The Relationship of CONUT Score and Blood Urea Nitrogen/Albumin Ratio With Survival and Neurological Outcome in Patients With Acute Ischemic Stroke Followed in Neurology Intensive Care
Abstract
Background: Malnutrition is a frequent but underrecognized factor influencing prognosis in acute ischemic stroke (AIS) patients, particularly in intensive care settings. This study is aimed at evaluating the prognostic value of the controlling nutritional status (CONUT) score and the blood urea nitrogen/albumin (BUN/ALB) ratio on neurological outcomes and in-hospital mortality.
Methods: This retrospective cohort study included 208 AIS patients admitted to a neurology intensive care unit between 2020 and 2022. Nutritional status was assessed within 24–48 h using the CONUT score, calculated from serum albumin, total lymphocyte count, and cholesterol. The BUN/ALB ratio was used as an additional marker. Neurological outcomes were evaluated on day 60 using the modified Rankin Scale (mRS). Statistical analyses included univariate and multivariate logistic regression, Cox regression, ROC curve analysis, and Kaplan–Meier survival analysis.
Results: Higher CONUT scores and BUN/ALB ratios were significantly associated with poor neurological outcomes and increased in-hospital mortality. In multivariate analysis, both markers remained independent predictors of poor prognosis (CONUT OR = 3.8, p = 0.013; BUN/ALB OR = 7.4, p = 0.014). ROC analysis showed strong predictive value, especially for the CONUT score (AUC = 0.898 for progression; AUC = 0.860 for mortality).
Conclusion: Early assessment of nutritional status using CONUT and BUN/ALB ratio may help identify AIS patients at higher risk of poor outcomes. These cost-effective and easily obtainable markers can support clinical decision-making and improve prognostic accuracy in intensive care management.
1. Introduction
Acute ischemic stroke (AIS) remains one of the leading causes of mortality and long-term disability worldwide. Despite substantial advancements in acute stroke care, outcomes for patients admitted to neurology intensive care units (NICUs) continue to be poor, particularly among the elderly and those with comorbid conditions. One often underestimated yet clinically significant factor influencing these outcomes is malnutrition. Neurological deficits, impaired consciousness, dysphagia, and systemic inflammation contribute to a high prevalence of malnutrition in stroke patients, reported in up to 60% of cases [1–4].
In intensive care settings, early detection of malnutrition is vital, as poor nutritional status has been associated with increased infection rates, prolonged hospitalization, poor functional recovery, and higher mortality. However, conventional nutritional screening tools, such as the Subjective Global Assessment (SGA) and the Nutrition Risk in the Critically Ill (NUTRIC) score, have limitations in critical care environments. These methods often rely on clinical judgment, patient cooperation, or complex parameters that are not always feasible in unconscious or intubated patients. In contrast, the controlling nutritional status (CONUT) score offers a rapid, objective, and laboratory-based alternative, using serum albumin, lymphocyte count, and cholesterol level values routinely obtained upon ICU admission [5, 6].
Although the CONUT score has been validated as a prognostic tool in various clinical contexts including oncology, heart failure, and general ICU populations, its prognostic value in AIS patients, particularly those admitted to NICUs, remains underexplored [7, 8]. Moreover, another biochemical marker, the blood urea nitrogen to albumin (BUN/ALB) ratio, has gained attention as a potential indicator of inflammation, catabolic state, and fluid imbalance, all of which are relevant in the acute stroke setting. Despite emerging evidence linking this ratio to mortality in pneumonia and general ICU patients, data regarding its prognostic significance in AIS are scarce [9].
In light of these gaps, our study is aimed at investigating the predictive value of the CONUT score and the BUN/ALB ratio in determining in-hospital mortality and neurological outcomes in patients with AIS followed in a NICU. By utilizing simple, cost-effective, and widely available laboratory markers, we aim to provide clinicians with practical tools to enhance risk stratification and guide early nutritional and clinical interventions in this high-risk population.
2. Materials and Methods
2.1. Participants
This retrospective observational study included 208 patients diagnosed with AIS and admitted to the NICU of İzmir Katip Çelebi University Atatürk Training and Research Hospital between 2020 and 2022. Stroke diagnosis was confirmed by neurologists based on clinical evaluation and neuroimaging findings.
Inclusion criteria were as follows: (1) age ≥ 18 years, (2) first-time admission to the NICU with AIS confirmed by neuroimaging, and (3) availability of complete biochemical and clinical data within the first 48 h of admission. Exclusion criteria included hemorrhagic stroke, previous ICU admissions for stroke, COVID-19 positivity, brain death, or incomplete records. Patients who developed any exclusion condition during follow-up were also removed from the final analysis.
2.2. Nutrition and Care Standards
Nutritional protocols were applied according to institutional guidelines. Patients capable of oral intake received individualized diets providing 25–30 kcal/kg/day and 1.0–1.5 g/kg/day of protein. Patients unable to eat orally were started on enteral nutrition within 48 h.
2.3. Ethics Committee Approval
The study’s protocol was reviewed and adheres to the principles of the Helsinki Declaration, as reflected by the approval granted by the Clinical Research Ethics Committee of İzmir Katip Çelebi University (Decision Number: 0476; Date: 26/10/2023).
2.4. Data Collection and Research Design
Baseline blood samples were obtained within the first 24–48 h of admission. The CONUT score was calculated using three laboratory parameters: serum albumin (grams per deciliter), total lymphocyte count (×109/L), and total cholesterol (milligrams per deciliter), based on standard scoring criteria as defined by de Ulíbarri et al.
Scoring for each parameter was as follows: serum albumin: ≥3.5 g/dL = 0 points; 3.0–3.49 g/dL = 2 points; 2.5–2.99 g/dL = 4 points; <2.5 g/dL = 6 points. Total lymphocyte count (×109/L): ≥1.6 = 0 points; 1.2–1.59 = 1 point; 0.8–1.19 = 2 points; <0.8 = 3 points. Total cholesterol: ≥180 mg/dL = 0 points; 140–179 mg/dL = 1 point; 100–139 mg/dL = 2 points; <100 mg/dL = 3 points. The total CONUT score ranges from 0 to 12, with higher scores indicating worse nutritional status. Patients were categorized into four groups based on their total score: normal (0–1), mild (2–4), moderate (5–8), and severe (9–12) malnutrition. Additionally, the BUN/ALB ratio referred to as BALB was calculated by dividing BUN (milligrams per deciliter) by serum albumin (grams per deciliter), providing an index of nutritional and inflammatory status.
2.5. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics version 22.0. A p value of < 0.05 was considered statistically significant. The normality of data distribution was assessed using visual methods (histograms, Q-Q plots) and analytical tests, including the Kolmogorov–Smirnov and Shapiro–Wilk tests. Variables following a normal distribution were presented as mean ± standard deviation, while nonnormally distributed variables were expressed as median and interquartile range (IQR).
Categorical variables were summarized using frequencies and percentages. Comparisons between groups were made using the Chi-square test or Fisher’s exact test, where appropriate. Survival analysis was conducted using the Kaplan–Meier method. Univariate analyses were performed to identify potential predictors of neurological progression and in-hospital mortality.
Significant variables (p < 0.05) identified in univariate analyses were included in multivariate logistic regression models to evaluate independent predictors of neurological outcomes. Similarly, Cox proportional hazards regression analysis was used to determine factors independently associated with overall survival.
Receiver operating characteristic (ROC) curve analysis was used to assess the prognostic performance of the CONUT score and the BUN/albumin (BALB) ratio for survival and neurological outcomes. The area under the curve (AUC) and corresponding 95% confidence intervals (CIs) were calculated. Optimal cutoff points were identified using the Youden index. Graphs were generated using Python libraries Matplotlib and NumPy for visual representation of survival curves and predictive models.
3. Results
This study included 208 patients diagnosed with AIS and admitted to the NICU for the first time. After a follow-up period of 40 months, 104 patients (50.0%) died. Cardiac-related complications were the leading cause of death (n = 38, 36.5%), followed by sepsis (n = 29, 27.9%), unknown reasons (n = 26, 25.0%), gastrointestinal bleeding (n = 6, 5.8%), and acute kidney failure (n = 5, 4.8%).
The mean age of the study population was 67.9 years, and the median age was 70.0 years. Of the patients, 122 (58.7%) were male, and 86 (41.3%) were female. Regarding comorbidities, 131 patients (63.0%) had hypertension, 127 (61.1%) had diabetes mellitus, 49 (23.5%) had coronary artery disease, 52 (25.0%) had atrial fibrillation, and 45 (21.6%) were smokers. The median NIHSS score on admission was 9.0, and the median mRS score at 60 days was 3.0. According to TOAST classification, large artery atherosclerosis was the most common etiology (n = 119, 57.2%), followed by cardioembolic stroke (n = 34, 16.3%), small artery occlusion (n = 19, 9.2%), and other or undetermined causes (n = 36, 17.3%).
The median laboratory findings were as follows: hemoglobin (Hb) = 12.0 g/dL, platelet count = 231 × 109/L, lymphocyte count = 1.48 × 109/L, leukocyte count = 9.9 × 109/L, albumin = 3.4 g/dL, and BUN = 19.0 mg/dL. The median BALB ratio was 5.6. The CONUT score categorized patients into four nutritional risk groups: normal (n = 73, 35.1%), mild (n = 59, 28.4%), moderate (n = 56, 26.9%), and severe (n = 20, 9.6%) (Figure 1); mild (n = 59, 28.4%), moderate (n = 56, 26.9%), and severe (n = 20, 9.6%).

Comparison of clinical and laboratory characteristics according to CONUT categories (Table 1) revealed no significant differences between the groups in terms of smoking status, hypertension, diabetes mellitus, or coronary artery disease. However, statistically significant differences were found for age (p < 0.001), Hb level (p = 0.001), lymphocyte count (p < 0.001), NIHSS score at admission (p < 0.001), mRS score at 2 months (p < 0.001), BUN (p < 0.001), BALB ratio (p < 0.001), and mortality rate (p < 0.001). A higher CONUT score was significantly associated with older age and poorer clinical outcomes.
| Total | CONUT Normal |
CONUT Light |
CONUT Moderate |
CONUT Severe |
p | |
|---|---|---|---|---|---|---|
| n: 208 | n: 73 | n: 59 | n: 56 | n: 20 | ||
| Age year median (IQR) | 70 (20.0) | 60.5 (22.0) | 70 (18.2) | 74.5 (12.0) | 77 (16.0) | < 0.001 |
| Male n (%) | 122 (58.6) | 44 (60.0) | 32 (54.2) | 33 (58.4) | 13 (65.0) | 0.82 |
| Smoking n (%) | 45 (21.6) | 16 (21.9) | 12 (20.3) | 10 (17.8) | 7 (35.0) | 0.45 |
| Hypertension n (%) | 130 (62.5) | 42 (57.5) | 41 (69.4) | 35 (62.5) | 13 (65.0) | 0.56 |
| Diabetes mellitus n (%) | 81 (38.9) | 23 (31.5) | 22 (37.2) | 24 (42.8) | 12 (60) | 0.11 |
| In-hospital death n (%) | 104 (50.0) | 14 (19.1) | 23 (38.9) | 47 (83.9) | 20 (100) | < 0.001 |
| Coronary artery diasease n (%) | 49 (23.6) | 12 (16.4) | 15 (25.4) | 14 (25.0) | 8 (40.0) | 0.15 |
| Atrial fibrillation n (%) | 52 (25.0) | 14 (19.1) | 16 (27.1) | 16 (28.5) | 6 (30.0) | 0.63 |
| NIHSS on admission, median (IQR) | 10 (11.0) | 5.5 (10.0) | 9 (11.0) | 10.5 (10.0) | 16 (6.0) | < 0.001 |
| MRS score at 2 months | 3 (4.0) | 1.5 (2.0) | 3 (3.0) | 5 (2.0) | 6 (1.0) | < 0.001 |
| TOAST classifications, n (%) | ||||||
| Large-artery atherosclerosis | 119 (57.2) | 47 (64.3) | 41 (69.4) | 22 (39.3) | 9 (45.0) | |
| Small-artery oclusion | 19 (9.2) | 11 (15.0) | 2 (3.3) | 4 (7.2) | 2 (10) | 0.815 |
| Cardioembolic | 34 (16.3) | 9 (12.4) | 4 (6.7) | 13 (23.3) | 8 (40) | 0.001 |
| Other and unknown causes | 36 (17.3) | 6 (8.3) | 12 (20.6) | 17 (30.2) | 1 (5.0) | 0.069 |
| Hemoglobin mean (SD) | 12.0 (4.0) | 13.0 (2.25) | 12.0 (4.0) | 4 (2.7) | 2 (3.7) | 0.001 |
| Platalet count (109/L), median (IQR) | 232 (101) | 237 (105) | 243 (97) | 223 (116) | 222 (60) | 0.35 |
| Lymphocyte count (109/L), median(IQR) | 1.5 (1.2) | 2.1 (0.7) | 1.5 (0.8) | 1.0 (0.6) | 0.7 (0.3) | < 0.001 |
| Leukocyte (109/L), median (IQR) | 10 (4.1) | 9.0 (3.1) | 9.9 (5.8) | 10.5 (6.9) | 11.5 (8.6) | 0.056 |
| Albumin (g/dL) mean (SD) | 3.3 (0.6) | 3.9 (0.3) | 3.4 (0.4) | 2.8 (0.3) | 2.3 (0.3) | < 0.001 |
| Total cholesterol | 168 (65) | 203 (49) | 170 (56) | 141 (35) | 110 (40) | < 0.001 |
| BUN (mg/dL) median (IQR) | 19 (15) | 16 (8.0) | 19 (14.0) | 24.0 (19.0) | 44.5 (46.0) | < 0.001 |
| BALB (< 6.18) n (%) | 112 (53) | 62 (84) | 33 (55) | 16 (28) | 1 (5) | < 0.001 |
- Note: BALB for the ratio of blood urea nitrogen to albumin. Bold values indicate statistically significant results (p < 0.05).
- Abbreviations: BUN: blood urea nitrogen, CONUT: controlling nutritional status, IQR: interquartile range, MRS: Modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, SD: standard deviation, TOAST: Trial of Org 10172 in Acute Stroke Treatment.
Univariate analyses demonstrated that several factors were significantly associated with poor neurological outcomes (defined as mRS > 3 at 60 days), including age (OR = 4.0, p < 0.001), diabetes mellitus (OR = 3.20, p = 0.001), coronary artery disease (OR = 4.40, p < 0.001), NIHSS score at admission (OR = 5.60, p < 0.001), Hb level (OR = 0.81, p = 0.002), lymphocyte count (OR = 0.16, p < 0.001), albumin level (OR = 0.02, p < 0.001), BUN (OR = 3.1, p = 0.002), BALB ratio (OR = 8.3, p < 0.001), total cholesterol level (OR = 0.98, p < 0.001), cardioembolic stroke (vs. large artery atherosclerosis) (OR = 7.2, p < 0.001), and CONUT score (moderate vs. normal: OR = 4.3, p < 0.001).
Multivariate logistic regression revealed that coronary artery disease (OR = 6.9, p = 0.031), NIHSS score at admission (OR = 1.2, p = 0.003), albumin level (OR = 0.05, p = 0.022), BALB ratio (OR = 7.4, p = 0.014), cardioembolic stroke (OR = 7.7, p = 0.018), and CONUT score (moderate vs. normal: OR = 3.8, p = 0.013) were independently associated with poor neurological outcomes (Table 1).
ROC analyses were conducted to evaluate the predictive abilities of CONUT score and BALB ratio for survival and neurological progression. The AUC values for CONUT score were 0.860 for mortality and 0.898 for neurological progression, indicating excellent predictive power. The AUC values for BALB ratio were 0.772 for mortality and 0.815 for progression, suggesting good predictive ability. The optimal cutoff values were determined using the Youden index. These findings are presented in Figures 2.
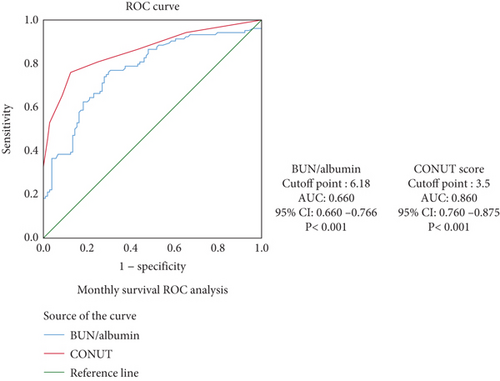
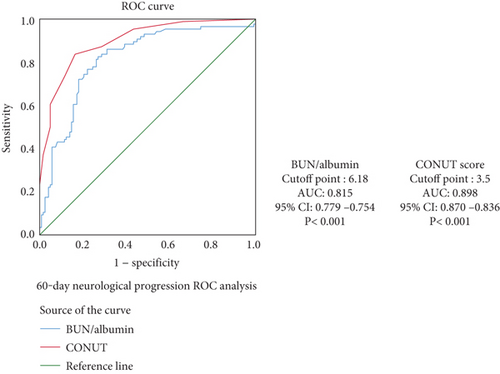
Kaplan–Meier survival curves showed that higher CONUT scores and BALB ratios were significantly associated with reduced survival times (log-rank p < 0.001 and p = 0.003, respectively). These findings are presented in Figure 3.
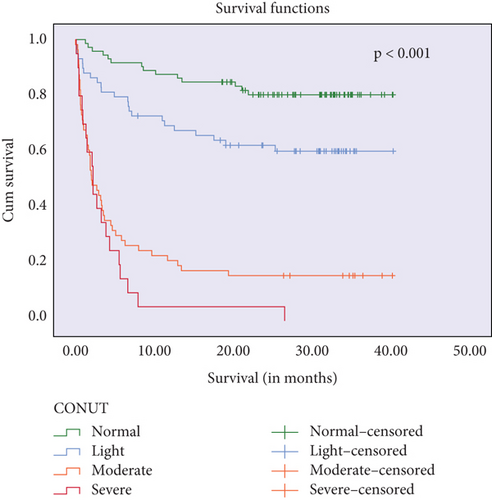
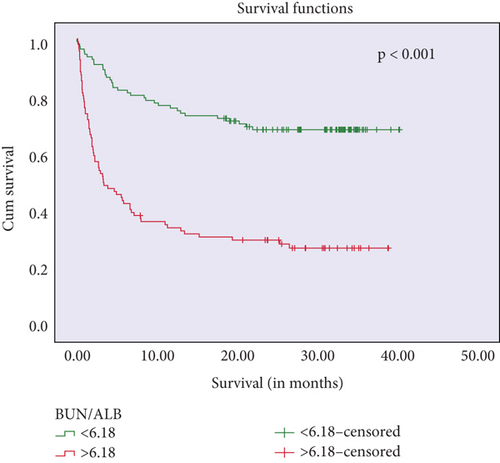
Further univariate analyses for overall survival revealed associations with age (OR = 11.0, p < 0.001), coronary artery disease (OR = 2.27, p = 0.014), NIHSS score at admission (OR = 1.2, p < 0.001), lymphocyte count (OR = 0.66, p < 0.001), leukocyte count (OR = 1.48, p < 0.001), albumin level (OR = 0.07, p < 0.001), BUN (OR = 13.9, p < 0.001), BALB ratio (OR = 5.62, p < 0.001), total cholesterol level (OR = 0.09, p < 0.001), cardioembolic stroke (OR = 4.8, p < 0.001), and CONUT score (severe vs. normal: OR = 14.7, p < 0.001) (Table 2). Cox regression analyses identified lymphocyte count (HR = 0.48, p = 0.019), BALB ratio (HR = 1.9, p = 0.009), cardioembolic stroke (HR = 2.0, p = 0.013), and CONUT score (moderate vs. normal: HR = 5.4, p = 0.003) (Table 2) as independent predictors of overall survival.
| Variables | Neurological progression | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| OR (95% CI) | p | HR (95% CI) | p | OR (95% CI) | p | HR (95% CI) | p | |
| Age | 4.0 | < 0.001 | 11.0 | < 0.001 | ||||
| Gender (man) | 1.92 | 0.05 | 0.85 | 0.37 | ||||
| Smoking | 0.93 | 0.87 | 1.18 | 0.61 | ||||
| Hypertension | 1.19 | 0.62 | 1.13 | 0.66 | ||||
| Diabetes mellitus | 3.20 | 0.001 | 1.69 | 0.06 | ||||
| Coronary artery diasease | 4.40 | < 0.001 | 6.9 | 0.031 | 2.27 | 0.014 | ||
| Atrial fibrillation | 1.90 | 0.09 | 1.3 | 0.85 | ||||
| Cause of death | 1.9 | 0.31 | ||||||
| TOAST classifications | ||||||||
| Large-artery atherosclerosis | Reference | |||||||
| Small-artery oclusion | 0.93 | 0.89 | 2.3 | 0.418 | 1.07 | 0.88 | 1.7 | 0.17 |
| Cardioembolic | 7.2 | < 0.001 | 7.7 | 0.018 | 4.8 | < 0.001 | 2.0 | 0.013 |
| Other and unknown causes | 4.6 | < 0.001 | 0.7 | 0.85 | 2.3 | 0.03 | 1.1 | 0.66 |
| NIHSS on admission | 5.60 | < 0.001 | 1.2 | 0.003 | 1.20 | < 0.001 | ||
| Hemoglobin gr/dL | 0.81 | 0.002 | 0.89 | 0.09 | ||||
| Platalet count (109/L) | 0.99 | 0.168 | 0.99 | 0.35 | ||||
| Lymphocyte count (109/L) | 0.16 | < 0.001 | 0.66 | < 0.001 | 0.48 | 0.019 | ||
| Leukocyte (109/L) | 1.70 | 0.083 | 1.48 | < 0.001 | ||||
| Albumin (g/dL) | 0.02 | < 0.001 | 0.05 | 0.022 | 0.07 | < 0.001 | ||
| BUN (mg/dL) | 3.1 | 0.002 | 13.9 | < 0.001 | ||||
| Total cholesterol (mg/dL) | 0.98 | < 0.001 | 0.09 | < 0.001 | ||||
| CONUT | 1.61 | <0.001 | ||||||
| Normal | Reference | Reference | ||||||
| Light | 2.6 | 0.001 | 1.30 | 0.26 | 2.3 | 0.01 | 1.7 | 0.41 |
| Moderate | 4.3 | < 0.001 | 3.8 | 0.013 | 9.8 | < 0.001 | 5.4 | 0.003 |
| Severe | 24.7 | 0.99 | 22.4 | 0.93 | 14.7 | < 0.001 | 4.8 | 0.011 |
| BALB | 8.3 | < 0.001 | 7.4 | 0.014 | 5.62 | < 0.001 | 1.9 | 0.009 |
- Note: BALB for the ratio of blood urea nitrogen to albumin. Bold values indicate statistically significant results (p < 0.05).
- Abbreviations: BUN: blood urea nitrogen, CONUT: controlling nutritional status, NIHSS: National Institutes of Health Stroke Scale, TOAST: Trial of Org 10172 in Acute Stroke Treatment.
A separate multinomial logistic regression analysis evaluated the relationship between NIHSS, mRS, and CONUT scores. While the NIHSS score was significantly associated with the CONUT score in univariate analysis (p < 0.001), this relationship was not statistically significant in the multivariate model (p = 0.330). In contrast, the 60th-day mRS score remained significantly associated with the CONUT score (OR = 11.53, p = 0.044), suggesting a strong link between functional outcome and nutritional status. No significant interaction effect was found between NIHSS and mRS scores (p = 0.502). Full results are detailed in Table 3, which includes the relationship between NIHSS, mRS, and CONUT scores.
| Variable | Std. error | Wald | df | Sig. | Odds ratio (Exp(B)) | 95% CI lower | 95% CI upper |
|---|---|---|---|---|---|---|---|
| NIHSS at admission | 0.450 | 0.949 | 1 | 0.330 | 1.550 | 0.642 | 3.741 |
| MRS | 1.216 | 4.040 | 1 | 0.044 | 11.53 | 1.063 | 125.1 |
| Interaction NIHSS∗MRS | 0.080 | 0.451 | 1 | 0.502 | 0.948 | 0.810 | 1.109 |
| CAD | 6.975 | 3.844 | 1 | 0.050 | 1.140 | 1.32 | 0.995 |
| HT | 0.786 | 0.044 | 1 | 0.833 | 1.180 | 0.253 | 5.502 |
| DM | 0.736 | 0.740 | 1 | 0.390 | 0.531 | 0.125 | 2.247 |
- Note: Bold value indicates statistically significant results (p < 0.05).
- Abbreviations: CAD: coronary artery disease, DM: diabetes mellitus, HT: hypertension, MRS: Modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale.
4. Discussion
This study comprehensively evaluates acute stroke patients and examines the complex relationship between their nutritional status and stroke outcomes. Our research divided patients into four main categories based on their CONUT scores, as defined in the original study [5]. Comparisons between these groups show significant differences in stroke outcomes and in-hospital mortality rates. When we evaluated the relationship between CONUT scores and stroke outcomes in detail, we observed that variables known to increase stroke risk, such as smoking habits, hypertension, patient gender, and diagnosis of atrial fibrillation, did not affect overall mortality and neurological progression in patients.
Age is the primary nonmodifiable risk factor for stroke, and mortality and morbidity are higher in elderly ischemic stroke patients [10, 11]. Another notable finding in our study is that patients with severe CONUT scores were generally older. This confirms the impact of age on stroke outcomes, as old age can increase the risk of stroke and negatively affect the prognosis. In this study, patient age was a risk factor for overall survival and neurological prognosis, consistent with the literature.
The most commonly observed subtype of stroke is large artery atherosclerosis [12, 13]. The frequency of stroke occurrences can differ based on geographical regions and age demographics. Specifically, strokes of the cardioembolic variety tend to occur more frequently in individuals over the age of 70 [14]. In a study conducted by Gosse De et al. in 2003, the highest mortality stroke subtype was found to be cardioembolic stroke [15]. In our study, when examining stroke types according to TOAST classification, patients diagnosed with cardioembolic stroke had higher CONUT scores, more neurological progression, and lower survival compared to those with large artery atherosclerosis types. This suggests that patients with initial cardiac problems may have a higher risk of malnutrition. Similarly, this could explain poor neurological outcomes in patients with coronary artery disease. Current data indicate the need for more comprehensive studies on this subject.
The risk of ischemic stroke in diabetic patients is approximately twice as high as in nondiabetics [16, 17]. In a study conducted on ischemic stroke patients (n: 4390), 4-week mortality due to ischemic stroke was observed more frequently in diabetic patients compared to nondiabetic patients [18]. In our study, neurological progression was observed more in diabetic patients, but the diagnosis of diabetes was not a statistically significant predictor in multivariate analysis.
Atrial fibrillation and coronary artery disease are well-known risk factors for ischemic stroke. The presence of atrial fibrillation and coronary artery disease in patients who have had an ischemic stroke has also been associated with poor survival and mortality [19, 20]. In our study, coronary artery disease was associated with poor neurological outcomes in univariate and multivariate analyses. In acute and subacute stroke, cardiac biomarkers such as cardiac Troponin T (cTnT) or natriuretic peptides can rise [21]. Similarly, the development of structural myocardial damage, including acute coronary syndrome, is often observed in acute and subacute stroke, which could explain the worsening prognosis of poststroke patients [22].
NIHSS is a systematic and quantitative assessment tool designed to be easy to use by healthcare professionals in less than 10 min [23]. NIHSS provides important prognostic information about the 30-day mortality risks of individuals with AIS. Patients with high NIHSS scores have been observed to have increased poststroke morbidity rates [24–26]. In this study, the NIHSS score was statistically significant with mortality and morbidity, in line with the general literature. Interestingly, in a separate single-variable analysis, the NIHSS score at admission showed a statistically significant impact on the Conut score (p < 0.001), indicating a strong relationship between initial stroke severity and nutritional status. However, this significant effect was not observed in the multivariate logistic regression analysis (p = 0.330), suggesting that the effect of NIHSS might be modulated by other factors included in the model. This finding highlights the complexity of stroke outcomes, where multiple interrelated factors, rather than a single parameter, influence the prognosis.
Data from studies on the association between Hb levels and poststroke functional outcomes in the existing literature are conflicting. A study by Furlan et al. showed that high Hb levels were associated with more severe strokes at presentation, more severe disabilities at discharge, and 30-day mortality following AIS [27]. In their cohort study and meta-analysis of similar studies published between 2007 and 2013, Hao et al. found no relationship between anemia and morbidity [28]. Mechanisms proposed to affect the pathophysiology and outcomes of ischemic stroke in anemia include decreased oxygen transport capacity to penumbral areas, especially hyperkinetic thrombogenic conditions arising from acute blood loss, and proinflammatory processes [29, 30].
Our study detected a significant relationship between Hb level and neurological progression; an increase in Hb level showed a negative correlation with neurological progression. However, in multivariate analysis, Hb level was not identified as an independent risk factor. Also, no relationship was observed between survival time and Hb level.
Recent research has revealed that the nutritional status prior to a stroke significantly influences patient prognosis and mortality. A study conducted by Dennis et al. examined the impact of nutritional status at the time of admission on long-term health outcomes in stroke patients. Analysis of 3012 patients demonstrated that 37% of those with inadequate nutrition at the time of admission died during the follow-up period, whereas the mortality rate among those with normal nutritional status was recorded at 20% [31]. Furthermore, patients with inadequate nutrition were found to have a higher likelihood of developing complications such as pneumonia, other infections, and gastrointestinal bleeding while hospitalised. In a similar vein, a meta-analysis of 26 studies involving acute stroke patients (n = 156,249) indicated that underweight patients had an increased risk of long-term mortality, whereas overweight or obese patients exhibited a decreased risk [32].
These findings corroborate the hypothesis that nutritional status at admission has an independent effect on poststroke health outcomes, supporting our hypothesis that prestroke nutrition can influence stroke outcomes. These studies utilized anthropometric nutritional measurements.
In evaluating the impact of the CONUT score and the BALB ratio in ischemic stroke, independently assessing the components of these scores, namely albumin, cholesterol, lymphocytes, and BUN, can facilitate a more effective interpretation of the results.
The serum albumin level is the cornerstone for both the CONUT score and the BUN/albumin ratio calculation. Similarly, low pre-event albumin levels, a key component of the CONUT score, have been significantly associated with mortality [33, 34]. Additionally, patients with hypoalbuminemia were found to have a higher risk of stroke recurrence [35]. In a study conducted by Idicula et al. on a large cohort of patients with AIS (n = 27,500), a direct correlation was observed between the level of serum albumin at the time of admission and mortality [36]. Recent studies also support these findings [37]. The protective effect of serum albumin has been explained through various mechanisms. Albumin reduces the permeability of the blood–brain barrier (BBB) and prevents post-ischemic edema, thereby providing neurological protection. Moreover, albumin expands the vascular volume without altering osmolality, facilitating the redistribution of blood in conditions of low flow [38]. Additionally, albumin’s ability to maintain vascular flow postthrombolysis and prevent reocclusion postthrombolysis are significant indicators of its prothrombolytic effects [39]. It is also hypothesised that albumin contributes to brain protection by binding to lysophosphatidylcholine (liso PC) [40].
Based on these protective effects, several pilot studies have even been designed to use albumin in the treatment of ischemic stroke [41]. In light of these findings, the significance of serum albumin levels in enhancing the predictive power of both the CONUT and the BUN/albumin ratio is evident.
The lymphocyte count constitutes a fundamental component of the CONUT score and is esteemed as a crucial determinant of neurological status. Antecedent research has consistently shown that diminished lymphocyte counts serve as prognostic indicators, significantly correlating with increased mortality and adverse neurological progression [42, 43]. Lymphopenia emerges during the hyperacute phase of ischemic stroke, and animal models have demonstrated an associated increase in the risk of immunodeficiency and susceptibility to infections [44].
Following the acute phase of stroke, intravascular inflammation characterized by disruption of the BBB and leukocyte infiltration into ischemic tissue initiates inflammatory processes within the brain parenchyma. One of the initial events of this inflammatory cascade may be the release of danger/damage-associated molecular patterns (DAMPs) from neurons that are damaged and undergoing cell death. The HMGB1 molecule and the RAGE signaling pathway play critical roles in this context [45]. A recent meta-analysis also supports the utility of lymphocyte counts in stroke prognosis [46]. Diverse studies with varying outcomes highlight the complex relationship between serum cholesterol levels and stroke risk. Ebrahim et al. found a positive correlation between high cholesterol and the occurrence of ischemic stroke and myocardial infarction [47]. Conversely, Vauthey et al. discovered that high cholesterol levels may confer a reduced risk of death and better functional outcomes poststroke [48]. Pan et al. associated high total cholesterol with improved long-term outcomes in first-time ischemic stroke patients [49], while Zuliani et al. noted increased short-term mortality in patients with lower cholesterol [50]. Olsen et al. observed that higher cholesterol levels correlated with less severe strokes and greater 10-year survival rates [51].
The protective effect of elevated cholesterol has been attributed to its antioxidant and neuroprotective properties [52], while low cholesterol may indicate poorer overall health and nutrition [53]. Our study contributes to this discussion, noting a negative relationship between cholesterol levels and neurological progression and survival, calling for more research on this topic. In contrast, our literature review suggests that high cholesterol does not have a direct and significant impact on ischemic stroke risk compared to traditional coronary artery disease risk factors [54]. Our findings imply that stroke development may be more closely tied to the cardiac effects of hypercholesterolemia than previously thought. The risk of ischemic stroke is more clearly related to biomarkers such as albumin and lymphocytes, which can be understood through simpler mechanisms than cholesterol’s influence. Therefore, we underscore the need to consider a wider range of biomarkers beyond cholesterol in both understanding and managing the risk of ischemic stroke.
Our primary hypothesis regarding the effect of elevated BUN levels on prognosis in patients with ischemic stroke suggests significant roles of dehydration and increased inflammation. Dehydration has been shown to have a prevalence of about 7% among emergency department presentations [55]. The effects of dehydration on the development of cerebral infarction are believed to negatively affect blood flow to the brain by increasing blood viscosity and reducing fluid volume in the body. Furthermore, evidence suggests that higher hematocrit levels may lead to larger areas of damage in patients with cerebral infarction [56]. Dehydration has been demonstrated to be associated with various thrombotic events, including recurrent embolic strokes and postacute stroke venous thromboembolism [57]. Studies investigating the relationship between BUN levels and ischemic stroke have revealed that, in a study conducted by Kılıç et al. (n = 300), an inverse relationship was found between BUN levels and mortality in patients with ischemic stroke [58]. Another study led by Kelly et al. found that BUN ratios were successful in predicting mortality in cases of ischemic stroke [59]. Similarly, research by Ren et al. has identified a relationship between the 3-month outcome of ischemic stroke and BUN levels [60].
In our study, lymphocyte count, albumin level, and cholesterol levels, the basic calculating parameters of the CONUT score, were associated with neurological progression and survival in univariate analyses. However, the performance of the CONUT score in predicting neurological progression and survival was better than the performance of other parameters measured individually; this is especially evident in the moderate group compared to the normal reference group. According to the regression model, the risk level associated with the CONUT score increases with each step from the normal reference group to the severe group. In different studies conducted on ischemic stroke patients, significant relationships similar to our study have been found between the CONUT score and poor neurological outcomes and 3-month neurological progression [61]. In a study by Kokura et al., the CONUT score was a physical functional prognostic indicator for adult stroke patients [62]. In a study examining the CONUT score in AIS patients by Hiroyuki et al. in 2020, poor outcome rates in patients with malnutrition were found to be higher in those who had cardioembolic stroke and strokes due to other etiologies compared to those without malnutrition [63]. Recent studies have also highlighted its role in rehabilitation outcome prediction and in hospital mortality risk [64, 65].
5. Conclusion
Laboratory parameters such as BUN, albumin, and lymphocyte count are routinely obtained upon hospital admission in patients with AIS due to their cost-effectiveness, accessibility, and rapid turnaround. In line with current literature and our study findings, we recommend that total cholesterol levels also be routinely assessed in this patient population. Incorporating these parameters allows for rapid and practical calculation of CONUT and BALB scores, both of which can offer valuable prognostic insight.
Furthermore, our study categorized CONUT scores into four groups: normal, light, moderate, and severe, unlike the original three-category classification. Notably, the moderate and severe groups demonstrated similar clinical profiles and prognoses, suggesting that future research might benefit from consolidating these two into a single “high-risk” category to improve stratification models. Despite its strengths, our study has limitations. The retrospective, single-center design and reliance on laboratory data collected within the first 24–48 h postadmission limited the ability to capture dynamic changes in nutritional status. Additionally, the determination of mortality outcomes was challenged in patients discharged to external facilities, which may have introduced classification bias. Moreover, our multivariate regression analysis was constrained by the small sample size in the severe CONUT group, all of whom showed neurological progression, leading to potential overfitting. These findings underscore the need for larger, multicenter prospective studies that integrate anthropometric measurements and broader nutritional assessments to validate and expand upon our results.
Disclosure
This article has not been published in any journal before, but an earlier version has been presented as a preprint on ResearchSquare [66].
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no external funding.
Acknowledgments
We would like to express our heartfelt gratitude to all those who contributed to the realization of this project. Special thanks to Dr. Mensure Çakırgöz, whose expertise in statistics and design was invaluable in shaping the methodology and execution of our study. We are also deeply grateful to Zeynep Tanrıverdi, Dilan Düztaş, and Hatice Sevil for their dedication and significant contributions throughout the research process. Their collective efforts were instrumental in the success of our work.
Open Research
Data Availability Statement
All data, including figures and statistical analyses, are available upon reasonable request from the corresponding author, Dr. Eren Mingsar.




