1 kg of salmon. In contrast, Site B had the worst feed utilization which could be linked to the higher mortality and higher stress levels that this fish seemed to experience. This higher stress most likely led many individuals to have reduced appetite or cease feeding for periods [43, 44], resulting in increased wasted feed and the elevated FCR observed. However, despite these differences in feed utilization, feed was always available in excess in the three sites, and therefore, it would be unlikely that ration impacted growth to the extent we saw in our results. Finally, the differences in stocking densities at the sites are also unlikely to be related with the differences in fish growth. Poorer growth performance has been observed at increased stocking densities, for example, over 75 kg/m3 for postsmolts in land-based facilities [45] or over 26.5 kg/m3 for adult salmon in sea pens [46]. However, in our study, the highest growth occurred in HFS operating at the highest stocking density of the three. In any case, this higher stocking density in HFS (42.6 kg/m3) was still within reasonable limits for land-based facilities (75 kg/m3 according to Calabrese et al. [45]).
After discarding the previous factors, the significantly better growth in HFS than in Sites A and B must then be linked to the various disturbances and stressors, both acute and long-term, that fish in sea sites experienced. First, salmon in Sites A and B were subjected to operations that must have directly and indirectly limited their scope for growth, while this did not occur to salmon in HFS. For example, the two sea sites groups experienced a transfer in a well-boat from the HFS to the pens. Well-boat transport entails crowding and pumping of fish in both the loading and the unloading, processes known to elicit significant amounts of stress in the fish, more than the transport itself [22, 32, 47, 48]. Both processes can also cause physical trauma in some fish, for example, causing mucus and scale loss that leave areas in the skin vulnerable to infections, as happened in Site B. After a stressful loading and unloading for the transfer, this stressed fish are released in a new open environment where they are exposed to new conditions they may have not experienced before. In addition, it is possible that, as happens with RAS salmon [20], the “over-protected” land-based environment that fish in HFS have experienced for a long period makes their adaptation to a new open marine environment challenging. As a result, this vulnerable fish will require a period to adapt to the new marine environment, where performance will be poor until they start feeding normally and eventually reach optimal growth performance. The actual duration of that adaptation period, its impact on appetite, and the resulting delay in growth is difficult to estimate. Older studies reported that following a stressful event such as transfer to sea cages, smolts did not resume to normal feeding until up to 4–8 weeks [43, 44], but this was highly dependent on an individual basis (10% individuals feeding 1 week post-transfer and 65% 5 weeks post-tranfer, according to Stradmeyer [43]). More recent work with postsmolts of 600–1000 g [49] indicated that returning to normal feeding after transfer to sea pens took 4–8 weeks respectively. A hypothetical ceased feeding period of only 2 weeks could pose a relevant impact on growth, with the extent of this impact being dependent on the fish size and temperature. For example, according to salmon SGR models for different body weights and temperatures generated by Skretting and AquaSim, SGR for salmon of 2 kg at 10°C is 0.71% of the body weight per day. Accordingly, every day the fish does not eat normally could directly entail missing a gain of 0.71% of the body weight. A rough calculation with these numbers suggests that, in 2 weeks, the fish could miss gaining up to 10% of the body weight (~200 g).
Subsequent perturbations would become “additive” or “cummulative” over time in terms of detracting growth potential, thus resulting in the delayed growth observed. Among the most severe acute perturbations that fish in sea pens are likely to experience are de-lousings, which both Sites A and B were subjected to. Like transport, de-lousings take place in a well-boat and requires crowding, sometimes sorting, and pumping in- and outside the boat [22]. As previously mentioned, only this processes alone will elicit stress and may cause in cases physical damage to the fish. But in addition to this, de-lousing also entails the actual treatment applied to remove the sea lice from the salmon skin (i.e., Thermolicer, freshwater, hydrogen peroxide, or chemical drugs, among others); these treatments can cause mortality, further increase stress levels, and affect growth [50, 51]. The effect of this perturbation will most likely accumulate to the previous and further detract scope for growth. Indeed, recent research [52] has estimated that in a cage with 150,000 fish of 3 kg (450 ton biomass), a nonmedicinal de-lousing operation can result in a short-term loss of biomass gain of 32 ton, due to both the starvation pre-treatment and suboptimal feeding post-treatment. The discussed above suggests that usual operations implying fish crowdings, movements, and manipulation can potentially affect growth to a severe magnitude, indicating that such operations should be avoided as much as possible and carried out following more refined procedures.
In addition to specific or acute perturbations, the exposure to an open environment in marine sites implies the probability of subtle stressors whose impact is more diffuse, but continuous and additive to the stress elicited by the previous acute operations [41]. Our results in HFS show how fast salmon can grow when the combination of stressors are minimized, from 0.9 to 4.7 kg in approximately 7 months (gain of ~543 g/month), while in contrast in presence of those stressors (i.e., in traditional sea pens of Site A), reaching 4.7 kg required about 10 months (gain of ~380 g/month). This evidences the positive effect of a high quality and controlled environment with a minimized load of environmental stressors and perturbations to realize all the fish growth potential. Apart from on growth, the influence of the rearing system on the fish could also be deduced from proxies of energy allocation like body condition [53] or HSI [54]. The HFS environment allowed salmon to build energy reserves in the body even during a challenging period like winter, when they tend to lose lipids [55]; this was evidenced by the increasing trend in condition factor observed in HFS in winter and the subsequent higher values observed in March, in contrast to the declines seen in Sites A and B in the same period. Thus, fish in HFS grew at a fast rate over winter and still was able to save energy reserves in their body. This trend was also clearly correlated with the HSI increase seen in salmon in HFS during winter; this indicates that apart from in their body, fish in HFS were able to accumulate energy resources also in the liver, in contrast to fish in Site A. The high HSI in fish from Site B in March, however, did not correspond with the low body condition observed, and therefore, does not appear indicative of energy reserves in liver. Consequently, that high HSI in Site B requires a different explanation. We hypothesize that the high HSI was caused by an enlarged or inflammated liver in individuals suffering severe winter ulcers. Unpublished data that we collected in the same HFS facility during the production of a previous batch (May 2022–March 2023, raised from smolt to postsmolt of ~1 kg), data that are not part of this study, support this claim. In these data we noticed increased liver size and consequently higher HSI (generally over 2.0%) in fish suffering severe winter ulcers, similarly to what we observed now in fish with severe ulcers in Site B. This indicates that the bacteria causing winter ulcers may induce a systemic infection that affects organs like liver (as previously reported by Løvoll et al. [38]), resulting in its enlargement or inflammation. Finally, we must also highlight that the higher growth and energy reserves in HFS did not lead to early sexual maturation. Signs of testis maturation, like elevated GSI [56] were absent in males collected in HFS (males have higher tendency to mature early compared to females [57]). In contrast, early signs of testis development were noticed in few individuals in Site A and B in May; this resulted in some mature males at harvest, for example in the report provided by Site A (3.4%).
4.3. Aspects that Could be Improved in HFS
Despite all positive observations about salmon performance in HFS, some other aspects could still be improved. For example, the condition factor of the fish was very high in HFS from December, reaching on average about 1.7 in March and 1.5 in May. This very high body condition suggests an increased proportion of total lipid in the flesh [53], which is not optimal for consumers from a fillet quality perspective [58] or when used for smoking [59, 60]. Consistent with this high body condition and expected high lipid proportion, in these two samplings, we also observed considerable amounts of fat attached to the viscera (although viscerosomatic index was not measured). This is not positive at harvest, since in relative terms, it will entail a lower final head-on-gutted (HOG) weight and subsequently lower income. Finally, this large body mass in HFS resulted in reduced CSI, as the weight of the heart did not increase proportionally to the body mass. This was observed in March (2–3 months pre-harvest) and was not correlated with any increase in mortality or welfare issues; as such, we cannot assume that low CSI was negative for the fish. However, an excessively small heart in relation to a large body mass may not be optimal for the correct irrigation of all body and organs, nor to cope with increased efforts or stress, although further research would be required to elucidate this extent. All the above indicates that in HFS, there is potential for further optimization, for example, by manipulating feeding regimes, feed composition, introducing an exercise regime, or by a combination of all, during the last few months. Increasing the water velocity seems positive for growth [21] and may be useful to reduce levels of body fat. If necessary, this reduction could be further extended by introducing a fasting regime for a period [61], allowing the fish to use up part of their exceeding lipid reserves in the viscera and flesh, or by using a feed containing a reduced proportion of lipids. However, specific optimized protocols to reduce the amount of energy reserves in HFS are unknown and should be properly investigated before providing concrete recommendations.
In our view, one of the most severe challenges that production in HFS currently faces is the occurrence of skin ulcers. This is a well known issue in sea pen aquaculture specially in winter, as some responsible bacteria like M. viscosa are more infectious at low temperatures [34, 39]. Although the presence of winter ulcers in HFS in the present study was the lowest among the three sites, our experience indicates that incidence of ulcers can be as severe in land-based HFS as in sea pens. For example, as we have mentioned before, we followed a previous batch in the HFS facility from smolt to postsmolt size (~1 kg) between May 2022 and March 2023 (data not published). In this group, we observed that approximately 50% of all fish sampled in January and February 2023 (120 individuals of 500–700 g on average) were affected by winter ulcers and 23% were moderate or severe ulcers. We must acknowledge that this sample most likely overestimated the presence of ulcers due to the higher tendency of ulcered fish to swim in the surface, which made them easier to catch. However, even considering this, the presence of ulcers was important and impacted the group’s performance and welfare. Occurrence of ulcers is likely to be higher when smolts are moved into the HFS in late fall or winter, as high stress post-transfer may depress the inmune system and in combination with the low temperature, lead to development of the ulcers [39]. A possible solution could be heating up the water when fish are transferred close to or during winter periods, up to temperatures of 9–10°C at which bacteria like M. viscosa are not as infectious [38, 39]. This could also add a positive effect on growth rates, although at the expense of higher energy costs due to the heating that the company would have to assess. If the issue with winter ulcers is eventually solved, considering the great results reported in our study, HFS stands high chances to become a preferred aquaculture system for many producers in the future.
4.4. Future Perspectives
In the present study, we have only assessed fish performance and production parameters of three different farming strategies, but not their economic results or environmental performance in terms of carbon, water, or nutrient footprints and biodiversity impacts. As such, further academic reports should focus on covering these two key aspects during harvest-size salmon production in HFS and compare with production in traditional sea pens, given the lower costs and higher environmental risks in the latter. A previous report that compared production costs in open net pens and RAS suprisingly estimated similar running costs in both strategies, provided that interest and depreciation were not included in the analysis [62]. Considering that costs in HFS are expected to be lower than in RAS, then market-size production in HFS should be able to compete with the increasing production costs in sea pens. This is likely to be the case if the better results we observed in all aspects of production (i.e., faster growth and larger size in the same period, absence of disease, efficient use of feed, no need for de-lousing, and high share of “Superior” salmon at harvest) continue to be consistent over time, as the benefits would outweigh the higher operating costs in HFS than in sea pens. Reports from other companies using the same production system like Salmon Evolution AS point on that direction; this company has consistently achieved great harvest results (92%–96% “Superior” salmon), while claiming to be on track to reduce farming costs to similar levels as traditional production in sea pens [31]. These two commercial cases are evidence of the positive prospects that producing market-size salmon in HFS may have in the future.
The fact that salmon in HFS performs better than in traditional marine sites does not imply that the strategy of rearing postsmolts of different size in sea pens should be avoided, especially considering the early stage of HFS and the small number of companies currently operating this system. Certainly, growing postsmolts in sea pens presents more challenges and higher risks than in HFS, and therefore, more uncertainty in the predictability of economic results. To minimize these risks, traditional farmers nowadays aim to maintain the fish the shortest possible time in sea pens, for example 5–6 months (G. M. Knutsen, Bremnes Seashore AS, personal communication), thus reducing the impact of sea lice, welfare issues, and chances for disease outbreaks. This strategy fits adequately with the production in HFS of 2 kg salmon that are then transferred to traditional sea pens, allowing for that short 5–6 month grow-out period at sea. Until a paradigm shift takes place towards production of market-size salmon in land-based facilities, this postsmolt strategy is positive to maximize the production capacity of all parties and to minimize welfare challenges; land-based HFS facilities can stock up a large amount of smolts and later sell part of them as stocking density increases; marine farms can make a better use of their licenses, stock up their total capacity in cases when this had not been completely covered with their own smolt production, and minimize welfare issues and the huge impact of sea lice by having the fish at sea for a reduced period. As such, a combination of the different production strategies appears positive for the whole sector to maximize production capacity continuously over time and minimize health and welfare issues.
5. Conclusion
In this case study, we compared for first time growth, stress, welfare, KPIs, and harvest results in salmon reared commercially in land-based HFS from smolt to harvest size versus in salmon reared in traditional open net pens from 0.9 to 1.9 kg. Salmon in HFS grew significantly faster and displayed better welfare and stress indicators, resulting in better KPIs and earlier and better harvest results, than in the two sea sites. This positive effect of HFS on salmon performance can be linked to both the minimized amount of stressful operations and disturbances that the fish experience in this land-based rearing system and to the provision of a high-quality rearing environment with large volumes of renewed seawater and closed-system biosecurity measures that avoid external challenges like parasites or pathogens. Thus, HFS appears as a realistic option to produce harvest-size Atlantic salmon with high welfare standards and fast growth, while minimizing the impact of important challenges like sea lice infestations or disease outbreaks. If these results prove consistent in the future, the interest on this land-based rearing system will likely increase.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Sigurd. O. Handeland established the project and gathered the funding. Enrique Pino-Martinez and Sigurd. O. Handeland designed the study in collaboration with Bue Salmon AS and performed samplings at the different sites. Enrique Pino-Martinez carried out plasma cortisol analyses, data analysis, and wrote the manuscript. Sigurd. O. Handeland provided assistance in writing and agreed on the final version.
Funding
This study was funded by Bue Salmon AS through Innovasjon Norge, project number 2019/107275.
Acknowledgments
Authors wish to thank Asbjørn Dyrkorn Løland (R&D Manager) and all staff at Bue Salmon AS in Bulandet, Norway, for their invaluable contribution to this research. Similarly, authors acknowledge the contribution of all staff and management from the two marine sites that were also part of this study, for providing excellent assistance, logistical support, and production data.
Open Research
Data Availability Statement
The data collected by the authors during the samplings in the three facilities are available from the corresponding author upon reasonable request. All the rest of data provided by the companies contain commercially sensitive information and are, therefore, subjected to privacy restrictions.




