The Ameliorative Effects of Probiotics on the Sperm Quality and Testicular Structure After Ischemia/Reperfusion Injury Following Testicular Torsion/Detorsion
Abstract
Oxidative stress is a critical contributor to the pathophysiology of ischemia/reperfusion (I/R) injury associated with testicular torsion (TT) and detorsion (D) (TT/D). This study examines the antioxidative properties of the probiotics Lactobacillus (L.) reuteri and Lactobacillus (L.) rhamnosus in ameliorating TT/D-induced oxidative and histological damage. Male Sprague–Dawley rats (n = 6 per group) were allocated into five experimental groups: a sham-operated group, a torsion/detorsion (T/D) group, and three T/D groups treated with L. reuteri (T/D+LRe), L. rhamnosus (T/D+LRm), or a combination of both (T/D+LRe+LRm). TT was induced by rotating the right testis 720° clockwise for 2 h, followed by 60 days of reperfusion. The testis was then excised for further analysis. The evaluated parameters included sperm quality, hormone levels (FSH, LH, and testosterone), as well as levels of malondialdehyde (MDA) and glutathione (GSH). Additionally, the activities of myeloperoxidase (MPO) catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD), as well as histological changes, were assessed. The findings demonstrated that TT/D significantly increased the FSH and LH levels, reduced sperm quality and testosterone levels, elevated MDA levels, and enhanced MPO activity. Additionally, TT/D reduced the GSH levels and the activities of GPx, CAT, and SOD. Probiotic treatment, particularly with L. rhamnosus, significantly increased testicular GSH levels and the activities of antioxidant enzymes GPx and CAT while reducing MDA levels and MPO activity. Histopathological analysis revealed severe testicular damage, including reductions in spermatogenic, sertoli, and Leydig cells following TT/D, mitigated by L. rhamnosus. These results suggest that probiotics, especially L. rhamnosus, may protect against I/R injury in a rat model of testicular T/D, likely due to their antioxidant properties.
1. Introduction
Testicular torsion (TT) is a critical urological emergency predominantly affecting neonates, children, and adolescents. It arises from the spermatic cord twisting, which restricts blood flow to the testis, resulting in ischemia, and severe pain [1]. Research indicates that the degree of testicular injury is strongly correlated with both the duration and severity of the torsion. Early diagnosis and surgical correction are vital to preventing irreversible testicular damage and minimizing the risks of infertility and subfertility [2]. Damage caused by torsion can persist even after detorsion (D). Although D and reoxygenation of the testis are critical for mitigating severe testicular injury and preserving tissue viability, the restoration of blood flow generates high levels of reactive oxygen species (ROS). This process, in turn, can trigger reperfusion injury, further exacerbating damage to the ischemic testicle.
Ischemia/reperfusion (I/R) injury arises through the activation of intracellular cascades, including neutrophil activation and adhesion within testicular vessels, (ROS) production, pro-inflammatory cytokines release, and cell necrosis and apoptosis initiation. ROS, in particular, plays a significant role by inducing cellular and mitochondrial lipid peroxidation [3–5]. Sperm cells are particularly prone to damage caused by ROS due to the abundance of unsaturated fatty acids in their membranes. ROS triggers lipid peroxidation, which results in the accumulation of oxidative stress within the cells. Studies have demonstrated that prolonged torsion induces testicular ischemia, resulting in elevated oxidative stress within the affected testes. This oxidative stress is characterized by increased production of nitric oxide (NO) and hydrogen peroxide (H2O2), enhanced lipid peroxidation, reduced levels of antioxidant enzymes, and an elevated incidence of apoptosis mediated by mitochondria in germ cell lines [6]. The increased ROS levels resulting from TT/D disturb the normal function of essential cellular components, such as genomic DNA, membrane lipids, and proteins; alter membrane permeability; disrupt membrane integrity; and lead to DNA damage. This disruption adversely affects spermatogenesis, leading to diminished sperm production and compromised sperm quality [2].
Antioxidants and their enzymes play a vital role in neutralizing free radicals and reducing oxidative stress, a key factor in TT and reperfusion injury. For example, superoxide dismutase (SOD) converts reactive superoxide anions (O2−) into H2O2, which catalase (CAT) further breaks down into water and oxygen. The glutathione (GSH) system, including glutathione peroxidase (GPx) and glutathione reductase (GR), maintains redox balance by neutralizing H2O2 and lipid peroxides, protecting the cells from damage [7]. Several studies have demonstrated that TT/D significantly affects enzymatic activity, leading to decreased antioxidant enzyme levels (SOD and GPx) and increased oxidative stress markers (MDA), contributing to testicular tissue damage [8–10].
As a major inflammatory enzyme, myeloperoxidase (MPO) plays a pivotal role in oxidative stress and neuroinflammation, making it a critical target for therapeutic intervention in I/R injury [10–12]. MPO facilitates the production of hypochlorous acid (HOCl), exacerbating oxidative stress and promoting cellular injury [13]. Increased MPO levels serve as a biomarker for the severity of inflammation and oxidative damage induced by I/R in testicular tissue [10].
The gastrointestinal tract harbors a critical component known as gut microbiota (GM) [14]. Increasing evidence suggests that GM exerts diverse effects on various distant organs, including the male reproductive system [15]. GM plays a crucial role in preserving the structural stability of the testes and regulating immune functions within the testicular environment to ensure optimal conditions for spermatogenesis [16]. Moreover, the GM and the testis establish a gut-testis axis, contributing to the production of essential molecules through microbial metabolism or de novo synthesis [17]. These molecules play pivotal roles in nutritional support, immune system modulation, and hormonal balance, ultimately promoting male reproductive health via the circulatory system [18].
Probiotics, widely recognized as gut-associated dietary microorganisms, are known to modulate local immunity in the gastrointestinal tract and activate metabolic pathways that affect overall health [19]. Some studies propose that certain probiotics may positively influence the sperm motility and count [20, 21]. Recent studies have shown that probiotics provide a protective effect against I/R injury in various tissues, such as the kidneys, liver, and intestine [22–24]. Furthermore, the antioxidant effects of probiotics have been demonstrated in numerous studies. Probiotics have been shown to exert antioxidant effects by regulating the activity of free radicals, reducing the NO and MDA levels, and increasing the activity of antioxidant enzymes [25, 26]. Certain probiotic strains, particularly Lactobacillus, have been associated with antioxidant and anti-inflammatory properties [27]. These effects may help mitigate oxidative stress, a known factor affecting the sperm quality.
As indicated, oxidative stress plays an important role in the reduction of sperm quality, testicular damage, and potential infertility during I/R injury following TT/D; numerous studies have demonstrated the effectiveness of antioxidant therapies in mitigating tissue damage resulting from inflammation and oxidative stress triggered by TT [10, 28, 29]. Since the antioxidant and anti-inflammatory roles of certain species of Lactobacillus have been identified [30], this study focuses on two specific Lactobacillus probiotic strains: L. rhamnosus GG (AATCC 53103) and L. reuteri DSM 17938. Both strains are currently used as probiotics and have received approval as dietary supplements, with documented antioxidant effects [31, 32]. Therefore, the primary objective of this investigation is to explore the impacts of these probiotics on testicular structure, spermatogenic cells, sperm quality, sex hormones, oxidative stress index, and the activity of the MPO following TT/D in male rats.
2. Materials and Methods
2.1. Approval for Ethical Considerations
The Animal Care and Local Ethics Committee of Shiraz University of Medical Sciences reviewed and approved the research protocol, with ethics approval obtained in 2022 (Approval No. IR.SUMS.AEC.1401.117).
2.2. Animals and Research
In this investigation, 30 adult male Sprague–Dawley rats weighing 250–300 g were procured from the Center of Comparative and Experimental Medicine at Shiraz University of Medical Sciences. The rats were subjected to standard laboratory conditions, including a 12-h dark/light cycle, a temperature of 23 ± 2°C, and humidity between 30% and 45%. They had unlimited access to tap water and standard chow throughout the experiment.
- 1.
The sham group, which served as the sham-operated group, experienced only surgical stress without induction of T/D
- 2.
T/D group, consisted of T/D-operated rats
- 3.
T/D+LRe group involved T/D-operated rats which received L. reuteri DSM 17938 (1 × 109 CFU/kg body weight [b.w])
- 4.
T/D+LRm group involved T/D-operated rats which received L. rhamnosus GG (AATCC 53103) (1 × 109 CFU/kg b.w)
- 5.
T/D+LRe+LRm group were T/D-operated rats which received both probiotics.
The treated groups received daily probiotic supplements through oral gavage for 60 days. The doses of both probiotics used in this study were selected based on previous research [33, 34]. The remaining groups also underwent oral gavage with normal saline to mitigate potential stress induced by the gavaging procedure.
Because the entire spermatogenesis cycle in rats lasts approximately 54–56 days [35, 36], the duration of the probiotic supplementation (60 days) was selected to exceed the length of a complete spermatogenic cycle. At the end of the experiment, the animals received an excessive dose of a ketamine/xylazine combination (300/30 mg/kg). After inducing deep anesthesia, blood samples were provided via cardiac puncture. Upon centrifugation of the blood at 3000 × g for 10 min, the resulting serum samples were gathered and stored at −20°C for subsequent analysis of serum hormone levels (FSH, LH, and testosterone). Following these steps, the rats were euthanized humanely through decapitation under deep anesthesia, and the left testes of all animals were removed under sterile conditions. The testes were weighed and then longitudinally divided into two halves. One half was designated for histopathological assessment, while the other was allocated for evaluating oxidative stress. Samples intended for histopathological evaluation were fixed in a 10% neutral buffered formalin solution, while those designated for other assays were kept at −80°C until further analysis.
2.3. Preparation of Probiotic Bacterial Suspension
L. rhamnosus GG (AATCC 53103) and L. reuteri DSM 17938 were provided by the Pardis Roshd Mehregan Company Shiraz, Iran. To prepare individual bacterial suspensions of L. reuteri and L. rhamnosus at a concentration of 1 × 109 CFU/mL, we dissolved 100 mg of each lyophilized probiotic in 1 mL of normal saline. To prepare a combined probiotic solution with the same concentration, 50 mg of each probiotic was dissolved in 1 mL of normal saline.
2.4. T/D Surgery
The animals were deeply anesthetized with a ketamine/xylazine combination (80/5 mg/kg), and the depth of anesthesia was continuously monitored using the toe pinch method [37]. The T/D surgery began with a vertical incision in the scrotal area. The tunica albuginea was incised, and the right testis was rotated 720° clockwise around the spermatic cord for 2 h, securing it with a bulldog clamp [38, 39]. After that, the testis was rotated back to its normal anatomical position, repositioned within the scrotum, and anchored to the inner scrotal wall with absorbable sutures. This anchoring helps keep the testes in place, reducing the risk of their twisting again. Subsequently, the skin incision was sutured, and the animals were kept in cages until the removal time. Following orchiectomy, decapitation was carried out using anesthetic agents across all experimental groups.
2.5. Measurement of Body and Testis Weight, Gonadosomatic Index (GSI)
During the experiment, the body weight was weekly recorded. Additionally, the weight of the testes and the determination of the GSI were performed for each animal. The GSI was determined as the percentage by multiplying the testicular weight-to-body weight ratio by 100.
2.6. Sperm Collection and Analysis of Sperm Parameters
The epididymal tail was carefully removed and immediately placed in a petri dish containing 5 mL of phosphate-buffered saline (PBS) and then incubated at 37°C for 30 s to allow the sperm to disperse. The sperm solution was gently agitated to ensure a homogenous sperm mixture.
2.6.1. Sperm Count
A Neubauer counting chamber was used to count the sperm. The sperm samples were first diluted with an appropriate buffer solution to a ratio of 1:5 (v/v) to facilitate accurate counting. A small volume (10–20 µL) of the diluted sperm sample was loaded onto the chambers, and the sperms were counted under a light microscope at 400x magnification. The number of sperm cells in each quadrant of the chamber was counted, and the concentration was calculated by applying the appropriate formula based on the chamber’s dimensions and the dilution factor used. Then, the findings were reported as the total number of sperm per milliliter [40].
2.6.2. Sperm Motility
The sperm mixture was placed on pre-warmed microscope slides. In 10 randomly chosen fields of view at a magnification of 400×, 100–200 sperms for each specimen were assessed. Sperm movement was categorized as progressively motile, nonprogressively motile, or immotile, following standards of the sixth edition of the World Health Organization (WHO) (2021) [41]. The percentage of sperm motility was assessed by dividing the number of motile sperms by the total sperm count and then multiplying the result by 100.
2.6.3. Sperm Viability and Morphology
For viability and morphology testing, a sperm smear was prepared and stained with eosin and nigrosin dyes (Sigma Aldrich), following the guidelines set by the WHO [41]. For evaluating the sperm viability, live sperm heads remain unstained, whereas dead sperm heads pick up the dye. Between 100 and 200 stained and unstained spermatozoa per rat were analyzed across 10 randomly chosen microscopic fields at a magnification of 400×. The proportion of viable sperms was determined by dividing the number of unstained (live) sperms by the total count of stained (dead) and unstained (live) sperms and then multiplying by 100 (Figure 1).
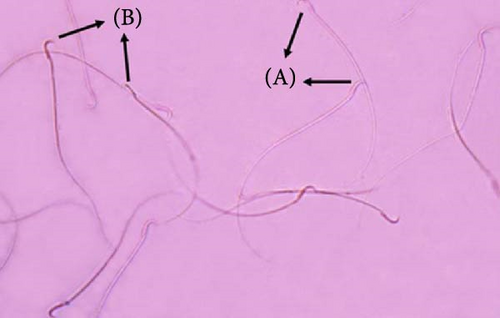
Sperm morphology was assessed by examining 200 spermatozoa per slide under a light microscope (400x). Abnormalities were categorized as head and tail defects and the percentage of normal sperm was calculated [42].
2.7. Analysis of Serum Sex Hormones
The serum concentrations of testosterone, FSH, and LH were determined using the enzyme-linked immunosorbent assay (ELISA) technique, according to the instructions provided by the manufacturer of the commercial kits (Cusabio Biotech Company, China). All measurements were conducted in duplicate.
2.8. Preparation of Testis Homogenate
A 100 mg sample of testicular tissue was finely chopped and rinsed with PBS with a pH of 7.4. The mixture was subsequently homogenized on ice with a homogenizer to keep the temperature low. To further break down the tissue, the mixture was subjected to sonication in an ice bath for 15 s to avoid heat buildup, followed by a centrifugation process with 1 mM ethylenediaminetetraacetic acid (EDTA) at 4000 rpm for 15 min at a temperature of 4°C, after which the supernatants were carefully collected and then carefully transferred into separate containers and frozen at −80°C until further analysis. These supernatants were used to analyze lipid peroxidation, enzyme activity, and protein levels.
2.9. Assessment of Oxidative Stress Indicators in Testicular Tissue
The MDA concentration in the supernatants of sample homogenate, an indicator of lipid peroxidation, was determined using the thiobarbituric acid reactive substances (TBARS) method. Briefly, 100 µl of the supernatant from the centrifuged testicular tissue homogenate was mixed with 400 µl of trichloroacetic acid (TCA, 20%) and thiobarbituric acid (TBA, 0.8%). The mixture was heated at 87°C for 1 h, cooled to room temperature, and centrifuged at 12,000 rpm for 15 min at 4°C. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The results were expressed as nmol MDA/mg protein of the tissue.
Reduced GSH levels in the tissue homogenate were determined using Ellman’s reagent (5,5′-dithiobis-[2-nitrobenzoic acid], DTNB) following previously established protocols, with slight modifications [11]. This oxidation leads to the creation of the yellow product called 5’-thio-2-nitrobenzoic acid (TNB). Briefly, 20 µL of the testicular tissue supernatant was mixed with 80 µL of the DTNB reagent and 40 µL of the glutathione reductase (GR) solution in 20 µL buffer solution (0.1M potassium phosphate buffer with 5 mM EDTA disodium salt, pH 7.5). After allowing 30 s, 60 µL of β-NADPH was added to the solution. GR facilitates the conversion of glutathione disulfide (GSSG) to GSH in the presence of NADPH. The formation of the yellow-colored product (5’-thio-2-nitrobenzoic acid) was measured spectrophotometrically at 412 nm. The results were expressed as µmol GSH/mg protein of the tissue.
MPO activity was determined using 3,3′,5,5′-Tetramethylbenzidine (TMB, Sigma) as described by Pulli et al. [43]. In short, 10 µL of the supernatant was mixed with 60 µL of H2O2 (Sigma), 110 µL of TMB solution) Sigma), and 170 µL of PBS (50 mM, pH 6.0). The mixture was incubated at 37°C for 10 min. The reaction was stopped by adding 50 µL of 2 M sulfuric acid (H2SO4, Sigma), and the change in absorbance at 450 nm was measured. The linear increase in absorbance over time (∆A/min) was used to calculate MPO activity, which was expressed as units per milligram of protein of the sample.
The testicular SOD, GPx, and CAT activities were quantified through commercially available kits (Kiazist Life Sciences, Iran) based on colorimetric assays. The testicular protein content was measured using a BCA (bicinchoninic acid) protein assay kit (Pars Tous Biotech, Iran).
2.10. Histological Preparations and Stereological Analysis
2.10.1. Collection and Preparation of Testis Tissue
Following the administration of deep anesthesia and euthanization of the rats, their testes were weighed, and the initial volume of the testes was determined using the immersion technique in saline solution. It is essential to prepare isotropic uniform random sections to assess stereological parameters. Therefore, the testes were sliced according to the “orientation method” (Figure 2A–C). The tissue pieces were preserved in 10% neutral buffered formalin for further processing. Subsequently, these pieces (8–12 per testis) were processed, embedded in paraffin blocks, cut into sections (5 and 20 µm thick), and stained with Hematoxylin and Eosin (H&E) for stereological analysis and Masson’s trichrome for histological assessment. Following staining, 8–12 fields from each slide were selected using systematic uniform random sampling to compute stereological parameters [40, 44].
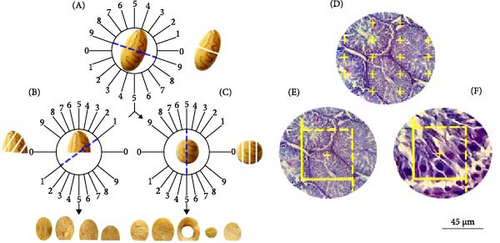
The primary testicular volume was measured by immersion in a saline solution before processing. By incorporating the degree of shrinkage into the calculation, the secondary volume provides a more accurate representation of the testis size after the histological processing, considering the reduction in size due to tissue processing methods. This adjusted volume is essential for accurate stereological assessments and subsequent analyses [44].
2.10.2. Evaluation of Volume
A total of 5 μm sections were prepared to estimate the volume of the lumen and epithelium of seminiferous tubules and interstitial tissue. Stereological analysis was conducted with a Nikon Eclipse E200 microscope (Tokyo, Japan) featuring an oil immersion objective lens paired with a Samsung SCB-2000P video camera (Hanwha Techwin, South Korea) linked to a computer.
This formula involves the summation of the points falling on the structure [∑P(structure)] divided by the total points counted [∑P(total)], multiplied by the secondary testicular volume [40].
The coefficient of error (CE) for the stereological parameters was calculated using the method described by Gundersen et al. [45]. On average, 250 points were counted in 8–12 sections per testis. The CE for estimating the volumes of the lumen and epithelium of seminiferous tubules and interstitial tissue (CE [V]) was 0.10–0.15.
2.10.3. Stereological Cell Count Estimation
Determination of the numerical density “Nv” of various testis cells was done by the optical disector method on sections 20 µm thick. This method involved the use of an Nikon Eclipse E200 microscope (Tokyo, Japan) equipped with an oil immersion lens (40×) with a high numerical aperture (NA = 1.3), linked to a video camera for broadcasting live images to a monitor. An electronic microcator (MT12, Heidenhain, Traunreut, Germany) was used to measure Z-axis movements.
An unbiased counting frame was deployed to enumerate the mentioned cells. Guard zones, established above and below the section surfaces, mitigate processing artifacts. Cells located within these zones were excluded from the count. The disector’s height was matched by the space between the guard zones. Each cell placed within the frame was counted (Figure 2F).
In 8–12 sections per testis, 30 spermatogonia, 90 spermatocytes, 420 spermatids, and 20 sertoli cells in 40 optical dissectors, as well as 20 Leydig cells in 10 optical disectors, were counted. The CE for estimating the number of cells (CE [N]) was 0.14–0.18.
2.10.4. Histopathological Evaluation
Histopathological and spermatogenesis evaluation of the testicular tissue was conducted using a semi-quantitative method known as the Johnsen score [46]. This scoring method categorizes spermatogenesis on a scale of 1–10 for each tubule cross section. At least 40–50 tubules were randomly assessed for each testis under a light microscope, and the mean Johnsen’s score per rat was calculated based on these evaluations.
2.11. Statistical Analysis
Data analysis was conducted using GraphPad Prism (version 8.4.3). Before analysis, data normality was evaluated using the Shapiro–Wilk test, confirming that all data were normally distributed. A one-way analysis of variance (ANOVA) was performed to compare body weight, biochemical parameters, sperm quality (including count, motility, morphology, and viability), and stereological parameters (such as volume and cell density) among the experimental groups. Tukey’s multiple comparison test was employed as a post hoc analysis to identify specific group differences. Results are presented as mean ± SEM, with statistical significance considered at p < 0.05.
3. Results
3.1. Body Weight, Testicular Weight, and GSI
Table 1 illustrates body weight, testicular weight, and GSI across different experimental groups. Comparative analysis of body weight among the various experimental groups indicated a statistically significant reduction in body weight in the T/D+Rt group compared to the sham group (p < 0.05). Additionally, body weight significantly differed between the T/D+Rt and T/D+Rt+Rm groups (p < 0.05). There were no significant differences in body weight among the other experimental groups.
| Parameters | Sham | T/D | T/D+Rt | T/D+Rm | T/D+Rt+Rm |
|---|---|---|---|---|---|
| Body weight (g) | 315.4 ± 7.62 | 299.8 ± 8.28 | 263.2 ± 10.96 ∗$ | 301.9 ± 9.05 | 315.2 ± 15.25 |
| Testis weight (g) | 1.916 ± 0.10 | 0.64 ± 0.07 ∗∗∗∗ | 0.64 ± 0.05 ∗∗∗∗ | 1.18 ± 0.03 ∗∗# | 1.36 ± 0.20 ∗∗# |
| GSI (%) | 0.64 ± 0.06 | 0.21 ± 0.02 ∗∗∗∗ | 0.26 ± 0.03 ∗∗∗ | 0.41 ± 0.03 ∗ | 0.38 ± 0.08 ∗ |
- Note: A one-way ANOVA with a Tukey post hoc test was performed to compare the animals among the different experimental groups. Each bar displays the mean ± SEM (n = 6). Sham, group of animals that had no testicular torsion after performing surgery; T/D, group of animals subjected to 2 h of testicular torsion and then detorsion; T/D+Rm, the T/D group was treated with L. rhamnosus; T/D+Rt, the T/D group was treated with L. reuteri; T/D+Rt+Rm, the T/D group was treated with both probiotics.
- ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. the Sham group.
- #p < 0.05 vs. the T/D group.
- $p < 0.05 vs. the T/D+Rt+Rm group.
The T/D group had considerably lower testicular weight than the sham group (p < 0.0001). Treatment with L. rhamnosus, alone or in combination with L. reuteri, led to a significant increase in testicular weight compared to the T/D group (p < 0.05); however, these groups still exhibited substantial differences in testicular weight compared to the sham group.
The comparison of GSI among the different experimental groups showed a significant difference in this parameter between the T/D and the sham groups. The use of probiotics in the T/D groups had no significant effect on GSI when compared to the untreated group.
3.2. Morphological Changes in the Sperm
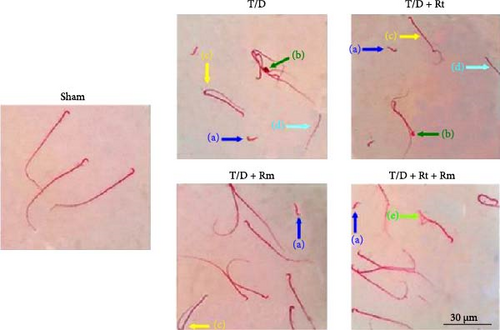
Compared with the sham group, the sperm cells in the T/D group exhibited various deformities, including detached heads, amorphous heads lacking hooks, twisted midpieces, headless sperm, and bent tails. The groups treated with L. rhamnosus (T/D+Rm) and the combination of L. rhamnosus and L. reuteri (T/D+Rt+Rm) significantly reduced sperm abnormalities compared to the T/D group (Figure 3).
3.3. Sperm Parameters (Count, Motility, Viability, and Morphology)
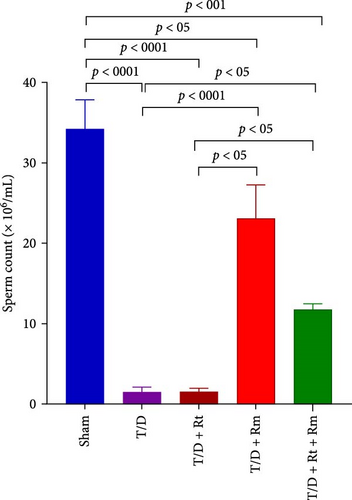
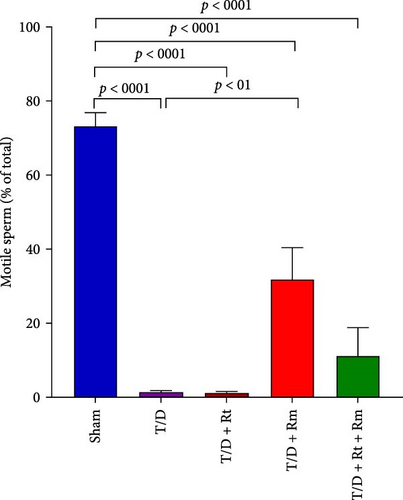
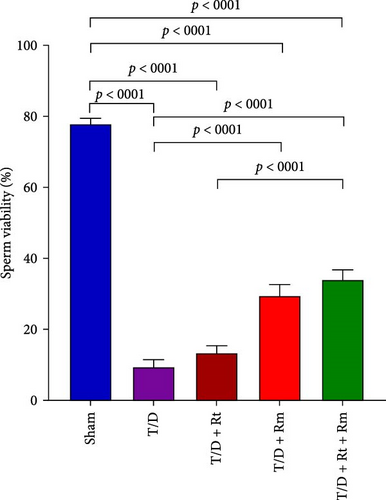
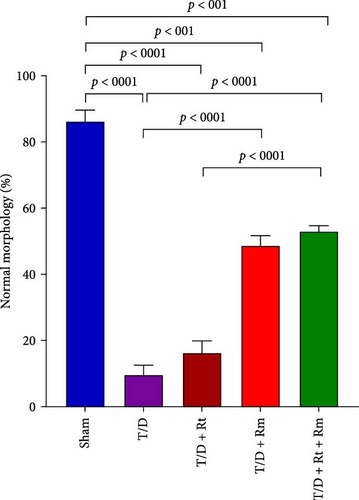
The study assessed the impact of L. reuteri and L. rhamnosus treatments on sperm parameters (Figure 4A–D). The T/D group had significantly lower sperm count (A), motility (B), viability (C), and normal morphology (D) compared to the sham group. Treatment with L. rhamnosus (T/D+Rm) and combining L. rhamnosus and L. reuteri (T/D+Rt+Rm) significantly increased the sperm count, viability, and normal morphology. Only L. rhamnosus (T/D+Rm) significantly improved the sperm motility, demonstrating a 22.8-fold increase compared to the untreated T/D group. The T/D+Rt group did not show significant differences in the above sperm parameters compared to the T/D group. There were significant differences in the sperm parameters between the probiotic T/D groups and the sham group.
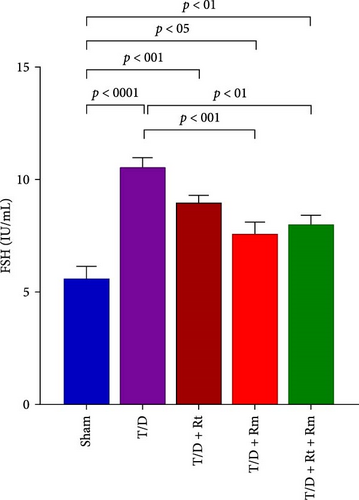
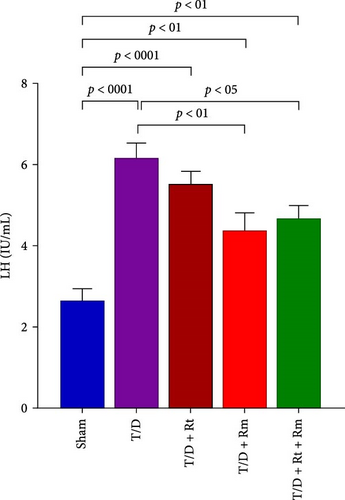
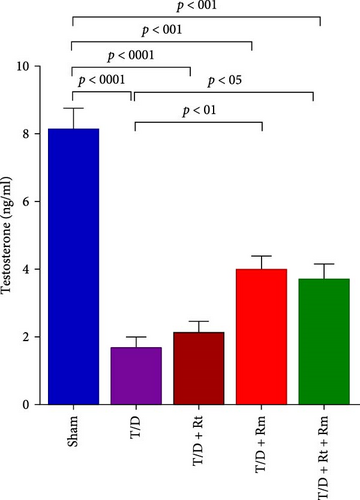
3.4. Serum Levels of LH, FSH, and Testosterone
As depicted in Figure 5A–C, the T/D group exhibited significantly reduced mean serum testosterone levels and elevated FSH and LH levels relative to the sham group. Treatment with L. rhamnosus (T/D+Rm) alone and in combination with L. reuteri (T/D+Rt+Rm) significantly elevated testosterone levels while reducing FSH and LH levels relative to the T/D group; however, these values were lower than those of the sham group. Treatment with L. reuteri alone (T/D+Rt) did not significantly affect testosterone, FSH, or LH levels compared to the T/D group.
3.5. Stress Oxidative Marker
As depicted in Table 2, The T/D group showed significantly higher MDA levels in testicular tissue than the sham group (p < 0.0001). Treatment with L. reuteri and L. rhamnosus reduced the MDA levels significantly in the T/D+Rm and T/D+Rt+Rm groups. GSH levels declined dramatically in the T/D group; only L. rhamnosus significantly elevated GSH in the probiotic-treated T/D group; however, it was below sham levels. CAT, GPx, and SOD activities were lower in the T/D group; both probiotics significantly improved CAT activity, with the T/D+Rm and T/D+Rt+Rm groups reaching sham levels. Only L. rhamnosus significantly increased GPx activity; it did not reach the sham levels, while SOD activity remained low across all probiotic-treated T/D groups.
| Parameters | Sham | T/D | T/D+Rt | T/D+Rm | T/D+Rt+Rm |
|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 24.44 ± 1.27 | 63.47 ± 4.05 ∗∗∗∗ | 56.69 ± 4.06 ∗∗∗∗ | 38.40 ± 3.09 ∗#### | 46.34 ± 46.34 ∗∗∗## |
| GSH (µmol/mg protein) | 89.30 ± 3.79 | 39.61 ± 3.71 ∗∗∗∗ | 45.12 ± 3.12 ∗∗∗∗ | 57.69 ± 3.78 ∗∗∗∗## | 44.48 ± 3.11 ∗∗∗∗ |
| CAT (IU/mg protein) | 5.80 ± 0.45 | 2.30 ± 0.31 ∗∗∗∗ | 3.69 ± 0.15 ∗∗∗# | 4.63 ± 0.19#### | 4.72 ± 0.23 #### |
| GPx (IU/mg protein) | 139.98 ± 6.03 | 74.33 ± 4.08 ∗∗∗∗ | 89.10 ± 5.99 ∗∗∗∗ | 115.42 ± 4.74 ∗### | 97.17 ± 6.73 ∗∗∗∗ |
| SOD (IU/mg protein) | 67.86 ± 3.47 | 42.33 ± 4.63 ∗∗∗ | 47.27 ± 2.84 ∗∗ | 49.55 ± 4.83 ∗ | 50.06 ± 3.64 ∗∗ |
- Note: A one-way ANOVA with a Tukey post hoc test was performed to compare the animals among the different experimental groups. Each bar displays the mean ± SEM (n = 6). Sham, group of animals that had no testicular torsion after performing surgery; T/D, group of animals subjected to 2 h of testicular torsion and then detorsion; T/D+Rm, the T/D group was treated with L. rhamnosus; T/D+Rt, the T/D group was treated with L. reuteri; T/D+Rt+Rm, the T/D group was treated with both probiotics.
- ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. the Sham group.
- #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. the T/D group.
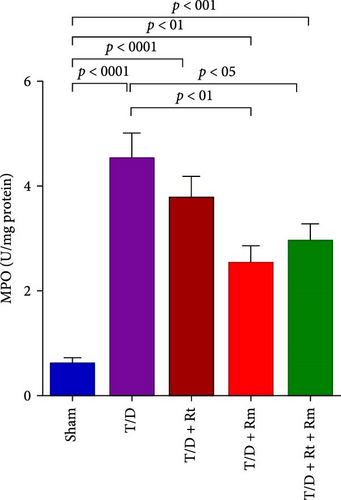
3.6. MPO Activity
Figure 6 illustrates that MPO activity in the testicular tissue of the T/D group was markedly elevated relative to the sham group (p < 0.0001). Treatment with the probiotic L. rhamnosus (T/D+Rm) alone and in combination with L. reuteri (T/D+Rt+Rm) significantly reduced MPO activity in the testis compared to the T/D group (p < 0.01 and p < 0.05, respectively). However, all the three probiotic-treated groups still exhibited significant differences in MPO activity compared to the sham group.
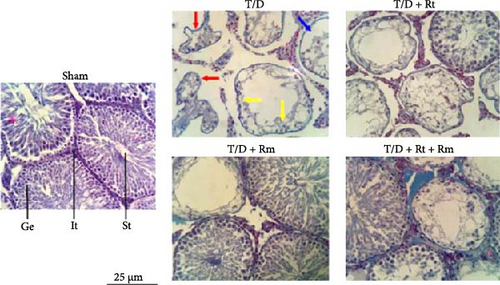
3.7. Testicular Histology and Johnsen Score (Histological Study)
Histological evaluation of testicular sections showed that the sham group had normal seminiferous tubules with intact seminiferous epithelium, spermatogenic, sertoli, and Leydig cells. The T/D group exhibited severe testicular damage, including atrophied and irregular tubules, loss of tubular epithelium, reduced spermatogenic cells, and decreased sertoli and Leydig cells. L. reuteri treatment provided minimal protection, but significant damage was still evident. However, treatment with L. rhamnosus, alone or combined with L. reuteri, partially prevented these structural changes, improving tubule organization and increasing tubular epithelium and testicular cells (Figure 7).
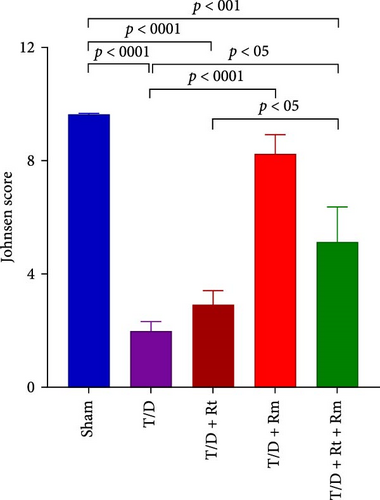
3.8. The Johnsen Score
The T/D group had a significantly lower Johnsen score than the sham group, indicating a significant decrease in spermatogenesis. Treatment with the probiotic L. rhamnosus (T/D+Rm) alone and in combination with L. reuteri (T/D+Rt+Rm) significantly increased the scores compared to the T/D group (p < 0.01 and p < 0.05, respectively). However, T/D+Rt+Rm still exhibited significant differences in this score compared to the sham group (Figure 8).
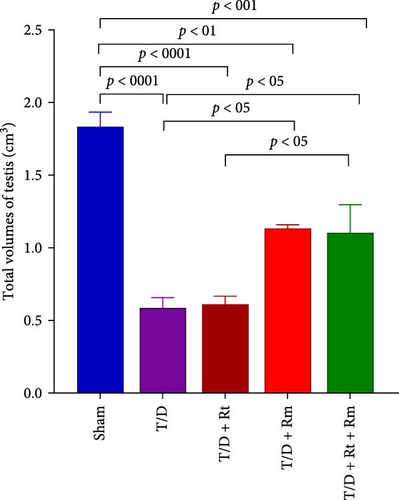
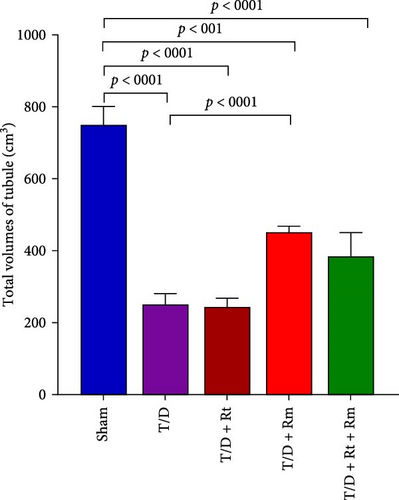
3.9. Volumes of the Testis, Seminiferous Tubules, Epithelium, and Interstitial Tissue
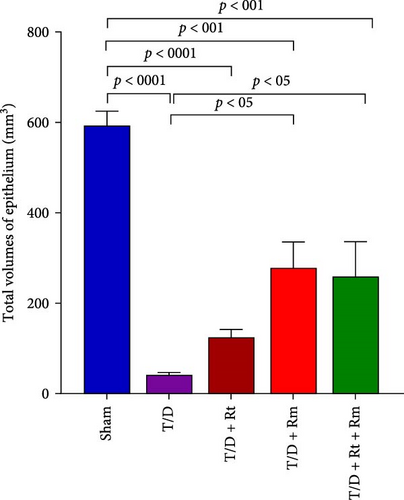
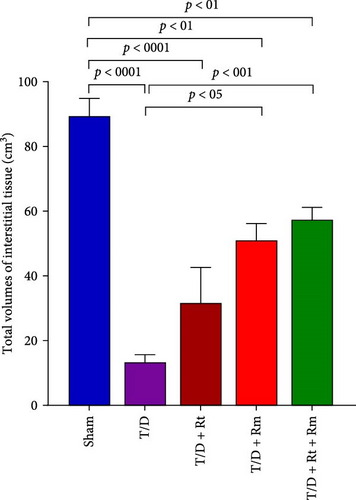
Results depicted in Figure 9A–D indicate that the average volumes of the testis (A), seminiferous tubules (B), tubular epithelium (C), and interstitial tissue (D) in the T/D group markedly diminished relative to the sham group (p < 0.0001). In contrast, the mean values for these four parameters in the T/D+LRm group, as well as the volumes of the testis, tubular epithelium, and interstitial tissue in the T/D+LRt+LRm group, were significantly higher compared to the T/D group (p < 0.05). Treatment with L. reuteri alone (T/D+Rt) did not significantly differ in these parameters in comparison to the T/D group.
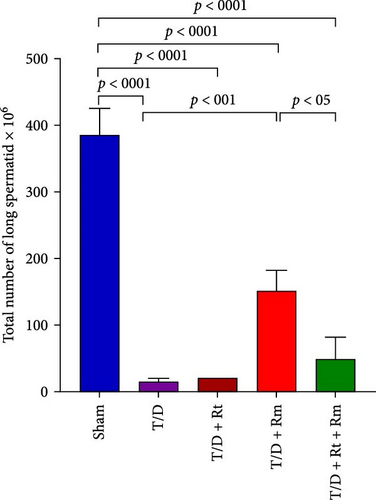
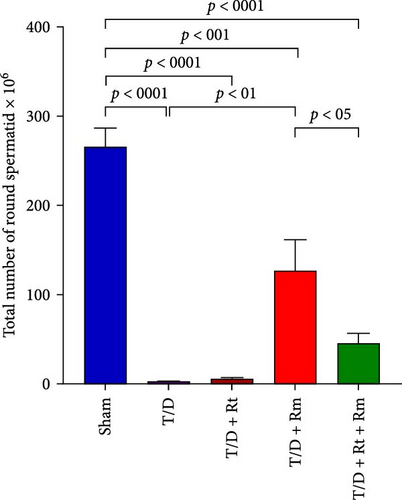
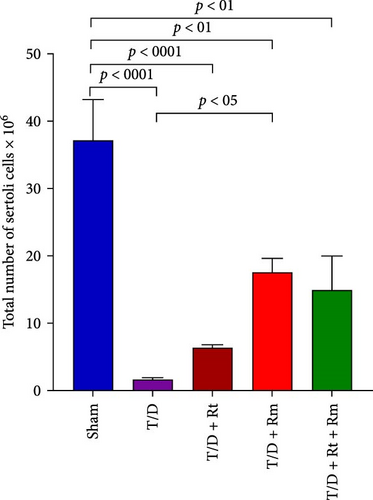
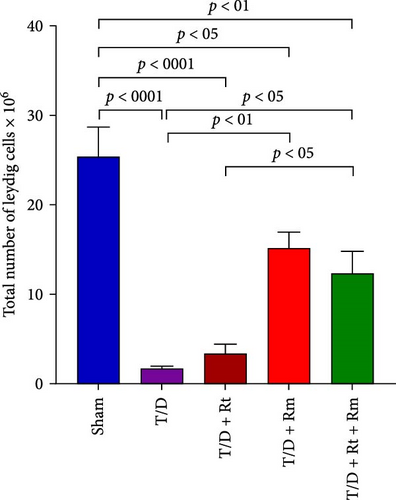
3.10. The Count of Spermatogonia, Spermatocytes, Spermatids (Round and Elongated), Sertoli, and Leydig Cells
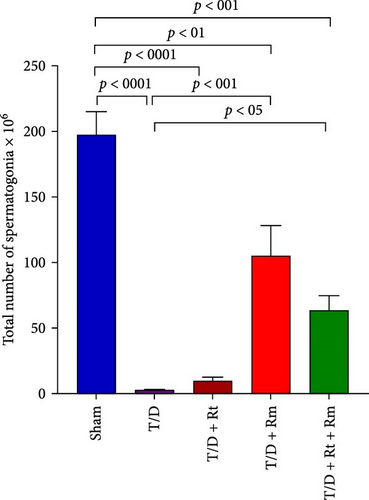
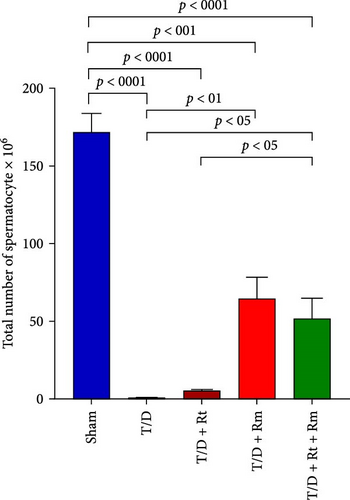
Figure 10 demonstrates that the T/D group had a significantly reduced quantity of spermatogonia, spermatocytes, spermatids (both round and elongated), sertoli cells, and Leydig cells in comparison to the sham group (p < 0.05). Treatment with L. rhamnosus (T/D+LRm) markedly elevated the quantities of these cell types relative to the T/D group. The T/D+LRt+LRm group had significantly higher levels of spermatogonia, spermatocytes, and Leydig cells than the T/D group. The stereological analysis revealed significant changes between the sham group and all probiotic-treated groups.
4. Discussion
In this research, we explored the potential of two types of probiotic strains, namely L. rhamnosus GG (AATCC 53103) and L. reuteri DSM 17938, in preserving and improving the testicular structure, sperm quality, and hormonal levels, as well as mitigating oxidative stress after I/R injury following TT/D in rats. Testicular I/R damage resulted in diminished sperm quality and reduced serum testosterone levels. Furthermore, T/D induced the generation of free radicals, decreased the antioxidant capacity of the testis, compromised sperm morphological integrity, and lowered testicular histomorphometric indices. Our results highlighted several positive effects, particularly with the administration of the probiotic L. rhamnosus. These include reducing oxidative stress, normalizing hormonal levels, and improving histological parameters in T/D rats after testicular I/R damage.
TT, considered a urological emergency, is a pathological event that results in obstruction of testicular blood flow due to the spermatic cord twisting. It causes testicular ischemia and necrotic damage. Failure to treat TT promptly, within several hours, can lead to significant damage to the testicle, often necessitating removal [2]. When blood flow is restored during D (reperfusion), the testicle experiences two forms of injury: ischemia damage from restricted blood flow and oxygen deprivation, and subsequently, oxidative injury triggered by oxygen radicals [47–49].
Under normal conditions, ROS levels are regulated by enzymatic and nonenzymatic antioxidant systems. However, during I/R injury caused by TT/D, the regulation of these defense mechanisms is impaired, leading to a significant release of ROS into the cells [50]. The predominant enzymatic sources that contribute to excessive ROS production following I/R include the enzymes xanthine oxidase, NADPH oxidase (Nox), mitochondria, and uncoupled NOsynthase, which are present in most tissues and likely contribute to the oxidative damage caused by reperfusion [51, 52]. Ischemia also shifts metabolism to anaerobic pathways, increasing lactic acid and reducing ATP levels. This disrupts cellular functions like the sodium-potassium pump, causing ion imbalances and a hyperosmolar environment. These conditions result in chromatin condensation, reduced enzyme activity, and impaired protein synthesis, weakening antioxidant defenses. As a result, ischemic tissues become highly vulnerable to oxidative damage during reperfusion [9, 52].
On the other hand, ROS plays a significant role in amplifying the inflammatory response, promoting neutrophil adhesion and migration to the testicular vascular endothelium during I/R injury. Once in the tissue, neutrophils release MPO, indicating a heightened neutrophil activity and inflammatory response during I/R [53, 54]. MPO catalyzes the conversion of H2O2 into ROS, including HOCl, a potent oxidative agent contributing to tissue damage during I/R injury. Therefore, activated neutrophils by releasing cytotoxic enzymes like MPO enhance ROS production and worsen oxidative stress; this inflammation, combined with excessive ROS, leads to germ cell apoptosis [53].
Consistent with several research studies [8–10, 55, 56], our results indicated that the I/R damage caused by T/D results in elevated oxidative stress and inflammation, as evidenced by increased MDA, enhanced MPO activity, and reduced GSH concentrations, along with diminished antioxidant enzyme activities of CAT, SOD, and GPx in the testicular tissue. Vakili-Sadeghi et al. [10] demonstrated that rats subjected to testicular I/R injury exhibited significantly elevated levels of MDA and MPO, accompanied by a reduction in antioxidant levels.
Given the efficacy of antioxidant-based therapies in alleviating oxidative tissue damage associated with TT/D [8, 9, 47, 57], this study explored the impact of probiotics on testicular tissue damage caused by I/R injury. Research has shown that probiotics possess antioxidant and anti-inflammatory properties, exerting these effects through various mechanisms, including chelating metal ions, producing antioxidants, altering the levels or activities of the host’s antioxidant systems, and regulating ROS-producing enzyme activities [30–32, 58, 59]. Our research found that only L. rhamnosus effectively reduced lipid peroxidation (MDA levels) and enhanced the antioxidant defense system in the rat testes after I/R. L. rhamnosus exhibited a significantly more potent antioxidant effect at equal concentrations than L. reuteri. The study suggests that the antioxidant action of L. rhamnosus may result from increased activity of enzymes like CAT and GPx or higher GSH synthesis in the testes.
L. rhamnosus administration partially mitigated the rise in MPO activity, suggesting a decrease in neutrophil activity and inflammation. Reducing MPO activity by probiotics, as observed in this study, leads to decreased oxidative stress, helping to preserve cellular integrity and function and reducing tissue damage in I/R injuries. This reduction in oxidative stress also contributes to a decrease in neutrophil infiltration and inflammation, providing additional protection against testicular damage following torsion and detorsion.
In line with other studies [60, 61], our research showed a significant decline in sperm count, motility, viability, and normal shape in the T/D group relative to the sham group. TT and D increase oxidative stress and inflammation in testicular tissues, leading to reduced spermatogenesis, impaired testicular function, and altered sperm parameters. Since glycolysis is essential for ATP production and sperm motility, TT significantly reduces glycolytic activity and ATP production in the sperm, affecting their motility [60, 62].
Probiotics, especially L. rhamnosus, improved the sperm quality in the T/D groups by increasing the sperm count, motility, and viability while reducing the abnormal sperms. For instance, the sperm count increased by ~15.4-fold in the T/D+Rm group compared to the untreated T/D group. Similarly, sperm motility, viability, and morphology showed 22.8-fold, 3.14-fold, and 5.02-fold improvements, respectively.
Various studies on humans and animals have shown that probiotics improve sperm parameters, elevate the proportion of normal sperm, and diminish morphological defects and DNA damage [20, 63, 64]. Additionally, L. rhamnosus and Bifidobacterium longum improved the sperm quality in asthenozoospermic cases by boosting motility, reducing DNA fragmentation, and lowering intracellular H2O2 levels [65]. Furthermore, in a diet-induced obesity model, L. rhamnosus raised the hormone levels, sperm velocity, and progressive motility [64].
The primary endocrine function of the testes is testosterone production. LH controls it through the hypothalamic–pituitary–gonadal (HPG) axis. In our study, 60 days after D following TT, the T/D group showed significantly lower serum testosterone levels and higher LH and FSH levels than the sham group. This decrease in testosterone aligns with findings from several studies [61, 66–68]. For example, in rats, Turner et al. [66] observed a significant drop in testicular vein testosterone levels 3 and 30 days after D. Similarly, another study reported a significant decrease in serum testosterone levels. It increased LH levels 30 days after TT alleviation in the rats exposed to 4 h of testicular ischemia [61].
Additionally, Akhigbe et al. [67] documented a significant drop in testosterone levels in the T/D group, attributed to decreased Leydig cell numbers and impaired testosterone biosynthesis from cholesterol. Our research showed reduced Leydig cells in the T/D group due to testicular I/R damage. This damage likely diminishes testosterone synthesis, impairing negative feedback on the pituitary and hypothalamus, resulting in increased LH and FSH levels.
The administration of L. rhamnosus significantly increased circulating testosterone levels and decreased LH levels in the probiotics-treated T/D group. Through its antioxidant properties, this probiotic mitigated the severe reduction in Leydig cells caused by I/R injury, thereby enhancing steroidogenesis and spermatogenesis in mice.
Histopathological and stereological evaluations of testicular tissue revealed that the T/D group exhibited detachment of the tubular epithelial layer, destruction of interstitial tissue, reduced volumes of both tubular epithelial and interstitial tissue, and a decrease in spermatogenic, Leydig, and sertoli cells. These findings explain the observed testicular atrophy and reduced testicular volume in the T/D group. The histopathological alterations in the testes of T/D rats are likely linked to oxidative stress affecting the testicular tissue. Hypoxia induced by TT significantly contributes to lipid peroxidation, activation of inflammatory pathways, and apoptosis of germ cells within the seminiferous tubules [3]. Furthermore, reperfusion after ischemia can lead to mitochondrial failure, intracellular calcium overload, increased ROS production, elevated pro-inflammatory cytokines, lipid peroxidation, and metabolic acidosis. These processes result in necrosis and apoptosis, as evidenced by studies [3, 69]. Altered blood flow initially leads to venous congestion, edema, and bleeding inside the seminiferous tubules, ultimately causing germ cell necrosis [61, 70]. Consistent with other studies, this research observed a notable reduction in the testicular weight in the T/D group relative to the sham group, likely due to elevated ROS generation after reperfusion and decreased testicular metabolism [71–73]. In this study, only L. rhamnosus effectively mitigated histopathological changes and tissue damage in the testes by reducing oxidative stress and inflammation.
In our study, L. rhamnosus provided more significant protection against oxidative stress and histological damage compared to Lactobacillus reuteri. This difference may be attributed to variations in the antioxidant capacity of these probiotics. L. rhamnosus has been reported to exhibit stronger antioxidant and anti-inflammatory activity, including reduced lipid peroxidation (MDA levels) and MPO activity, increased GSH production, and enhanced antioxidant enzyme activities of CAT and GPx. Additionally, L. rhamnosus may exert its protective effects more efficiently at the given concentration, while L. reuteri may require a higher dosage to achieve a comparable level of antioxidant protection.
Interestingly, the combined administration of L. rhamnosus and L. reuteri did not result in an additive or synergistic improvement in testicular recovery. This could be attributed to potential competitive interactions between the probiotics, differences in their mechanisms of action, competition for colonization sites, or alterations in GM composition. Additionally, probiotics may exert tissue-specific therapeutic effects, which can vary depending on mechanisms, such as immune system regulation, microbiome modulation, and the production of bioactive compounds. Therefore, further research is essential to better understand these differences and optimize probiotic combinations for targeted therapeutic applications.
Our results suggest that L. rhamnosus holds promise as a potential adjunct therapy for men experiencing infertility issues related to oxidative stress and inflammation. While immediate surgical intervention remains the standard treatment for TT, probiotic supplementation may offer a strategy to reduce long-term damage and enhance postoperative recovery. Additionally, the antioxidant and anti-inflammatory properties of L. rhamnosus could benefit other oxidative stress-induced testicular disorders that lead to infertility.
The therapeutic potential of probiotics in treating many disorders has attracted considerable interest recently, mainly because of their few adverse effects, making them a promising choice in other medical domains, including male fertility. Probiotics are typically considered safe for most individuals, although they may occasionally induce moderate gastrointestinal adverse effects, such as bloating and gas. These adverse effects are generally temporary and self-resolving. However, individuals with compromised immune systems, severe illness, or central venous catheters should exercise caution as, in rare cases, they may lead to systemic infections [74].
5. Limitations and Future Studies
While these findings highlight the potential of L. rhamnosus in reducing testicular damage caused by I/R in rats, several limitations must be considered before translating them to human applications. Although animal models are valuable for understanding biological mechanisms, they differ from humans in physiology, immune responses, and hormonal regulation. Moreover, the controlled experimental conditions in animal studies do not fully reflect the complexities of human health, including genetic diversity, lifestyle factors, and comorbidities. Future research should prioritize randomized controlled trials in humans to validate the efficacy and safety of L. rhamnosus in treating testicular damage and infertility associated with oxidative stress, inflammation, or a history of TT. Furthermore, as this study examined only a single probiotic dose, dose–response experiments are needed to determine the optimal dosage and potential effects. Investigating the mechanisms underlying the protective effects of probiotics on TT/D and comparing them with other therapeutic options, such as antioxidants or anti-inflammatory agents, is also recommended.
6. Conclusion
This study revealed that I/R resulting from TT/D induces significant structural and functional impairment in the testes, primarily due to increased oxidative stress and inflammation. These effects included tissue necrosis, seminiferous tubule atrophy, reduced seminiferous epithelium volume, decreased testicular cell count, impaired spermatogenesis, lower sperm quality, and diminished testosterone secretion.
Notably, L. rhamnosus was shown for the first time to partially mitigate these damages in a T/D rat model, demonstrating its protective role against I/R injury. The results indicated that probiotics, particularly L. rhamnosus, reduce oxidative stress and inflammation, thereby improving spermatogenesis and sperm quality. Furthermore, our results indicated that L. rhamnosus exhibited stronger antioxidant, anti-inflammatory, and tissue-protective properties compared to L. reuteri in reducing testicular I/R injury. In particular, L. rhamnosus demonstrated superior efficacy in enhancing the sperm quality, lowering oxidative stress markers, and maintaining the structural integrity of the testicular tissue. Probiotics may be a promising therapeutic option for mitigating testicular damage. However, further clinical trials are needed to optimize the dosage and evaluate the efficacy of different probiotic strains or their combinations for potential human applications
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zohresadat Akbari and Farhad Koohpeyma contributed to experimental investigation, data collection, and analysis. Narges Karbalaei, Saied Karbalay-Doust, Sourena Rastin, and Seyed Shahram Shekarforoush contributed to the supervision, conception, and design of the research, and interpretation of the findings. All authors read and approved the final manuscript.
Funding
This study was financially supported by Shiraz University of Medical Sciences as a grant (Grant No. 26826), Shiraz, Iran.
Acknowledgments
This study was financially supported by Shiraz University of Medical Sciences as a grant (Grant No. 26826), Shiraz, Iran. The authors would like to thank Shiraz University of Medical Sciences, Shiraz, Iran and also Center for Development of Clinical Research of Nemazee Hospital and Dr. Nasrin Shokrpour for editorial assistance.
Open Research
Data Availability Statement
All data used to support the findings of this study were analyzed and included in the article.