First Report on Vibrio harveyi Infection in Black Rockfish (Sebastes schlegeli): Skin Ulcers, Histopathology, and Immune Response
Abstract
In August 2023, a severe skin ulcer outbreak occurred in net-caged black rockfish (Sebastes schlegeli) of Nanhuangcheng Island, Bohai Sea. The causative bacterium, Vibrio harveyi, was identified through 16S rDNA and gyrB sequencing. Artificial infection experiments confirmed its high pathogenicity with a 7-day LD50 of 5.41 × 106 CFU/mL. Histopathological analysis revealed hepatocyte vacuolization, erythrocyte aggregation in the spleen leading to splenic congestion and reducing the contrast between red and white pulp regions, glomerular atrophy, renal damage, intestinal epithelial necrosis, and muscle fiber disintegration. Immunoenzymatic assays showed elevated alkaline phosphatase (AKP), acid phosphatase (ACP), and lysozyme (LZM) activity. Additionally, qRT-PCR analysis revealed downregulation of IL-12B and upregulation of TNF-α, CASP10, and RAFEA. Antibiotic susceptibility testing showed sensitivity to minocycline and doxycycline, but resistance to erythromycin and azithromycin. This study provides novel etiological and histopathological insights into V. harveyi infection in black rockfish, highlighting the bacterium’s impact on host tissues and immune response.
1. Introduction
With the growing demand for seafood and the limitations on land-based resources, mariculture is becoming increasingly essential as a sustainable source of food for the future [1]. Offshore farming, particularly in open sea net-cage systems, offers several advantages, including reduced environmental impact, and enhanced fish growth performance and improved fish quality [2]. This method has garnered international interest since the 1990s and has been rapidly adopted, especially in regions like China, where large-scale offshore net-cage farming of fish has become a dominant practice [3].
Sebastes schlegeli, commonly known as black rockfish, is a highly commercially valuable farmed-fish species in China, Japan, and Korea, with production having increased significantly in recent years. This species, a crucial marine fishery resource, has been artificially cultivated and bred in these countries since the 1980s [4]. Its high nutritional value, robust disease resistance, and rapid growth rate make it an ideal candidate for large-scale mariculture [5]. Moreover, its natural ability to overwinter in the northern maritime regions of China further cements its importance in northern marine cage farming [6]. In August 2023, a severe outbreak of skin ulcers was detected in black rockfish cultured in net cages in the Nanhuangcheng Island area of the Bohai Strait, one of the largest seawater net-cage fish farming zones in northern China. The outbreak occurred during the summer, characterized by elevated water temperatures, which may have contributed to the proliferation of pathogenic bacteria. Single net cages in this region house up to one million fish, predominantly black rockfish and Asian seabass (Lates calcarifer). This outbreak resulted in significant economic losses, with a mortality rate of ~35%. Affected fish exhibited distinct clinical signs, including widespread skin ulcers, fin rot, and lethargy.
Vibrio harveyi, a Gram-negative bacterium commonly found in marine environments, is a major pathogen in both wild and farmed marine fish. Its pathogenicity is primarily due to the combined action of several virulence factors, including hemolysins, extracellular enzymes (such as proteases, lipases, and phospholipases), as well as capsules and toxins. These factors enhance the bacterium’s infectivity by causing tissue damage and suppressing the host immune response [7]. Hemolysins, for instance, disrupt host cell membranes, leading to cell lysis, while proteases degrade tissue structures, exacerbating lesions [8]. The infection typically targets key tissues, including the skin, liver, kidneys, and gills. Skin infections result in severe ulcers and epidermal necrosis, commonly observed in fish [9]. Liver infections cause hepatocyte necrosis and fatty degeneration, while kidney infections are marked by tubular damage and interstitial inflammation. Gills are affected by filament necrosis, severely impairing respiratory function [10]. V. harveyi infections pose a significant threat to aquaculture, leading to economic losses, reduced fish quality, and increased disease management costs. Furthermore, the excessive use of antibiotics to control these infections may contribute to the development of antibiotic-resistant bacteria, which could disrupt marine ecosystems. The first documented cases of V. harveyi infection in fish and shrimp were reported in 1990 [11]. The pathogen’s impact extends beyond China, with notable outbreaks reported in regions such as the Mediterranean [12] and India [13]. More recent studies have shown that V. harveyi can influence the expression of immune-related genes in various fish species following infection, including red drum (Sciaenops ocellatus) [14], grouper (Epinephelus lanceolatus) [15], pearl dragon perch (Epinephelus fuscoguttatus♂× E. lanceolatus♀) [16], and Japanese pufferfish (Takifugu rubripes) [17].
However, research on the pathogenicity of V. harveyi in black rockfish, including its clinical signs and immune-related molecular changes postinfection, remains unexplored. In this study, we aimed to identify and evaluate the pathogenicity of V. harveyi strains to black rockfish through artificial infection experiments and histopathological examinations. To elucidate the immunological changes postinfection, we measured immune enzyme activity and the expression of immune-related genes (Figure 1). Additionally, we assessed the bacterial strain’s drug susceptibility, providing scientific insights for disease management and prevention.
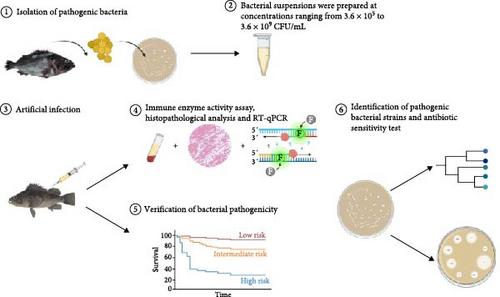
2. Materials and Methods
2.1. Isolation of Pathogenic Bacteria Pathogenic
Bacteria were isolated from diseased fish collected from a disease outbreak in the culture cages. Fish were anesthetized and dissected under aseptic conditions. Tissue samples from ulcerated skin, intestine, liver, and kidney were collected and separately homogenized in 1.5% sterile NaCl solution. The homogenates were cultured on tryptic soy broth (TSB) and thiosulfate citrate bile salts (TCBSs) sucrose agar plates, both containing 1.5% NaCl, using the streak plate method. The plates were incubated at 28°C for 24–36 h, and colony morphology was observed. The largest number of colonies with uniform morphology were considered dominant bacteria, and one colony was selected for further purification.
2.2. Experimental Design and Sample Preparation
A total of 154 black rockfish were purchased from a commercial aquaculture facility in Rizhao, Shandong Province, China. These fish were cultured for 2 weeks to ensure their health status. They were randomly divided into two parts for the experiments: 140 fish were used for bacterial strain pathogenicity verification, and the remaining 14 fish were used for histopathological analysis, gene expression, and immune enzyme activity analysis at 24 h postinfection. The fish were kept in eight 160 L tanks filled with natural seawater, which was obtained from the Yellow Sea near Qingdao, Shandong Province, China. The seawater was filtered through a 1-micron filtration system and sterilized using UV treatment before being used in the tanks. The water was static and renewed twice daily (morning and evening). The seawater was aerated using oxygen pumps. The water salinity was ~30–35 ppt, and the pH was maintained between 7.8 and 8.2. All fish were fed twice daily at 08:00 and 17:00 with Changxing brand grouper pellets (Changxing Feed Company, Changshu City, Jiangsu Province) at a daily ration of 1.5% of their body weight. Prior to the experiment, the fish were fasted for 24 h. After the experiment started, the fish were not fed further. Seven fish were randomly selected and intramuscularly injected with a bacterial suspension at a concentration of 3.6 × 107 CFU/mL, which constituted the infected treatment group. The remaining seven fish, which were not subjected to any treatment, served as the control group. Blood samples were collected from the caudal vein 24 h postinjection using a 1 mL syringe, transferred into centrifuge tubes containing sodium heparin, and centrifuged at 3000 rpm for 8 min. The resulting serum supernatant was stored at −80°C for immune enzyme activity assay. Concurrently, the liver, spleen, kidney, intestine, and muscle tissues were fixed in Davidson’s fixative for histopathological examination. Additionally, a portion of the kidney tissue was stored at −80°C for subsequent RNA extraction to analyze the expression levels of genes related to immune and stress responses. The remaining 140 fish were used for verification of the bacterial strain pathogenicity.
2.3. Verification of the Bacterial Pathogenicity
A total of 140 black rockfish were randomly assigned to seven groups (n = 20): five experimental groups, one negative control group, and one blank control group. The dominant bacterial strains isolated from diseased fish collected from aquaculture cages were cultured and serially diluted in sterile 1.5% NaCl solution to prepare bacterial suspensions with concentrations of 3.6 × 109, 3.6 × 108, 3.6 × 107, 3.6 × 106, and 3.6 × 105 CFU/mL. Each group, including the experimental and control groups, was intramuscularly injected with 0.1 mL of the respective bacterial suspension (experimental groups) or sterile 1.5% NaCl solution (negative control group) at the dorsolateral muscle, located just below the dorsal fin and above the lateral line, using a 0.45 × 16 mm needle and a 1 mL syringe. The blank control group received no injections. The infection experiment lasted for 7 days, during which fish vitality and mortality were monitored three times daily: in the morning, afternoon, and evening. The number of dead fish each day was recorded and used to calculate the LD50 [18]. Bacterial isolation was conducted on each group during the experiment and at the end of the experiment. All bacterial isolations were performed on live fish.
2.4. Histopathological Analysis
A total of 10 black rockfish were intramuscularly injected with 3.6 × 107 CFU/mL of the bacterial suspension. Seven fish were from the infected group, which were humanely euthanized using MS-222 (200 mg/L) at 24 h postinjection. Seven fish from the control group were euthanized in the same manner. Tissue samples from the liver, spleen, posterior kidney, intestine, and muscle were then collected and immediately fixed in Davidson’s fixative. The tissue samples were paraffin-embedded and processed following standard histological procedures. Sections of 3 μm thickness were stained with hematoxylin and eosin (H&E), a widely used method for examining tissue morphology and identifying cellular damage. This technique was chosen because of its ability to clearly differentiate between various tissue structures, making it ideal for observing pathological changes in infected tissues. To ensure the validity of the results, tissue samples from the control group were included for comparison, enabling clear identification of infection-induced lesions. All slides were examined using a light microscope equipped with an imaging system for detailed analysis.
2.5. Identification of Pathogenic Bacterial Strain
The isolated bacteria from each sample were identified through 16S rDNA and gyrB gene sequencing and alignment. Selected bacterial colonies were suspended in 100 µL of sterile water in a 1.5 mL centrifuge tube and incubated at 99°C for 10 min. After centrifugation at 12,000 rpm for 1–2 min at 4°C, the supernatant was used as a template for PCR. The sequences of the primers are listed in Table 1. The PCR reaction (50 µL) comprised 2 µL of DNA template, 25 µL of Taq DNA polymerase, 1 µL of each primer, and sterile double-distilled water. The PCR program included an initial denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 1.5 min. A final extension was conducted at 72°C for 5 min, with subsequent cooling to 4°C. PCR products were resolved by 1.0% agarose gel electrophoresis and sequenced by Shanghai Bio-Engineering Co. Ltd. The sequencing results were compared with the NCBI database using BLAST. Strains with high sequence homology were selected for phylogenetic tree construction using the neighbor-joining method in MEGA 11.0, with validation by 1000 bootstrap replicates.
2.6. Antibiotic Sensitivity Test
Antibiotic sensitivity of the bacterial strain was assessed using the Kirby–Bauer disk diffusion method. Bacterial cultures were adjusted to a concentration of 1 × 106 CFU/mL in sterile 1.5% NaCl solution. A 100 μL aliquot of this suspension was evenly spread across tryptic soy agar (TSA) plates. Antibiotic discs were then placed on the surface of the inoculated plates, which were incubated at 28°C for 24 h in an inverted position. After incubation, the diameters of the inhibition zones around the antibiotic discs were measured, and the strain’s susceptibility to the antibiotics was determined according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [21].
2.7. Immune Enzyme Activity Assay
For immune enzyme activity analysis, a total of 10 black rockfish were used, including seven from the infected group (intramuscularly injected with 3.6 × 107 CFU/mL of the bacterial suspension and sampled 24 h postinjection) and three from the control group. The same fish used for histopathological analysis were selected, but blood samples were collected for enzyme activity analysis. Immune enzyme activities were measured using commercial assay kits (Nanjing Jianchen Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. The analysis included several immune markers: alkaline phosphatase (AKP), acid phosphatase (ACP), lysozyme (LZM), secretory immunoglobulin A (SIgA), and pancreatic amylase (PAMY).
2.8. Bacterial Quantification and Immune- and Stress-Related Gene Expression
Total RNA was extracted from kidney tissues using the Vazyme RNA extraction kit (Kit V2), following the manufacturer’s protocol. cDNA synthesis was performed using the PrimeScript RT reagent Kit (Vazyme). Real-time quantitative PCR (RT-qPCR) was performed on a LightCycler 480 PCR System (Roche) in a 15 μL reaction volume, containing 7.5 μL SYBR Premix Ex Taq (Vazyme), 1.0 μL cDNA, and 0.5 μM primers. Reactions were performed in triplicate for each sample group. The PCR cycling conditions included initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. A melting curve analysis was conducted from 65°C to 95°C (5 s per step) to assess primer specificity. The RPL-17 gene, selected for its high stability in tissue analysis of black rockfish [22], was used as the reference gene to normalize the data.
For immune- and stress-response-related gene analysis, PCR protocols were adapted from Song et al. [23] and Tu et al. [24]. The study focused on immune-related genes TNF-α [25], CASP10 [26], RAFEA [27], IL-10 [28], and IL-12B [29] which are involved in inflammatory responses, as listed in Table 2.TNF-α, CASP10, and RAFEA are proinflammatory, IL-12B is anti-inflammatory.
| Genes | Primers (5′-3′) | Product size (bp) |
|---|---|---|
| Rpl-17 |
|
109 |
| TNF-α |
|
128 |
| Casp10 |
|
143 |
| IL-12B |
|
185 |
| RAEFA |
|
121 |
| IL-10 |
|
175 |
2.9. Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 10.0. Differences between the experimental groups were assessed using one-way ANOVA followed by Tukey’s post hoc test for pairwise comparisons. Gene expression data were analyzed to compare the levels of immune response genes between the infected and control groups. A significance level of p < 0.05 was set for all tests.
3. Results
3.1. Clinical Signs of Diseased Fish
In naturally infected black rockfish, clinical signs included skin redness (congestion), ulcerations, and muscle necrosis, with ulcers predominantly forming on the dorsal skin (Figure 2). Additionally, the dorsal and caudal fins also showed signs of ulceration and necrosis. In all treatment groups of artificially infected fish, sluggish swimming or inactivity near the tank walls was observed within 24 h postinjection. After 24 h, all groups, except for the 3.6 × 105 CFU/mL treatment group, exhibited varying degrees of balance impairment, redness (congestion) in the dorsal and caudal fins, and dorsal muscle ulcerations. The fish in the 3.6 × 107 CFU/mL, 3.6 × 108 CFU/mL, and 3.6 × 109 CFU/mL treatment groups ultimately developed fin necrosis and extensive dorsal muscle ulcerations. Both naturally and artificially infected fish exhibited similar necropsy findings, with the intestinal walls appearing transparent, significant fluid accumulation in the intestines, and liver discoloration, characterized by darkening and softening of the liver tissue. No abnormalities were observed in the 3.6 × 105 CFU/mL treatment group after dissection. Bacterial isolation from tissue samples revealed that V. harveyi was the predominant bacterium isolated from the liver, intestine, spleen, kidney, dorsal ulcerated muscle, and skin of both naturally and artificially infected fish.




3.2. Pathogenicity of the Strain
The infection experiment was conducted over a 7-day period, starting 24 h after injection. As shown in Figure 3, fish injected with V. harveyi at concentrations of 3.6 × 109 CFU/mL and 3.6 × 108 CFU/mL began to die within the first day. The group receiving the 3.6 × 109 CFU/mL dose reached 100% mortality by the second day, while the group receiving 3.6 × 108 CFU/mL reached 100% mortality by the third day.
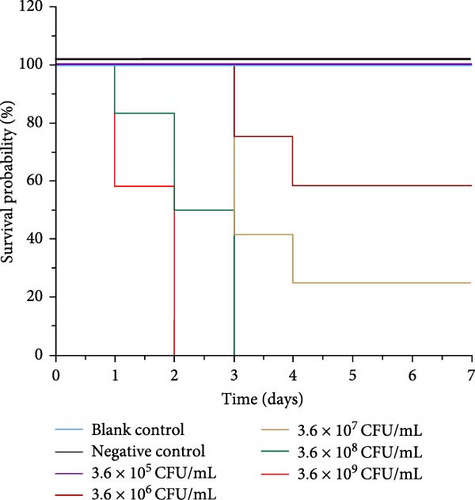
In contrast, no mortality was observed in the group injected with the lowest concentration of 3.6 × 105 CFU/mL. Both the negative control group and the blank control group showed no deaths or abnormalities. The 7-day LD50 for strain 0607V25 in black rockfish was determined to be 5.41 × 106 CFU/mL.
3.3. Identification of the Strain
Strain 0607V25 was identified through sequencing of the 16S rDNA and gyrB gene (Figure 4). The 16S rDNA gene was amplified, resulting in a 1299 bp product, while the gyrB gene yielded a 1211 bp product.
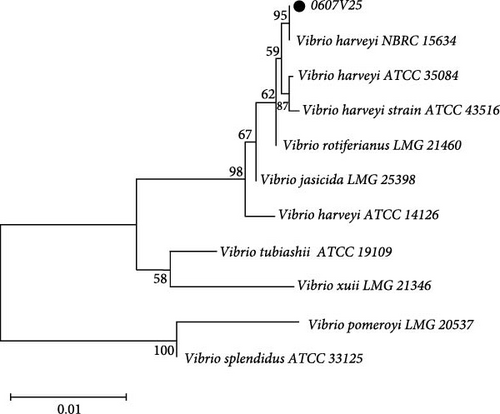
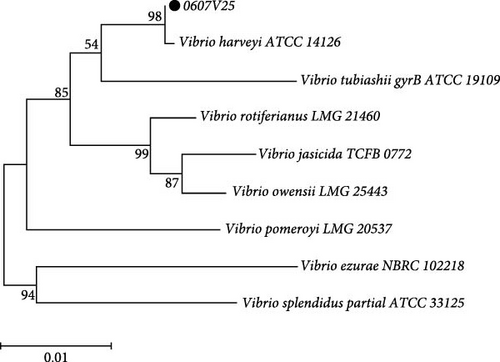
BLAST analysis and subsequent phylogenetic analysis demonstrated that strain 0607V25 clustered with V. harveyi in both cases. Specifically, the 16S rDNA gene sequence analysis showed a high similarity to V. harveyi, with a bootstrap confidence level of 84%. Similarly, the gyrB gene analysis confirmed a clustering with V. harveyi at a confidence level of 99%. These combined results definitively identify strain 0607V25 as V. harveyi.
3.4. Histopathological Observations
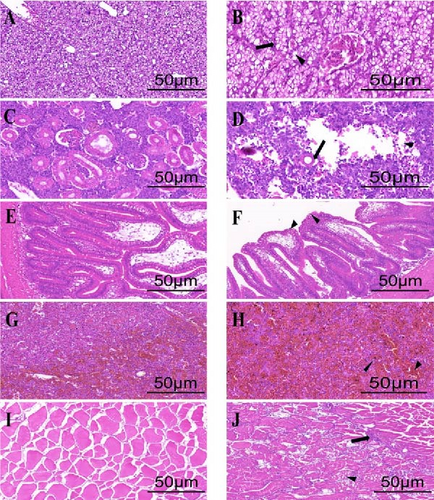
As shown in Figure 5, hepatocytes in healthy black rockfish are intact and densely arranged, forming a compact parenchyma. In contrast, the liver of diseased fish exhibits hepatocyte vacuolization, with vacuoles pushing the nuclei to one side. Healthy kidneys show well-defined glomeruli and orderly renal tubules, whereas the kidneys of diseased fish exhibit glomerular atrophy and widespread damage in the renal interstitium. In healthy intestines, the mucosa, submucosa, lamina propria, and muscle layers are well-organized. However, in diseased fish, severe dissolution of the lamina propria and a loosely organized submucosa are observed, with some areas showing ulceration. The spleen of healthy fish displays distinct red and white pulp regions, while in diseased fish, erythrocyte aggregation occurs, leading to splenic congestion and reducing the contrast between these regions. Muscle fibers are clearly defined in healthy fish, while in diseased fish, marked disintegration and atrophy of muscle fibers are evident. Additionally, infiltration of immune cells was observed between the muscle fibers, suggesting an inflammatory response associated with infection.
3.5. Immune Enzyme Activity
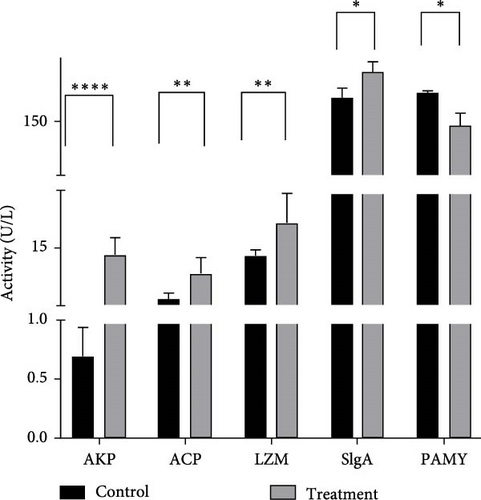
As shown in Figure 6, the experimental group exhibited significantly lower serum PAMY levels compared to the control group (p < 0.05). Conversely, levels of AKP, LZM, ACP, and SIgA were significantly elevated in the experimental group (p < 0.05).
3.6. Gene Expression Profile
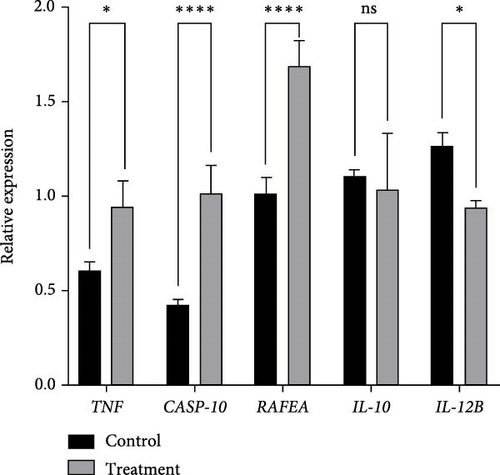
The transcriptional expression levels of five immune-related inflammatory response genes in the kidney of black rockfish are shown in Figure 7. The proinflammatory genes TNF-α, CASP10, and RAFEA were significantly upregulated in 24 h postintravenous injection (p < 0.05). In contrast, the anti-inflammatory gene IL-12B was significantly downregulated (p < 0.05). While IL-10 did not show a statistically significant change, a downward trend was observed.
3.7. Antimicrobial Susceptibility Test
The results of the antimicrobial susceptibility test for V. harveyi strain 0607V25 were provided in Table 3. The strain was found to be highly to moderately susceptible to 18 antibiotics, such as minocycline, doxycycline, nalidixic acid, and sulfamethoxazole. Resistance was observed to 20 antibiotics, including erythromycin, pipamperone, and azithromycin.
| Antibiotic name | Content (µg/disc) |
Inhibition zone standard (mm) (G−) | Inhibition zone standard (mm) (G+) | Inhibition zone diameter (mm) |
Sensitivity | ||
|---|---|---|---|---|---|---|---|
| R | S | R | S | ||||
| Erythromycin | 15 | ≤13 | ≥23 | ≤22 | ≥30 | 9 | R |
| Minocycline | 30 | ≤12 | ≥16 | ≤25 | ≥30 | 17.8 | S |
| Doxycycline | 30 | ≤10 | ≥14 | ≤23 | ≥28 | 17.7 | S |
| Tetracycline | 30 | ≤11 | ≥15 | ≤24 | ≥30 | 15.8 | S |
| Neomycin | 30 | ≤17 | ≥23 | ≤18 | ≥26 | 11 | R |
| Pipemidic acid | 30 | ≤22 | ≥28 | ≤ | ≥ | 16.4 | R |
| Nalidixic acid | 30 | ≤13 | ≥19 | ≤ | ≥ | 26.7 | S |
| Azithromycin | 15 | ≤13 | ≥18 | ≤21 | ≥26 | 11.2 | R |
| Clarithromycin | 15 | ≤13 | ≥18 | ≤26 | ≥32 | 14.7 | I |
| Acetylspiramycin | 30 | ≤ | ≥ | ≤22 | ≥30 | 19.6 | R |
| Streptomycin | 10 | ≤11 | ≥15 | ≤14 | ≥22 | 11.5 | I |
| Kanamycin | 30 | ≤13 | ≥18 | ≤19 | ≥26 | 13.3 | I |
| Gentamicin | 10 | ≤12 | ≥15 | ≤19 | ≥27 | 12.5 | I |
| Amikacin | 30 | ≤13 | ≥18 | ≤20 | ≥26 | 9.6 | R |
| Cefoperazone | 75/75 | ≤15 | ≥21 | ≤ | ≥ | 15.4 | I |
| Rifampicin | 5 | ≤16 | ≥20 | ≤24 | ≥26 | 16.2 | I |
| Furazolidone | 30 | ≤14 | ≥17 | ≤ | ≥ | 9.7 | R |
| Trimethoprim | 23.75/1.25 | ≤10 | ≥16 | ≤24 | ≥32 | 18.1 | S |
| Novobiocin | 30 | ≤17 | ≥22 | ≤22 | ≥31 | 17.6 | I |
| Polymyxin B | 300IU | ≤11 | ≥12 | ≤ | ≥ | 9 | R |
| Flumequine | 5 | ≤15 | ≥19 | ≤21 | ≥27 | 15.1 | I |
| Lomefloxacin | 10 | ≤18 | ≥22 | ≤ | ≥ | 14 | R |
| Ciprofloxacin | 5 | ≤15 | ≥21 | ≤22 | ≥30 | 12.6 | R |
| Ofloxacin | 5 | ≤12 | ≥16 | ≤24 | ≥28 | 18 | S |
| Norfloxacin | 10 | ≤12 | ≥17 | ≤ | ≥ | 12.4 | I |
| Cefuroxime | 30 | ≤21 | ≥25 | ≤27 | ≥35 | 17 | R |
| Cefotaxime | 30 | ≤22 | ≥26 | ≤25 | ≥31 | 16.6 | R |
| Ceftriaxone | 30 | ≤19 | ≥23 | ≤ | ≥ | 17.1 | R |
| Ceftazidime | 30 | ≤17 | ≥21 | ≤16 | ≥20 | 15.7 | R |
| Cephaloridine | 30 | ≤14 | ≥18 | ≤ | ≥ | 12 | R |
| Penicillin | 10 | ≤11 | ≥15 | ≤28 | ≥29 | 10.2 | R |
| Oxacillin | 1 | ≤14 | ≥20 | ≤10 | ≥13 | 11.6 | R |
| Ampicillin | 10 | ≤13 | ≥17 | ≤28 | ≥29 | 12 | R |
| Cefaclor | 30 | ≤14 | ≥18 | ≤29 | ≥37 | 13.5 | R |
| Cefazolin | 30 | ≤14 | ≥18 | ≤29 | ≥35 | 11.2 | R |
| Chloramphenicol | 30 | ≤12 | ≥18 | ≤19 | ≥26 | 21 | S |
| Enrofloxacin | 10 | ≤15 | ≥19 | ≤22 | ≥28 | 23 | S |
| Florfenicol | 30 | ≤12 | ≥18 | ≤ | ≥ | 22.3 | S |
- Abbreviations: I, intermediate; R, resistant; S, sensitive.
4. Discussion
In this study, we isolated V. harveyi from skin lesions of diseased black rockfish, confirming its identity through both 16S rDNA and gyrB gene sequencing. The calculated 7-day LD50 of the V. harveyi strain in black rockfish was 5.41 × 106 CFU/mL, V. harveyi is a common pathogen in aquaculture that infects various marine fish species, causing severe tissue damage and high mortality [30]. Previous studies have reported that this pathogen induces lesions in the skin, muscles, and internal organs of different fish species. For instance, Morone saxatilis infected with V. harveyi exhibits skin ulcers, muscle necrosis [31]. Paralichthys olivaceus develops not only skin ulcers but also hepatocyte degeneration and intestinal tissue damage [32], and Sparus aurata shows renal tubule atrophy and hepatocyte necrosis [33]. Moreover, in Salmo salar, V. harveyi infection not only leads to tissue lesions but also significantly upregulates host inflammatory genes, suggesting that this pathogen may exacerbate infection by modulating immune signaling pathways [34]. We found that V. harveyi infection in Sebastes schlegeli primarily resulted in skin ulcers, and damage to the liver, kidney, and intestine. These pathological manifestations closely resemble those reported in previous studies, suggesting that V. harveyi may employ similar pathogenic mechanisms across different hosts.
Histopathological analysis further revealed that the disease extended beyond skin ulcers, affecting internal organs such as the intestines, liver, kidneys, and gills. In large yellow croaker, V. harveyi infection has been shown to cause vacuolation and necrosis of renal tubular epithelial cells [35]. Similarly, infected turbot exhibited extensive organ damage, including vacuolar degeneration, hemocyte infiltration, and tissue necrosis in the intestines, liver, spleen, and kidneys [36]. These pathological changes align with our observations in black rockfish, where severe ulcers in the dorsal skin and muscle closely resemble the signs observed in pompano (Trachinotus ovatus) [37].
Additionally, we analyzed the immune responses of black rockfish following V. harveyi infection. The fish immune system primarily relies on innate immunity as the first line of defense [29, 30], with nonspecific immune responses often assessed through immune enzyme activity markers. Previous studies have shown that V. harveyi infection leads to increased AKP and LZM activity in various fish species. For example, in American black sea bass (Centropristis striata), AKP activity significantly increased 24 h postinfection [31], while in American eel (Anguilla rostrata), LZM activity was also upregulated within 24 h [32]. Consistent with these findings, we observed a significant increase in AKP, LZM, and SIgA levels in infected black rockfish, mirroring the immune response reported in Asian seabass (L. calcarifer) [33]. Notably, we also found a significant decrease in PAMY activity following infection, a phenomenon not previously reported. Since PAMY plays a key role in carbohydrate metabolism and digestion, its decline suggests a potential interplay between immune activation and host metabolism. This novel finding highlights an immune-metabolic regulatory mechanism in black rockfish during V. harveyi infection, suggesting that immune responses may influence metabolic processes. The observed increases in AKP, LZM, ACP, and SIgA levels indicate a robust innate immune response in black rockfish. AKP plays a crucial role in dephosphorylation and is involved in pathogen recognition and inflammation. LZM, a key antimicrobial enzyme, degrades bacterial cell walls and serves as a frontline defense. ACP is associated with lysosomal activity and pathogen degradation, while SIgA is essential for mucosal immunity, helping maintain gut barrier function and immune surveillance. These elevated immune markers suggest that black rockfish mount a strong innate immune response against V. harveyi, potentially limiting pathogen proliferation and reducing tissue damage.
In the study by Wang et al. [38], V. harveyi infection in pearl dragon grouper (E. fuscoguttatus ♂ × E. lanceolatus ♀) caused severe immune system damage after 7 days, leading to intestinal dysbiosis, altered intestinal tissue morphology, and changes in immune response-related gene expression. These results are similar to those observed in infected pearl dragon grouper. Qiao et al. [34] reported that 84 out of 151 immune-related genes in black perch exhibited differential expression 24 h postinfection. This study indicates that V. harveyi not only causes skin ulcer disease in black rockfish but also significantly impacts the expression of inflammatory factors.
We also conducted antibiotic susceptibility testing on the isolated V. harveyi strain, which demonstrated high to moderate sensitivity to 18 antibiotics, including minocycline, doxycycline, nalidixic acid, and trimethoprim-sulfamethoxazole. In contrast, the strain exhibited resistance to 20 other antibiotics, including erythromycin, ciprofloxacin, and azithromycin.
To mitigate the risks of V. harveyi infection in aquaculture, several strategies could be considered. The use of antibiotics such as minocycline and doxycycline has proven effective, but overuse raises concerns about the development of resistant strains [39]. Alternative approaches, such as the use of bacteriophages, probiotics, and immune modulators, could help reduce bacterial loads without contributing to resistance [40, 41]. Additionally, vaccine development targeting V. harveyi could offer long-term protection for farmed fish, reducing the need for antibiotics. These strategies, combined with regular monitoring of fish health, could provide a more sustainable approach to managing V. harveyi outbreaks [42].
However, there are several limitations to this study. First, the sample size for histopathological analysis was limited, which may reduce the generalizability of the findings. Furthermore, we used a single bacterial concentration in the infection model, which may not fully capture the range of infection dynamics. Future studies could explore different bacterial doses and longer exposure times to better understand the progression of V. harveyi infection and evaluate the efficacy of various treatment strategies under different conditions.
5. Conclusion
This study identified V. harveyi as the causative agent of skin ulceration in black rockfish and confirmed its pathogenicity through controlled infection experiments and detailed histopathological analyses. The pathogen caused significant systemic damage, including severe organ and tissue lesions. Immunological assessments revealed marked changes in enzyme activity and cytokine expression, emphasizing its profound effects on host immune responses. Antibiotic susceptibility testing provided essential insights, identifying effective treatments while highlighting resistance to certain antibiotics. These findings provide important insights into the pathogenesis of V. harveyi infection in black rockfish, offering potential guidance for the development of more effective diagnostic tools and disease management strategies. By identifying specific pathological markers and immune responses associated with the infection, this study may facilitate the early detection of outbreaks, enabling more timely and accurate interventions.
Ethics Statement
The experiment adhered to the European Directive (2010/63/EU), and all animal studies were approved (YSGF2024-08) by the Fish Ethics Committee of the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, China.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yindong Gu: conceptualization, investigation, methodology, validation, formal analysis, data curation, writing–review and editing, writing–original draft. Yongxiang Yu: review and editing, formal analysis, investigation. Chunyuan Wang, Zhiqi Zhang, and Xiaojun Rong: investigation, writing–review and editing, formal analysis, data curation, methodology. Yingeng Wang: formal analysis. Meijie Liao: funding acquisition, writing–review and editing. Bin Li: investigation. Lei Qin: writing–review and editing, methodology, data curation, supervision, formal analysis. Zheng Zhang: investigation, funding acquisition, writing–review and editing, methodology, data curation, supervision, formal analysis.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFD2400704), Natural Science Foundation of Shandong Province (ZR2021MC027), Key Research and Development Program of Shandong Province (Science and Technology Demonstration Project) (2021SFGC0701), and the Central Public-Interest Scientific Institution Basal Research Fund (2023TD29).
Acknowledgments
Financial supports for this study were provided by the National Key Research and Development Program of China (2023YFD2400704), Natural Science Foundation of Shandong Province (ZR2021MC027), Key Research and Development Program of Shandong Province (Science and Technology Demonstration Project) (2021SFGC0701), and the Central Public-Interest Scientific Institution Basal Research Fund (2023TD29).
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Data requests can be sent to [email protected].




