Curcumin Ameliorates Hydrocortisone-Induced Leydig Cell Injury and Testicular Dysfunction by Upregulating the Nrf2 Pathway
Abstract
Curcumin has antifragmentation, antivirus, anti-infection, and antioxidation properties. Curcumin has been proven to prevent the peroxidation of sperm and testis membranes, thus enhancing sperm motility and reducing sperm abnormalities. This study aims to explore the role of curcumin in improving hydrocortisone (HCT)-induced Leydig cell injury by upregulating the Nrf2 pathway. Thirty male rats were divided into three groups: control group (CG, n = 10), model group (MG, n = 10), and curcumin treatment group (CTG, n = 10). CG rats were fed 2.5 mL/kg carboxymethyl cellulose as a blank control. MG rats were fed with 10 mg/kg HCT for 2 weeks. CTG rats were fed with 10 mg/kg HCT for 2 weeks and 100 mg/kg curcumin orally every day for 4 consecutive weeks. The ultrastructural score of Leydig cells was higher in the MG than in the CG (p < 0.05) and was lower in the CTG than in the MG (p < 0.05). The levels of Nrf2 and HO-1 were lower in the MG than in the CG (p < 0.05) and higher in the CTG than in the MG (p < 0.05). Curcumin can improve the biochemical and histological structural damage of rat testes induced by HCT by upregulating Nrf2 expression. This protective effect relies on the Nrf2 pathway inhibiting oxidative stress in the body, increasing testosterone levels and enzyme activity, thereby improving testicular dysfunction caused by interstitial cell damage.
1. Introduction
Leydig cells are the main sites of androgen composition and excretion, which are integral for the establishment and maintenance of the testis microenvironment [1, 2]. Leydig cell damage is an important element leading to testicular impaired functioning, accompanied by decreased reproductive health [3–5]. Glucocorticoids are extensively used to treat many sicknesses. However, long-term glucocorticoids may cause Leydig cells to lose regular morphology [6, 7]. Oxidative stress is caused by undue production of reactive oxygen species (ROS), which is an important factor in inducing apoptosis. The nursing evaluation of testicular injury should consider the patient’s condition, cooperation, self-care ability, psychological status, etc. and examine the scrotal skin, pain, and swelling. The initial treatment includes immediate cold compress to reduce swelling, lifting and fixing the testicles to relieve pain, and performing ultrasound examination as soon as possible to determine the degree of injury. Curcumin is a natural phenolic antioxidant extracted from the roots and stems of ginger plants such as turmeric and turmeric. Its chemical formula is C21H20O6, its molecular weight is 368.380, and it is an orange yellow crystalline powder with a slightly bitter taste. It is insoluble in water and is a commonly used natural food pigment and pharmacological ingredient. Curcumin has pharmacological mechanisms such as antioxidant, anti-inflammatory, and antitumor effects and can inhibit platelet aggregation. Curcumin has significant antioxidant, anti-inflammatory, and antiapoptotic properties, which can effectively alleviate testicular damage caused by ischemia-reperfusion, formaldehyde, and other factors, and protect testicular spermatogenic function. It has shown great potential in the treatment of testicular injury by regulating enzyme activity, promoting the expression of antioxidant enzyme factors, and other mechanisms [8, 9]. At the same time, the excessive production of ROS induces organ fibrosis by activating the fibrosis signaling pathway. Moreover, glucocorticoids can guide ROS accumulation and lead to testicular redox imbalance [10–12]. Nrf2 plays a vital role in maintaining the redox state balance in cells. Nrf2 can boost the level of antioxidant factors. Previous studies have confirmed that melatonin’s antiapoptosis influence on H2O2-induced apoptosis of Leydig cells is greatly reduced after siRNA-mediated Nrf2 silencing. Therefore, the regulation of the Nrf2 pathway is a key approach to reduce oxidative stress and cure interstitial cell injury. Curcumin is the main active ingredient of Curcuma longa. Curcumin has antifragmentation, antivirus, anti-infection, and antioxidation properties. Curcumin has been proven to prevent the peroxidation of sperm and testis membranes, thus enhancing sperm motility and reducing sperm abnormalities. This study aims to use hydrocortisone (HCT) to induce testicular injury in rats and estimate the effect of curcumin in regulating Nrf2 and improving testicular interstitial cell injury and testicular dysfunction.
2. Methods
2.1. Experimental Rats
Thirty male Sprague–Dawley (SD) rats, 180–220 g each, aged at 6 weeks, were obtained from the Animal Experimental Center of the Provincial Academy of Agricultural Sciences (Wuhan, China). They were kept at 23 ± 2°C and a relative humidity of 50% ± 10%. During this experiment, the rats were allowed with free access to feed and water.
2.1.1. Testicular Injury Model and Grouping
After 1 week’s adaptation, the rats were stochastically separated into three groups: control group (CG) (rats fed only 2.5 mL/kg carboxymethyl cellulose as a blank control, n = 10), model group (MG) (rats fed with 10 mg/kg HCT for 2 weeks, n = 10), and curcumin treatment group (CTG) (rats fed with 10 mg/kg HCT for 2 weeks and 100 mg/kg curcumin orally every day for 4 weeks, n = 10). After treatment, all rats were anesthetized.
2.2. Experimental Methods
2.2.1. Organization Sampling
The blood samples were taken from the posterior orbital plexus of rats under mild ether anesthesia, and the serum was resolved. The collected testes were chopped, washed in an ADounce glass homogenizer in HEPES-KOH/EGTA buffer and phosphatase inhibitor. The supernatant was collected for experiment.
2.2.2. Determination of Serum Testosterone and Testicular Enzyme Markers
According to the instructions, the testosterone level in serum samples was tested with an ELISA kit (Shanghai Youxuan Biotechnology Co., Ltd.). The LDH measurement was conducted given the mutual conversion of pyruvate and lactic acid. The ACP and ALP activities were tested according to the previously described methods using kits provided by Quimica Clinica Aplicada (Anna Poorna) and Biolabo SA. The determination of ACP and ALP was conducted by the hydrolysis of p-nitrophenyl phosphate. The released p-nitrophenol was quantified by a 420 nm spectrophotometer.
2.2.3. Detection of Oxidative Stress Level
A proper amount of homogenate was admixed with Tris-HCl buffer to prepare 10% homogenate. The supernatant was collected to evaluate the TAC. The glutathione (GSH) level was calculated. GSH reductase can use NADPH to catalyze GSSG to produce GSH, and the activity level of GSH peroxidase can be calculated by detecting the decrease in NADPH.
2.2.4. ELISA
The supernatant was obtained from rat testis homogenate to test the horizontals of secreted interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), MCP-1, and IFN-α. ELISA kit (Minneapolis) was used to detect the above inflammatory factors using Bio-Rad Laboratories 680.
2.2.5. Western Blot Analysis
Cells were rinsed with phosphate buffer saline (PBS) and then lysed with a lysis buffer. After 30 min of lysis at a low temperature, the lysate was centrifuged, and the supernatant was collected to obtain the total protein. The protein concentration was determined using the BCA assay, with bovine serum albumin as the standard for the calibration curve. After SDS-PAGE gel electrophoresis, the proteins were transferred to a PVDF membrane. The membrane was then blocked in a 5% skim milk powder solution for 40 min. The primary antibody was added and incubated overnight at 4°C. The following antibodies were used: beclin1 (1:5000, Cell Signaling Technology, USA), LC3 II (1:1000, Abcam, UK), LC3 I (1:500, Abcam, UK), and p62 (1:500, Santa Cruz Biotechnology, USA). The following day, the membrane was washed and incubated with a secondary antibody (rabbit antihuman polyclonal antibody, 1:2000, Thermo Fisher Scientific, USA).
2.2.6. Quantitative Analysis of Leydig Cells
Testicular tissue was fixed with 4% paraformaldehyde. The samples were dehydrated and embedded for H&E staining. The slides were scanned with a digital scanning microscope panoramic MIDI (3DHISTECH, Hungary). 3DHISTECH digital scanning microscope panoramic MIDI was used to quantitatively analyze the number of Leydig cells containing the entire seminiferous tubule in five consecutive 400× field areas.
2.2.7. Ultrastructural Analysis
Testicular samples were observed under a transmission electron microscope. To determine the extent of testicular stromal cell damage. The testicles were semiquantitatively scored by three observers in the blind semiquantitative evaluation. In short, three categories (0–3 points for each score) were considered: the extent of nuclear membrane gap expansion, mitochondrial damage, and endoplasmic reticulum swelling. The sum of these three categories was counted.
2.2.8. Immunofluorescence Staining
After OTC embedding, the thickness of the frozen slicing machine preparation was 10 μM per slice. The sectioned specimens were permeated with PBS containing 1% Triton X-100, blocked with 1% BSA for 1 h at room temperature, discarded the blocking solution, and added diluted primary antibody: rabbit anti-Nrf2 polyclonal antibody (1:500, Abcam company) and mouse anti-HO-1 monoclonal antibody (1:800, mo15013, neuromics company). Then the sectioned specimens were incubated overnight at 4°C. The next day, the primary antibody was washed off; diluted fluorescent secondary antibody Alexa-488 donkey antimouse IgG (1:1000, Invitrogen company) or Alexa-546 donkey antirabbit IgG (1:1000, Invitrogen company) and DAPI (1:1000, sigma company) were added and incubated. The antiquencher was sealed, observing and taking photos under a laser scanning confocal microscope. If positive areas were quantified by Image-Pro Plus software, the antibody was obtained from ABclonal Technology. DAPI-labeled nuclei were blue. According to the fluorescent label used, the positive cells were red.
2.3. Statistical Analysis
The monitoring data were analyzed by SPSS19.0 statistical software. The results of data analysis were represented as mean ± standard deviation (mean ± SD). Multigroup data analysis was conducted based on one-way ANOVA. LSD test was used for subsequent analysis. p < 0.05 indicates the statistical significance of the difference.
3. Results
3.1. The Improvement Effect of Curcumin on Testicular Injury Induced by HCT
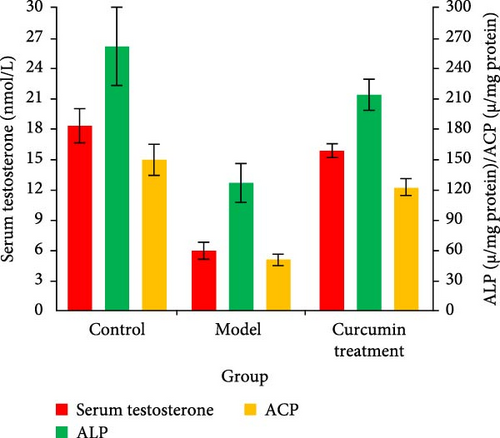
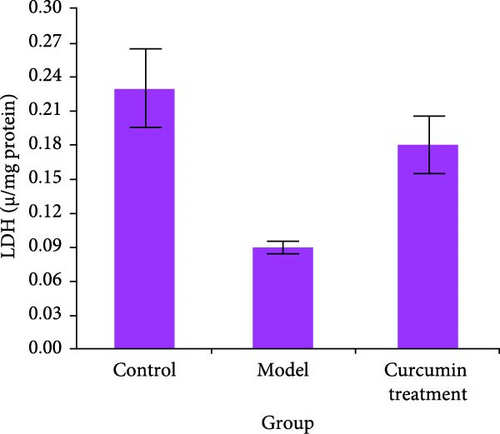
The levels of serum testosterone and testicular enzyme markers in rats were tested by ELISA and commercial kit. Compared with the CG, the serum testosterone level, ALP, ACP, and LDH activities of the MG rats decreased (p < 0.05), while those of the CTG rats increased (p < 0.05). The data indicated that the curcumin improved the testicular injury induced by HCT (Figure 1).
3.2. Morphological Analysis of Rat Testis
The morphology of rat testes was analyzed by H&E staining. H&E staining showed that compared with the CG, a large number of Leydig cells in the MG shrank and vacuolized, and the number was significantly reduced (p < 0.05). Curcumin showed a significant improvement on these injuries after treatment (p < 0.05) (Figure 2).

3.3. Curcumin Reducing Oxidative Stress of the Testis Induced by HCT
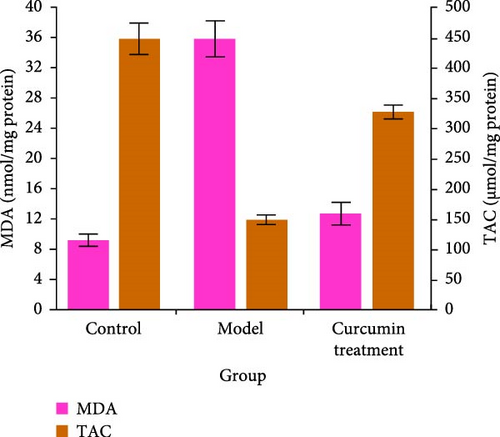
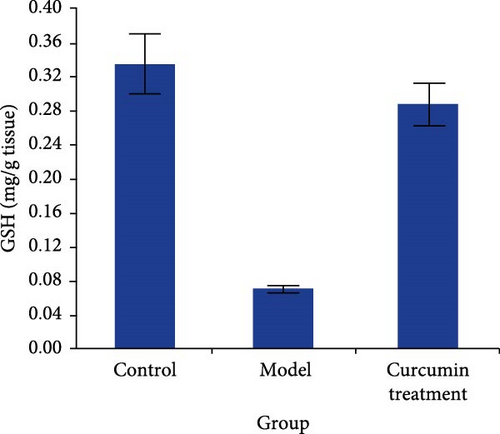
The oxidative stress level of the testis was detected by commercial kit. Compared with the CG, the levels of TAC and GSH in the testis of rats in the MG decreased (p < 0.05), while those in the CTG increased (p < 0.05). The level of MDA in the testis of the MG increased (p < 0.05), while that in the CTG decreased (p < 0.05) (Figure 3).
3.4. Curcumin Reduced the Inflammatory Response of the Testis Induced by HCT
ELISA was used to test the inflammatory factors of rat testis mesenchymal cells. The inflammatory factors of IL-1β, TNF-α, MCP-1, and IFN-α in rat testis homogenate were higher in the MG than the CG (p < 0.05), while those of IL-1β, TNF-α, MCP-1, and IFN-α were lower in the CTG than the MG (p < 0.05) (Table 1).
| Groups | IL-1β (pg/mL) | TNF-α (pg/mL) | MCP-1 (pg/mL) | IFN-α (pg/mL) |
|---|---|---|---|---|
| Control group | 18.24 ± 3.62 | 37.54 ± 8.32 | 26.48 ± 6.67 | 113.63 ± 15.87 |
| Model group | 846.13 ± 68.25 | 578.23 ± 35.14 | 824.51 ± 54.29 | 745.15 ± 43.18 |
| Curcumin treatment group | 273.49 ± 34.49 | 166.08 ± 14.56 | 115.32 ± 25.44 | 256.32 ± 26.40 |
| F value | 229.420 | 89.785 | 181.004 | 113.256 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
- Abbreviations: IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
3.5. Western Blot Analysis
The levels of Bax and caspase-3 in the MG rats were higher than those in the CG (p < 0.05). The levels of Bax and caspase-3 in the CTG were lower than those in the MG (p < 0.05), and the level of Bcl-2 was lower than that in the CG (p < 0.05) (Figure 4, Table 2).
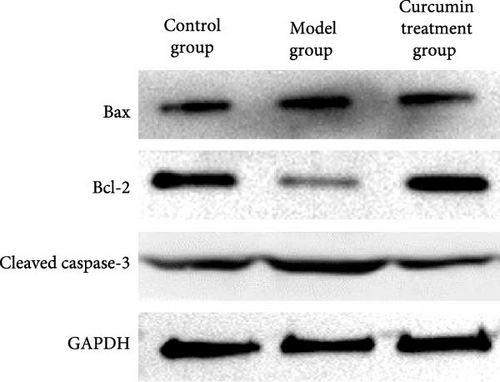
| Groups | Bax | Bcl-2 | Cleaved caspase-3 |
|---|---|---|---|
| Control group | 1.02 ± 0.01 | 1.93 ± 0.16 | 1.05 ± 0.02 |
| Model group | 1.95 ± 0.18 | 1.13 ± 008 | 1.91 ± 0.14 |
| Curcumin treatment group | 1.14 ± 0.11 | 1.87 ± 0.13 | 1.08 ± 0.05 |
| F value | 15.584 | 19.029 | 19.772 |
| p value | <0.001 | <0.001 | <0.001 |
3.6. Quantitative Analysis of Leydig Cells
Digital scanning microscope was used to quantitatively detect the number and ultrastructure of interstitial cells in tissue sections. Compared with the MG, the quantity of Leydig cells in the MG reduced (p < 0.05), and the number of Leydig cells in the CTG increased (p < 0.05). Compared with the CG, the ultrastructural score of Leydig cells in the MG was higher (p < 0.05), while that in the CTG was lower (p < 0.05). Testicular slices of the CG showed that the morphology of Leydig cells was normal, and the nuclear membrane, mitochondria, and endoplasmic reticulum were intact. Compared with the CG, the nuclear membrane gap in the MG widened, the mitochondrial cristae narrowed, the mitochondrial membrane was incomplete, and the endoplasmic reticulum was slightly swollen (p < 0.05). However, the changes of Leydig cells in the CTG almost returned to normal (p < 0.05) (Figure 5, Table 3).
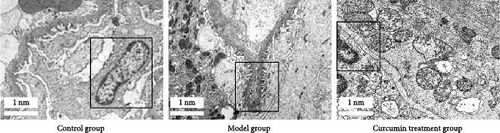
| Groups | Leydig cell number | Leydig cell ultrastructural score |
|---|---|---|
| Control group | 14.47 ± 3.35 | 0.32 ± 0.01 |
| Model group | 6.52 ± 1.86 | 2.45 ± 0.35 |
| Curcumin treatment group | 11.56 ± 2.69 | 1.02 ± 0.14 |
| F value | 20.751 | 23.442 |
| p value | <0.001 | <0.001 |
3.7. Curcumin Promoted the Upregulation of Nrf2/HO-1 Pathway
The level of Nrf2/HO-1 pathway protein in rat testis was analyzed by immunofluorescence technique. Compared with the CG, the levels of Nrf2 and HO-1 in the MG decreased (p < 0.05), while those in the CTG increased (p < 0.05). It was obvious that curcumin promoted the upregulation of Nrf2/HO-1 pathway. Immunofluorescence images further confirmed the horizontals of Nrf2 in Leydig cells of the testis (Figure 6, Table 4).

| Groups | Nrf2 | HO-1 |
|---|---|---|
| Control group | 1.92 ± 0.16 | 1.97 ± 0.19 |
| Model group | 1.13 ± 0.08 | 1.06 ± 0.05 |
| Curcumin treatment group | 1.87 ± 0.12 | 1.91 ± 0.16 |
| F value | 19.629 | 21.475 |
| p value | <0.001 | <0.001 |
- Note: The values in the table represent the fluorescence levels of Nrf2 and HO-1 in testicular interstitial cells.
4. Discussion
Curcumin attracts people’s attention because of its antidiabetes, anti-inflammatory, antitumor, and antioxidation properties. Previous studies have shown that curcumin can improve erectile dysfunction induced by HCT in rats, increase testosterone levels in TM3 cells treated with H2O2, and alleviate testicular fibrosis and oxidative stress [13]. This suggested that curcumin might treat testicular dysfunction by inhibiting oxidative stress [14].
In this research, it was found that curcumin reversed HCT-induced testosterone secretion impairment in rats. These results suggested that DP1 might alleviate HCT-induced testicular impaired functioning by restoring testicular form. Leydig cells were mainly responsible for the compound. Glucocorticoid-mediated testicular dysfunction was accompanied by the destruction of Leydig cells. Excessive HCT-induced testicular dysfunction had the ability to inhibit testosterone production. Concerning testicular morphology, this study proved that curcumin could effectively alleviate HCT-induced seminiferous tubule atrophy. Increasing the proportion of Bcl-2/Bax was essential to prevent apoptosis. It was found that curcumin reversed the HCT-induced decrease in the amount of Leydig cells and ultrastructural damage in the testis [15–18]. The testicles were more vulnerable to peroxide damage [19, 20]. The reduction of TAC and GSH in the testis and the increase of MDA standard might be caused by mitochondrial dysfunction caused by HCT, which can induce the release of cytochrome C from the mitochondria into the cytosol. In this study, the decrease in GSH level and the high standard of MDA in the testis of model rats reflected that the testis could not eliminate the excessive ROS produced by HCT and its metabolites. The increase in oxidative stress would lead to ROS-induced macromolecular damage, playing a part in testicular steroid production and sperm production. The decrease of LDH vitality induced by HCT in rats indicated the defect of spermatogenesis and testicular maturation. The decrease of ACP and ALP activity in the testis might bring about the destruction of spermatogenic epithelial cells. This study showed that HCT administration could cause testicular oxidative damage and testicular dysfunction, indicating that testicular TAC and GSH levels decrease with increasing MDA. In addition, HCT significantly reduced the serum testosterone level and the vitality of LDH, ALP, and ACP in the testis, while curcumin significantly increased the activities of marker enzymes in the testis of model rats, reduced the level of oxidative stress in vivo, and finally improved testicular function. Testicular inflammation makes the testicles susceptible to various pathogens. This antibacterial reaction was the result of the natural immunity of organs. When the immune function of the body was impaired, the innate immunity of the testicles was very important. The function of curcumin in radio resistance of various cancers was related to its role in maintaining the opposition of cancer stem cells to DNA damage and the regeneration of progenitor cells. This study found that HCT-induced testicular cell inflammation led to an increase in inflammatory factors such as IL-1β, TNF-α, MCP-1, and IFN-α in rat testicular homogenate. However, these inflammations were inhibited by curcumin, indicating the inhibitory effect of curcumin on testis inflammation.
The Nrf2/HO-1 pathway is an important antioxidant stress pathway, in which Nrf2 is the main factor regulating cellular redox balance and HO-1 is a key antioxidant enzyme [21, 22]. This pathway maintains cellular redox homeostasis by regulating the expression of antioxidant proteins, thereby protecting cells from oxidative damage [23]. The Nrf2/HO-1 pathway is a key mechanism for resisting oxidative stress damage. Under oxidative stress, Nrf2 dissociates from Keap1 and transfers to the nucleus, inducing the expression of antioxidant proteins such as HO-1, thereby restoring cellular redox homeostasis and reducing damage caused by oxidative stress. The Nrf2/HO-1 pathway is a key antioxidant stress pathway that can maintain cellular redox homeostasis and has various effects such as anti-inflammatory and antiapoptotic effects. It has an important impact on cellular homeostasis and disease progression and is an important target for biological research and disease treatment [24]. The activation of the Nrf2 pathway is beneficial to the recovery of testicular affect. The results found that curcumin could increase the levels of Nrf2 and HO-1 in the testis and Nrf2 could induce the levels of various cell protection and detoxification genes, such as cell protection enzyme HO-1 and other phase II enzymes. It has been reported that Nrf2 upregulated the regulation of glutamyl cysteine ligase and the catalytic unit of glutamyl cysteine ligase. When exposed to ROS or electrophilic, Nrf2 dissociated from its inhibitor, and gene expression was driven by antioxidant response elements. This study found that curcumin boosted the horizontal of Nrf2 and HO-1 in the testis of HCT-treated mice. These results suggest that the Nrf2 pathway may be interrelated to the curcumin’s ability to protect the testis from HCT-induced oxidative stress. In this study, curcumin boosted its antioxidant capacity by increasing the levels of Nrf2/HO-1 protein, so the levels of GSH, TAC, and MDA in the testis of the CTG decreased [25]. However, this study only used rats as the experimental objects. Although rats are a commonly used model for studying testicular dysfunction, physiological differences between different species may affect the extrapolation of the results. In the future, different mouse or mammalian models such as cattle and sheep can be selected to verify the protective effect of curcumin in different species.
Curcumin has low bioavailability, making it difficult to reach effective concentrations in the body, which in turn affects its pharmacological effects. This not only reduces the therapeutic effect of curcumin but may also increase the dosage, bringing potential drug side effects and economic burden. Therefore, improving the bioavailability of curcumin is crucial. New formulations of curcumin, such as nanoparticles, liposomes, and micelles, can significantly improve its solubility and bioavailability. These formulations enhance the absorption and distribution of curcumin in the body by changing its crystal structure, thereby improving its efficacy and reducing its toxicity to normal cells.
5. Conclusion
In summary, this study suggests that curcumin can improve the biochemical and histological structural damage of rat testes induced by HCT by upregulating Nrf2 expression. This protective effect relies on the Nrf2 pathway inhibiting oxidative stress in the body, increasing testosterone levels and enzyme activity, thereby improving testicular dysfunction caused by interstitial cell damage.
Ethics Statement
All the animal experiments were performed in line with the Beijing Chaoyang Hospital of Capital Medical University and Yuying Children’s Hospital of Wenzhou Medical University guidelines using approved procedures of the Institutional Animal Care and Use Committee at Beijing Chaoyang Hospital of Capital Medical University and Yuying Children’s Hospital of Wenzhou Medical University.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Open Research
Data Availability Statement
The data presented in this study are available on request from the corresponding author.