Exploration of Four MicroRNA Expressions (miR-371a-3p, miR-34c-5p, miR-146b-5p, and miR-199a-3p) in the Seminal Plasma of Asthenozoospermic Infertile Tunisian Patients
Abstract
Asthenozoospermia (ASZ) is a semen condition characterized by reduced sperm motility; it is observed in ~40% of male infertility cases, and it is related to various factors including genetic and epigenetic factors such as microRNAs (miRNAs). Although miRNAs play a crucial role in spermatogenesis, their function in seminal plasma and their relationship with ASZ remain largely unknown, with a considerable amount of controversial and contradictory data. This study aimed to explore the expression variation of miRNAs (miR-371a-3p, miR-34c-5p, miR-146b-5p, and miR-199a-3p) in relation to ASZ. We collected seminal plasma samples from Tunisian infertile patients with ASZ (progressive sperm motility <32%) and without ASZ (normospermic patients) and performed quantitative real-time PCR to identify differentially expressed miRNAs. Three miRNAs showed dysregulated expression in the seminal plasma of patients with ASZ (miR-34c-5p, miR-146b-5p, and miR-199a-3p). Two miRNAs were upregulated (miR-34c-5p and miR-146b-5p; p < 0.05), while miR-199a-3p was downregulated (p < 0.05) compared to patients without ASZ. The expression levels of miR-199a-3p and miR-34c-5p were significantly correlated with sperm motility and vitality percentage with an area under the receiver operating characteristic (ROC) curve (area under the curve [AUC]) of 0.75 and 0.71, respectively. These findings indicate that the three miRNAs (miR-34c-5p, miR-146b-5p, and miR-199a-3p) are implicated in the etiology of ASZ and could be recognized as potential indicators of disrupted sperm function and male infertility.
1. Introduction
Infertility has become a global reproductive health problem, affecting ~15% of couples trying to conceive, and 50% of the infertility cases are attributed to male factors [1].
Asthenozoospermia (ASZ), one of the most common causes of male infertility (40% of cases) [2], is characterized by reduced or absent sperm motility (progressive sperm motility <32%), in fresh ejaculate, as outlined by the World Health Organization (WHO) guidelines [3].
ASZ can be caused by various factors, including varicocele, bacterial infections, genetic defects (chromosomal aberrations, mutations in genes related to sperm motility, mitochondrial DNA mutations, etc.) as well as epigenetic factors [4]. In fact, for years, sperm was considered merely as a carrier for delivering genetic material to the oocyte. It is now known that it plays a dual role by providing both genetic and epigenetic contributions to fertilization. This paradigm shift arises from a heightened awareness of the biological significance not only of sperm DNA but also of sperm RNA [5], such as microRNAs (miRNAs), which are short noncoding RNAs, generally composed of 18–24 nucleotides, with a single-stranded structure. These molecules are generated endogenously in cells [4] and play a crucial role in regulating gene expression [6].
Scientific evidence has demonstrated that miRNAs play a crucial role in various biological processes, including embryonic development, proliferation, differentiation, metabolism, apoptosis, and spermatogenesis [7].
In light of the important role that miRNAs play in cellular functions, dysregulation of miRNAs has been observed in various diseases, including infertility [8], making them potential targets for the diagnosis and treatment of this disease.
To date, numerous studies have been conducted to identify miRNAs exhibiting distinct expression patterns in infertile men as promising new biomarkers and to unravel their roles in spermatogenesis and fertility. Despite these efforts, numerous questions remain regarding the functions of miRNAs in sperm cells [4, 9].
It was demonstrated that certain miRNAs, miR-34c and miR-146a, exhibit preferential expression in spermatogonial stem cells [10, 11]. Recently, miR-199a-3p was reported to inhibit spermatogonial stem cell proliferation [12]. In 2005, Ostermeier et al. [13] identified miRNAs in the spermatozoa for the first time. Additionally, a notable correlation has been shown between the expression levels of miR-371a-3p and both sperm concentration and total sperm count [14]. The authors suggested that the level of this miRNA in ejaculate could serve as a novel biomarker for the noninvasive assessment of male infertility.
On the other hand, increasing and controversial evidence suggests that miRNAs may play a crucial role in the regulation of sperm motility [15]. Indeed, Wang et al. [16] reported a significant upregulation of seven miRNAs (miR-34c, miR-122, miR-146b, miR-181a, miR-374b, miR-509, and miR-513a) in ASZ patients.
These results have been confirmed by other studies including the recent study by Yeh et al. [17] in which the authors demonstrated that sperm from ASZ men had significantly higher levels of four miRNAs (miR-34b/miR-34c, miR-122, and miR-429) than those from non ASZ men. However, Mokánszki et al. [18] showed that three miRNAs (miR-15b, miR-34b, and miR-122) were significantly lower in the spermatozoa and seminal plasma of ASZ men compared to normospermic men. Similarly, Liang et al. [19] reported seven downregulated miRNAs in ASZ patients (miR-146b- 5p, miR-34b- 5p, miR-34c- 5p, miR-135a-5p, miR-449a, miR-509-3p, and miR-196b- 5p).
The controversial and contradictory data on the diversity and expression levels of miRNAs in sperm of ASZ patients highlight the necessity of conducting further research on larger and more diverse populations. This will help explore significant variations in miRNA expression related to infertility and to investigate the mechanistic roles of identified miRNAs and determine their potential use as biomarkers for assessing fertility or sperm quality.
In line with these ideas, we aimed to evaluate the expression of four miRNAs (miR-371a-3p, miR-199a-3p, miR-34c-5p, and miR-146b-5p), which are involved in spermatogenesis, in ejaculated sperm from Tunisian patients with ASZ.
2. Materials and Methods
2.1. Patient Collection and Preparation of Semen Samples
-
Normospermic group as control: (progressive sperm motility >32%, n = 20).
-
Asthenozoospermic group: (progressive sperm motility <32%, n = 20).
Patients with other semen pathologies such as oligozoospermia, necrozoospermia, or teratozoospermia, genetic abnormalities (cystic fibrosis, Klinefelter syndrome, etc.), varicocele, chemotherapy, AZF microdeletions, and history of cryptorchidism were excluded from the study.
After semen analysis, fresh semen samples were centrifuged at 4°C for 10 min at 2000 g. The supernatant was carefully removed and stored at −80°C before miRNA analyses.
2.2. RNA Extraction
Total RNA, including miRNAs, was isolated from frozen seminal plasma using the miRNeasy Mini Kit (QIAGEN) according to the protocol recommended by the manufacturer. The purity (A260/A280) and quantity of the extracted RNA were measured using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, USA).
2.3. Reverse Transcription (RT) and Quantitative Real-Time PCR for miRNA Quantification
Total RNA was reverse-transcribed into single-stranded cDNA using the miRCURY LNA Reverse Transcription Kit (Qiagen, United States, ID: 339340) according to the protocol recommended by the manufacturer. The cDNA was stored at −20°C.
Relative quantitative real-time PCR was performed on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems) using miRCURY LNA TM SYBR Green PCR Kit (Qiagen, USA, ID: 339346) and miRNA-specific primers for hsa-miR-371a-3p (5′AAGUGCCGCCAUCUUUUGAGUGU), hsa-miR-199a-3p (5′ACAGUAGUCUGCACAUUGGUUA), hsa-miR-34c-5p (5′AGGCAGUGUAGUUAGCUGAUUGC), and hsa-miR-146b-5p (5′UGAGAACUGAAUUCCAUAGGCUG).
Reaction steps were as follows: denaturation at 95°C for 15 min, followed by 40 cycles of amplification (denaturation at 94°C for 15 s, primer annealing at 55°C for 30 s, and primer extension at 70°C for 30 s), and terminated with a melting curve analysis (65–95°C; with 0.5°C increments) to confirm specific amplification.
All reactions were prepared according to the manufacturer’s recommendations and carried out in duplicate with standard negative controls. Data were normalized to the average housekeeping gene (RNU6-2).
The relative expression of target miRNAs was determined according to the 2−∆∆CT method using UniSp6 as the exogenous control. To normalize the scale between the four miRNAs, we plotted the log of the relative expression levels of miR-371a-3p, miR-199a-3p, miR-34c-5p, and miR-146b-5p.
2.4. Statistical Analysis
The SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) and GraphPad Prism 5.04 (GraphPad Software) were used for the data analysis and for plotting graphs.
The nonparametric Mann–Whitney U test was used to evaluate the differences in miRNA expression levels between cases and controls.
Spearman’s correlation was used to assess the association between sperm parameters and the normalized Ct value of each miRNA.
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminatory power of the miRNAs in distinguishing patients with abnormal sperm motility. The area under the ROC curve (area under the curve [AUC]) was obtained and an AUC of 0.7 was considered indicative of a good biomarker.
All data were expressed as means ± SD. For multiple testing correction, the adjusted p-values were calculated using the Benjamini–Hochberg (BH) method, and statistical significance was considered for a p-value < 0.05.
2.5. Target Prediction and Functional Analysis
Target genes were predicted using miRDB (https://mirdb.org) and TargetScan (https://www.targetscan.org). Both tools consider only experimentally validated targets. The Venny tool was used to identify common genes between the two lists. Gene Ontology and PANTHER pathway analysis were performed using online Gene Ontology resources (http://geneontology.org/) and PANTHER DB (https://www.pantherdb.org). PANTHER pathways with a false discovery rate (FDR) and a p-value < 0.05 were considered significant.
3. Results
3.1. Demographic and Clinical Characteristics
The comparative demographic and semen parameters of the two patient groups are presented in Table 1. There were no significant differences in age, semen volume, and sperm concentration as well as leukocyte levels between the two groups. However, the percentage of sperm vitality was significantly reduced in ASZ patients (p < 0.01), as were the percentages of progressive motility and total sperm motility (p < 0.001).
| Parameters | Study population | p-Value | |
|---|---|---|---|
| Control group (n = 20) | ASZ group (n = 20) | ||
| Age (years) | 36.11 ± 5.18 | 36.65 ± 7.79 | p = 0.61 |
| Volume (mL) | 3.79 ± 1.25 | 3.7 ± 1.54 | p = 0.69 |
| Progressive motility (A + B) (%) | 31.32 ± 9.48 | 20.22 ± 6.54 | p < 0.001 ∗ |
| Total motility (%) | 45.36 ± 5.59 | 29.52 ± 8.07 | p < 0.001 ∗ |
| Concentration (×106/mL) | 78.32 ± 43.05 | 86.23 ± 11.67 | p = 0.12 |
| Vitality (%) | 76.04 ± 7.74 | 61.13 ± 13.44 | p = 0.01 ∗ |
| Leukocytes (×106 WBC/mL semen) | 1.27 ± 1.74 | 1.09 ± 0.86 | p = 0.59 |
- Note: All numbers are reported as mean ± SD. ASZ group, asthenozoospermic group; control group, normospermic group.
- ∗ p-Value ≤ 0.05.
3.2. miRNA Expression
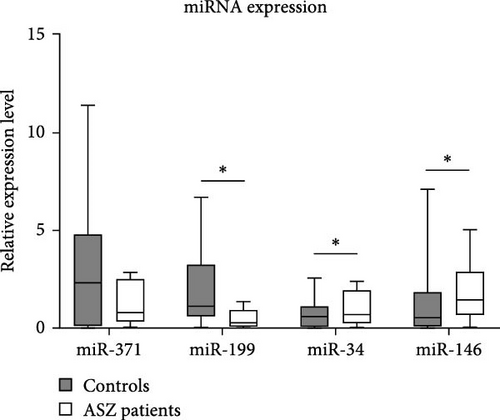
Regarding miRNA expression profiles, we observed a significant downregulation of miR-199a in the ASZ group compared to the normospermic group (p = 0.035). In contrast, the expression levels of miR-34c-5p and miR-146b-5p were significantly upregulated in ASZ patients compared to the control group (p = 0.036 and p = 0.022, respectively).
However, no significant difference was observed in miR-371a-3p expression between the two infertile groups (p = 0.853) (Figure 1, Table 2).
| miRNAs | Epigenetic dysregulation status (means ± SD) | p-Values (Mann–Whitney U test) | Adjusted p-values (Benjamini–Hochberg method) | |
|---|---|---|---|---|
| Control group | ASZ group | |||
| miR-371a-3p | 4.57 ± 4.40 | 7.91 ± 17.32 | p = 0.853 | p = 0.853 |
| miR-199a-3p | 2.72 ± 3.49 | 0.49 ± 0.40 | p = 0.035 ∗ | p = 0.048 ∗ |
| miR-34c-5p | 0.66 ± 0.52 | 2.38 ± 3.77 | p = 0.036 ∗ | p = 0.048 ∗ |
| miR-146b-5p | 1.57 ± 1.18 | 10.88 ± 17.96 | p = 0.022 ∗ | p = 0.048 ∗ |
- Note: ASZ group, asthenozoospermic group; control group, normospermic group.
- ∗ p-Value ≤ 0.05.
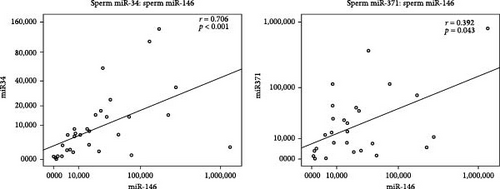
The correlation analysis showed that miR-146b-5p expression was positively correlated with the miR-371a-3p expression levels (r = 0.392, p = 0.043) and also miR-34c-5p (r = 0.706, p < 0.001).
3.3. Evaluation of miRNA Potential Role as Sperm Quality Biomarkers
Since the statistical analysis did not show significant differences in miR-371a-3p expression between the two studied groups, consequently, the remaining biostatistical and bioinformatics analyses were focused solely on the three miRNAs (miR-34c-5p, miR-146b-5p, and miR-199a-3p). Therefore, the ROC curve and the AUC showed a good accuracy with high AUC scores reached by the miR-199a-3p and miR-34c-5p in the ASZ group. For miR-199a-3p, AUC was 0.75% with 95% CI 0.55–0.95. At the cutoff (0.13), the sensitivity and specificity were 55% and 95%, respectively (p = 0.03). For miR-34c-5p, AUC was 0.71 with 95% CI 0.54–0.89. At the cutoff (0.07), the sensitivity was 94%, and specificity was 66%, with a p = 0.03.
For miR-146b-5p, the AUC was 0.69 with 95% CI 0.54–0.85. At the cutoff (0.11), the sensitivity was 95% and specificity was 73%, with a p = 0.02.
Logistic binary regression analysis showed that the three miRNAs (miR-199a-3p, miR-34c-5p, and miR-146b-5p) are risk factors for ASZ with a high AUC (0.95), 95% CI 0.95–1, and a statistical significance of p = 0.004. At the cutoff (0.005), the sensitivity was 100% and specificity was 80% (Figure 2).

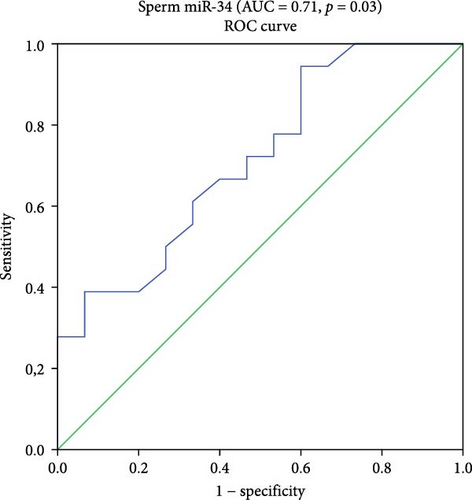
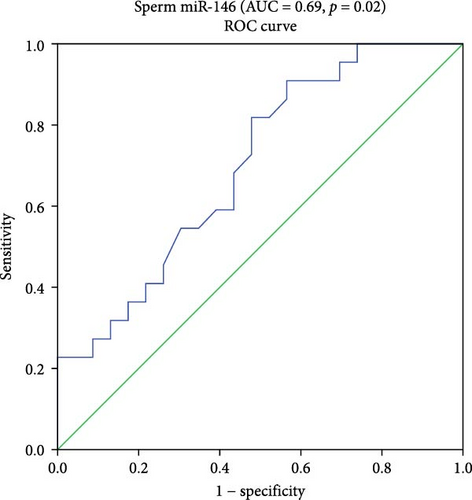
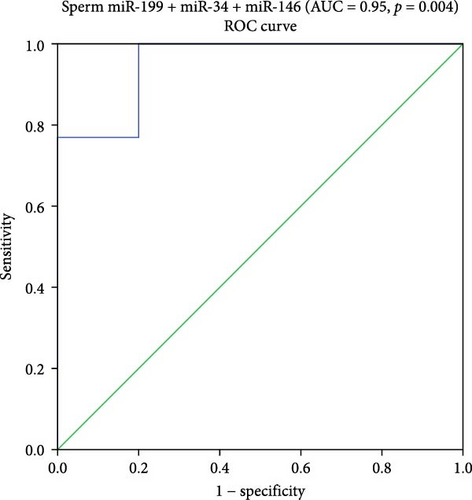
4. AUC
4.1. The Relationship Between miRNA Expression and Semen Parameters
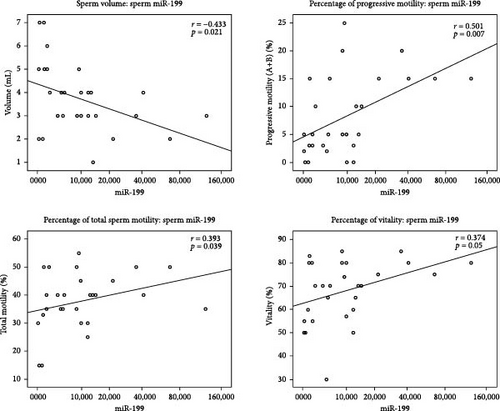
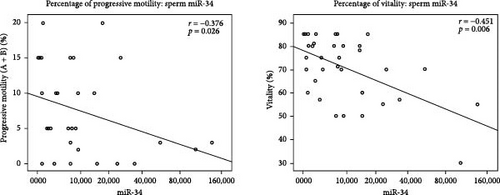
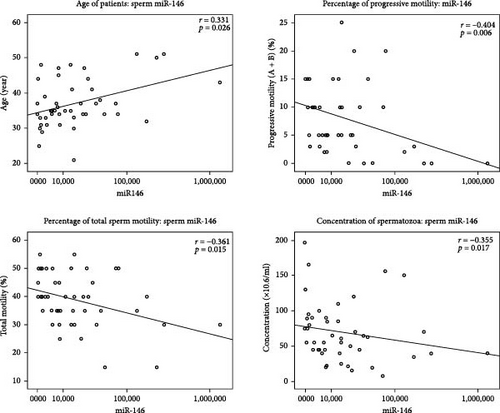
In all the studied samples, we observed significant negative correlation between the expression of miR-199a-3p and the sperm volume (r = −0.433, p = 0.021). In contrast, this miRNA was positively correlated with progressive motility (r = 0.501, p = 0.007), total sperm motility (r = 0.393, p = 0.039), and vitality (r = 0.374, p = 0.05). In addition, the Spearman test results showed a significant negative correlation between the expression of miR-34c-5p and progressive motility (r = −0.376, p = 0.026) and also sperm vitality (r = −0.451, p = 0.006). There was a significant negative correlation between miR-146 b-5 p expression level and sperm count (r = −0.355, p = 0.017), progressive motility (r = −0.404, p = 0.006), and total sperm motility (r = −0.361, p = 0.015) among all studied samples. Moreover, the expression of miR-146b-5p was positively correlated with the age of patients (r = 0.331, p = 0.026) (Table 3, Figure 3).
| miRNAs | Age (year) | Volume (mL) | Progressive motility (A + B) (%) | Total motility (%) | Concentration (×106/mL) | Vitality (%) |
|---|---|---|---|---|---|---|
| miR-199a-3p | ns |
|
|
|
ns |
|
| miR-34c-5p | ns | ns |
|
ns | ns |
|
| miR-146b-5p |
|
ns |
|
|
|
ns |
- Abbreviation: ns, no significant.
4.2. Target Prediction, Pathway Enrichment, and Functional Analysis
Using miRDB and TargetScan to predict target genes of the three miRNAs that showed differential expression in seminal plasma (miR-199a-3p, miR-34c-5p, and miR-146b-5p) and using Venny to identify common genes for each miRNA, we found that these three miRNAs share more than 100 genes (Table 4).
| miRNAs | miRDB | TargetScan | Venny (common genes) |
|---|---|---|---|
| miR-199a-3p | 477 genes | 474 genes | 271 |
| miR-34c-5p | 803 genes | 754 genes | 430 |
| miR- 146b-5p | 487 genes | 238 genes | 101 |
After functional enrichment analysis using the Gene Ontology resource, we identified significant PANTHER pathways for miR-199a-3p and miR-34c-5p, which cover a broad spectrum of cellular processes, including cell cycle (integrin signaling pathway), mitogenesis, differentiation, survival, apoptosis, cell migration (FGF signaling pathway), neuromodulation (5HT1-type receptor-mediated signaling pathway), cell growth, and proliferation (EGF receptor signaling pathway) as well as pubertal development and reproduction (gonadotropin-releasing hormone receptor pathway) (Table 5).
| miRNAs | PANTHER pathway | Accession | Total number of genes | Number of target genes | Row p-value | FDR |
|---|---|---|---|---|---|---|
| miR-199a-3p | Integrin signaling pathway | P00034 | 191 | 13 | 3.76E−06 | 6.06E−04 |
| FGF signaling pathway | P00021 | 123 | 8 | 3.77E−04 | 3.04E−02 | |
| EGF receptor signaling pathway | P00018 | 138 | 8 | 7.75E−04 | 4.16E−02 | |
| miR-34c-5p | Circadian clock system | P00015 | 10 | 3 | 2.14E−03 | 4.92E−02 |
| Notch signaling pathway | P00045 | 44 | 6 | 5.51E−04 | 4.43E−02 | |
| Metabotropic glutamate receptor group II pathway | P00040 | 48 | 6 | 8.35E−04 | 3.36E−02 | |
| 5HT1-type receptor-mediated signaling pathway | P04373 | 49 | 6 | 9.21E−04 | 2.47E−02 | |
| 5HT2-type receptor-mediated signaling pathway | P04374 | 68 | 7 | 9.02E−04 | 2.90E−02 | |
| Gonadotropin-releasing hormone receptor pathway | P06664 | 229 | 14 | 5.60E−04 | 3.00E−02 | |
| miR-146b-5p | Synaptic vesicle trafficking ∗ | P05734 | 31 | 2 | 9.26E−01 | 9.26E−01 |
| p53 pathway by glucose deprivation ∗ | P04397 | 22 | 1 | 1.07E−01 | 1.00E00 | |
| Notch signaling pathway ∗ | P00045 | 44 | 2 | 2.16E−02 | 1.00E00 | |
| Cell cycle ∗ | P00013 | 22 | 1 | 1.07E−01 | 1.00E00 | |
| Axon guidance mediated by Slit/Robo ∗ | P00008 | 25 | 1 | 1.21E−01 | 1.00E00 | |
| Toll receptor signaling pathway ∗ | P00054 | 58 | 2 | 3.54E−02 | 1.00E00 | |
| Alzheimer disease–presenilin pathway ∗ | P00004 | 126 | 4 | 3.93E−03 | 6.33E−01 | |
| General transcription regulation ∗ | P00023 | 39 | 1 | 1.79E−01 | 1.00E00 | |
| p38 MAPK pathway ∗ | P05918 | 40 | 1 | 1.84E−01 | 1.00E00 | |
| TGF-beta signaling pathway ∗ | P00052 | 102 | 1 | 9.33E−02 | 1.00E00 | |
| Transcription regulation by bZIP transcription factor ∗ | P00055 | 59 | 1 | 2.57E−01 | 1.00E00 | |
| Cadherin signaling pathway ∗ | P00012 | 164 | 2 | 1.97E−01 | 1.00E00 | |
| T cell activation ∗ | P00053 | 84 | 1 | 3.44E−01 | 1.00E00 | |
| Wnt signaling pathway ∗ | P00057 | 309 | 3 | 2.00E−01 | 1.00E00 | |
| EGF receptor signaling pathway ∗ | P00018 | 138 | 1 | 4.98E−01 | 1.00E00 | |
| Angiogenesis ∗ | P00005 | 171 | 1 | 5.74E−01 | 1.00E00 | |
| CCKR signaling map ∗ | P06959 | 172 | 1 | 5.76E−01 | 1.00E00 | |
- Note: p-value and FDR < 0.05.
- ∗Significant pathways based only on a p-value < 0.05.
Enrichment analysis for miR-146b-5p did not identify a significant PANTHER pathway (p-value and FDR < 0.05) due to the limited number of common genes ( = 101 common genes). Considering only p-value < 0.05, different pathways were revealed, related to cell cycle, angiogenesis, cellular homeostatic mechanisms (p53 pathway by glucose deprivation), cell growth and proliferation (EGF receptor signaling pathway), and immune system pathways (Toll receptor signaling pathway and T cell activation) (Table 5).
Considering molecular functions (Gene Ontology), the target genes shared by the three miRNAs are associated with ATP-dependent activity, binding, transcription regulator activity, and translation regulation activity. In cellular processes, these targets are related to cellular anatomical entity and protein-containing complexes. In reproduction, these targets are associated to meiotic cell cycle process, mitotic cell cycle, cytoplasmic microtubule organization, spermatogenesis, embryo development, organelle organization, piRNA processing, and spermatid development. The enrichment analysis revealed also that among the shared target genes by the three miRNAs, two genes (TUBGCP3 and TDRKH) are related to the reproduction pathway. The TUBGCP3 gene encodes gamma–tubulin complex component 3, and the TDRKH gene encodes tudor and KH domain-containing protein.
5. Discussion
Conventional clinical assessments of male fertility are considered to have limited diagnostic efficacy, prompting the search for more accurate molecular markers that better reflect men’s reproductive abilities. The development of new diagnostic and prognostic biomarkers has become crucial to improving the understanding of the mechanisms involved in male infertility and the management of infertile patients. Therefore, the exploration of novel sperm biomarkers, indicative of male fertility potential, has emerged as a significant focus of research. Thus, many assays have been developed to detect epigenetic variations, furnishing molecular data to elucidate the pathogenesis of unexplained infertility. In this context, the involvement of miRNAs in regulating the expression of genes involved in spermatogenesis has been highlighted, and extensive research has been conducted to explore the substantial link between miRNAs and various aspects of male infertility [20].
Among the earlier investigations on sperm miRNAs, Yuan et al. [21] reported that miR-34b/miR-34c is essential for normal spermatogenesis and male fertility. In the same year, Munoz et al. conducted an analysis of 23 miRNAs in sperm samples and revealed significant changes in the expression patterns of four miRNAs including miR-34c-5p in sperm collected from infertile individuals with mild spermatogenic failure. They suggested that the efficiency of the spermatogenic process significantly impacts the cellular miRNA content of developed germ cells [22]. In another study, miR-34c-5p was statistically associated with sperm concentration [23, 24] and positively correlated with age [25]. In addition, Abu-Halima et al. [26] suggested that five miRNAs including miR-34c-5p (hsa-miR-34b ∗, hsa-miR-34b, hsa-miR-34c-5p, hsa-miR-429, and hsa-miR-122) could serve as potential noninvasive biomarkers for diagnosing patients with subfertility.
Other studies have also explored the involvement of miRNAs in ASZ with diverse and contradictory findings and data. In 2011, a significant alteration in the expression of miRNAs in seminal plasma, including miR-34c-5p and miR-146b-5p, was reported for the first time in ASZ [16]. Other researchers proved that specific miRNAs, counting miR-34c-5p and miR-146b-5p, were significantly reduced in the samples of ASZ and azoospermic patients [4, 27, 28]. Also, in a small cohort study (15 infertile and 15 fertile individuals), Dorostghoal et al. [29] demonstrated a significant reduction (p = 0.019) in sperm miR-34c-5p levels among Iranian infertile patients with low sperm motility compared to fertile controls.
In this study, we explored the expression profiles of miR-371a-3p, miR-199a-3p, miR-34c-5p, and miR-146b-5p in seminal plasma samples from Tunisian ASZ infertile patients compared to normospermic patients. Our findings showed that three of these miRNAs (miR-199a-3p, miR-34c-5p, and miR-146b-5p) were dysregulated in ASZ patients. The expression of miR-34c-5p and miR-146b-5p was significantly higher in sperm of ASZ patients compared with controls. These two miRNAs (miR-146b-5p and miR-34c-5p) were positively correlated. In addition, there was a significant negative correlation between sperm miR-34c-5p expression and both progressive motility and vitality percentage. Furthermore, miR-146b-5p expression was negatively correlated with sperm count and motility. All these results suggest that the three miRNAs could serve as useful diagnostic biomarkers in the sperm of patients with ASZ. Our data are consistent with the findings of Wang et al. (2011) findings which showed that seven miRNAs, including miR-34c-5p and miR-146b-5p, were upregulated in ASZ patients but downregulated in azoospermic patients among infertile Chinese men. The ROC curve analysis revealed that the AUC for these miRNAs ranged from 0.733 to 0.921, significantly higher than that of routine biochemical parameters (0.510–0.622) [16].
Regarding miR146b-5p, it has been shown that this miRNA is involved in spermatogenesis and is regulated during spermatogonial differentiation. In the study performed by Huszar and Payne (2013) in Chicago, miR-146b-5p was found to undergo significant regulation during spermatogonial differentiation, which is influenced by retinoic acid (RA) signaling. Transcript levels of miR-146b-5p were reduced by nearly 180-fold in differentiating spermatogonia compared to undifferentiated ones. Based on these findings, the authors proposed that miR-146b-5p plays a modulatory role in the effect of RA on spermatogonial differentiation [30].
In our study, miR-146b-5p was correlated positively with the age of patients. Paoli et al. [31] reported that this miR showed a threefold lower expression (p < 0.001) in the elderly men.
Concerning miR-199a-3p, our findings showed a significant decrease in the sperm of ASZ patients compared to normal controls, with an AUC of 0.75. It has been shown that miR-199a-3p, along with other miRNAs, is initially present at low levels in testicular sperm populations, but its levels increase upon entering the epididymis, and its relative abundance significantly rises during epididymal maturation [32]. Currently, no literature data are available regarding the expression profile of this miRNA in the seminal plasma of ASZ infertile men. However, it was reported that the elevated levels of miR-199-5p interfered with the development of sperm flagella during spermiogenesis of male allotriploid crucian carp, by downregulating the expression of the Tekt1 gene, leading to structural abnormalities in the sperm [33].
Our findings indicated that the miR-371a-3p expression ratio was lower in ASZ patients compared to controls, without reaching statistical significance. Currently, no literature data are available regarding the relationship between this miRNA and ASZ, but several studies have recognized miR-371a-3p as a potential indicator of disrupted spermatogenesis [14, 34]. Paoli et al. [31] reported a 14-fold decrease in expression of miR-371 in semen samples obtained from 40 elderly Italian men compared to 40 young controls (p < 0.001). However, the authors were unable to completely exclude the presence of asymptomatic or idiopathic conditions. Lian et al. [35] observed a downregulation of miR-371 in the testicular tissue of nonobstructive azoospermic (NOA) patients. The authors suggested that the decreased expression of this miRNA might contribute to the increased apoptosis in the testes of NOA patients. Additionally, in the study by Radtke et al. [14] which focused on the exploration of miR-371a-3p expression in germ cell tumors patients (n = 20) and healthy men as controls (n = 30), a very low serum expression and high seminal plasma expression were observed in controls. Moreover, the expression of miR-371a-3p in seminal plasma was correlated with sperm concentration but not with motility, leading the authors to suggest that spermatozoa could be a possible source of miR-371a-3p production [14]. Supporting these data, Boellaard et al. [34] detected miR-371a-3p levels in semen from normospermic (n = 11) and oligospermic (n = 3) Dutch men, independent of motile sperm count. However, the small sample size was considered a notable limitation of this study.
Considering the findings of our study and all data reported in the literature about the variation of miRNAs expression in semen, we suggest that the set of miRNAs, including miR-34c-5p, miR-146b-5p, and miR-199a-3p, could affect sperm motility and may potentially serve as diagnostic indicators for ASZ, as well as disrupted semen quality and function.
To explore the potential mechanism involving miRNAs in the regulation of sperm motility and in ASZ, we performed a functional analysis using bioinformatics tools. The enrichment analysis revealed two target genes (TUBGCP3 and TDRKH) in pathways mainly related to reproduction. The TUBGCP3 gene encodes gamma–tubulin complex component 3 which plays a crucial role in the formation and function of microtubules that are essential for the proper function of sperm flagella [36]. We suggest that mutations in TUBGCP3 or dysregulation of its expression may disrupt microtubule formation in sperm, leading to reduced motility. The TDRKH gene encodes tudor and KH domain-containing protein is involved in spermatogenesis [37, 38]. We speculate that genetic variations in the TDRKH gene could potentially impact the function of the TDRKH protein and its role in spermatogenesis, leading to alterations in sperm parameters and male infertility.
In trying to understand the molecular mechanisms of ASZ patients, numerous studies have suggested that the effects of altered miRNAs on ASZ may occur through diverse mechanisms [39–42]. Zhou et al. [43] showed that miR-27a and miR-27b negatively regulate the expression of cysteine-rich secretory protein 2 (CRISP2). Furthermore, the same group demonstrated in 2017 that decreased levels of CRISP2 protein were significantly associated with reduced abnormal sperm morphology, impaired progressive motility, and infertility in men with ASZ [44]. Another research group from China indicated that the downregulated let-7b- 5p could repress glycolysis in ASZ by targeting AURKB [45]. On the other hand, many authors reported that target prediction showed a significant association between miR-199a-3p and miR-34c-5p and several pathways that play critical roles in spermatogenesis and postfertilization development, including integrin, FGF, EGF receptor, circadian clock system, and Notch signaling pathways [46–49]. HIF-1 and FoxO signaling pathways were also reported in association with ASZ [50]. In a study by Liang et al. [19], 13 dysregulated miRNAs in ASZ were linked to key pathways, including PI3K-Akt, MAPK, HIF-1, and FoxO. Zhang et al. [51] demonstrated that through PI3K-Akt pathway activation, leucine enhances sperm motility by promoting autophagosome formation and inhibiting its fusion with lysosomes. Similarly, Yu et al. [52] revealed that the activating of P38 MAPK, cytochrome P450, and COX pathways reduces sperm motility, highlighting a potential pathological mechanism of ASZ. These findings may advance diagnostic, prognostic, and therapeutic approaches, paving the way for new therapeutic possibilities in ASZ and in the field of personalized medicine.
In light of all the above data, which highlight the complexity of miRNA involvement and the mechanisms they mediate in the etiology of ASZ, further research is needed to clarify the precise role of miRNAs in this condition. Future research should focus on (i) studies involving larger and more diverse populations to ensure findings are generalizable, (ii) the use of standardized methodologies for miRNA detection and analysis, (iii) experimental validation of the computational predictions to confirm miRNA targets, and (iv) functional studies to elucidate the precise roles of miRNAs in sperm physiology.
6. Conclusion
In conclusion, our findings support numerous previous studies regarding the role of miRNAs as epigenetic factors regulating spermatogenesis and semen quality. They highlight the role of miR-34c-5p, miR-146b-5p, and miR-199a-3p in sperm as potential novel biomarkers for assessing male infertility, particularly ASZ. However, the small sample size constitutes a limitation of this study, and further research into the proposed target genes is essential for a more comprehensive understanding.
Ethics Statement
The Ethical Committee of the Faculty of Medicine of Sfax approved all the experiments under code n.74/24.
Consent
A signed consent form was obtained from all participants who donated semen samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Rihab Derbel and Imen Belguith. The first draft of the manuscript was written by Rihab Derbel, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
There is no financial or commercial involvement in this study. This project is carried out under the MOBIDOC scheme, funded by the EU through the SWAFY Project and managed by the ANPR.
Acknowledgments
We extend our gratitude to the Ministry of Higher Education and Scientific Research in Tunisia for their support. This project is carried out under the MOBIDOC scheme, funded by the EU through the SWAFY Project and managed by the ANPR.
Open Research
Data Availability Statement
All relevant data are in the manuscript. The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.