Analytical Characterisation of Selected Alternative Wood Species and Their Suitability for Oenological Applications Using Austrian Grüner Veltliner Wine
Abstract
In response to growing consumer expectations for greater diversity and novel flavour profiles in wines, this study explored the use of alternative wood species in oenology. The effects of six alternative wood species – sweet chestnut, common hazel, Robinia, blackcurrant, vineyard peach, and grapevine – on Austrian Vitis vinifera L. cv. Grüner Veltliner wine were examined after a 98-day contact period using both toasted and untoasted wood chips. The aim was to characterise the similarities of these alternative wood species to traditionally used oak. A comprehensive analytical profiling was conducted, quantifying over 50 wood-associated compounds, including hydroxycinnamic and benzoic acids, ellagitannins, lactones, carbonyl compounds and volatile phenols. Currently, only oak and sweet chestnut are approved by the International Organisation of Vine and Wine for oenological applications, with wood chips permitted exclusively from the genus Quercus. Based on the results, toasted sweet chestnut was the most promising alternative species for use as wood chips. It enhanced the wine colour intensity more effectively than oak, while its phenolic composition, particularly catechin levels and hydroxycinnamic acids, aligned moderately with oak. Furthermore, wine treated with toasted sweet chestnut presented comparable concentrations of vescalagin and castalagin. The similar levels of carbonyl compounds, such as furfural, further underlined its suitability. Although volatile phenol levels, including vanillin, exceeded those of oak, wine treated with toasted sweet chestnut remained comparable in its overall composition. Based on these findings, toasted sweet chestnut is recommended for approval as wood chips, although additional sensory and toxicological studies are necessary to substantiate this recommendation.
1. Introduction
The Austrian grape variety Vitis vinifera L. cv. Grüner Veltliner, also known as Weissgipfler, is by far the most important white wine grape variety in the country, with a cultivation area of 14,409 ha (32.4% of the total vineyard area in Austria) [1]. Presumably originating from Lower Austria, it is the result of a cross between Vitis vinifera L. cv. Traminer and Vitis vinifera L. cv. St. Georgen [2]. In addition to several monoterpenes, the sesquiterpene rotundone has also been identified as characteristic of Grüner Veltliner [3]. Given the significant challenges facing the wine sector and the necessity to adapt to new, alternative taste trends, there is increasing interest regarding the use of innovative vinification methods for this prominent white wine grape variety.
In winemaking, oak species such as Quercus alba, Quercus petraea, and Quercus robur have traditionally dominated. Specifically, wine is matured in barrels made of these species or wood chips, cubes, blocks, or staves of these species are used to enhance wine quality [4, 5]. Oak is favoured in the wine industry due to its flexibility, ease of handling, durability, and low permeability [6]. Recently, alternative wood species such as cherry wood (Prunus avium), black locust (Robinia pseudoacacia), mulberry (Morus L.), and ash (Fraxinus excelsior) have been investigated, either due to their lower costs or their specific sensory contributions [7–11]. The use of such alternative wood species offers a range of advantages and disadvantages. They can be more cost-effective, introduce new sensory properties and aromas to the wine, and reduce environmental impacts. However, they also challenge traditional preferences and require further research to understand their long-term effects on wine quality [12, 13].
Wood chips have been established as a popular alternative to the use of wooden barrels in winemaking. They enable quicker extraction of aromas and are more flexible in their application, imparting the characteristic aromas and flavours of wood to the wine. According to the International Organisation of Vine and Wine (in French: Organisation Internationale de la vigne et du vin [OIV]) OENO-Resolution 3/2005, oenological wood chips must exclusively come from the genus Quercus and are used to release specific substances into the wine [14]. This release occurs through direct ethanolic extraction as well as the breakdown of macromolecules and chemical reactions between wood compounds and the wine matrix [15]. The use of wood chips offers several advantages: first, they allow for faster aroma extraction due to their larger surface area compared with wooden barrels, leading to shortened maturation times, which are especially beneficial for producers seeking a quicker market launch [16]. Second, the use of wood chips enables controlled aroma development in wine, as winemakers can precisely manage the intensity and duration of wood aromas by adjusting the amount and contact time with the chips. Third, wood chips are generally more cost-effective than wooden barrels, making them an economical choice, particularly for smaller producers. The cost savings enable winemakers to produce high-quality wines without exceeding budget constraints [12].
Unlike barrels, wood chips do not allow natural micro-oxygenation, which plays a crucial role in stabilising wine colour, reducing astringency, and enhancing phenolic structure. Wines aged with wood chips often exhibit less complex sensory profiles and faster degradation of phenolic compounds and colour stability during long-term storage. Additionally, the extraction of compounds from wood chips is quicker and less controlled, resulting in a less refined maturation process. Furthermore, the contribution of wooden barrels to wine texture and mouthfeel is significantly diminished when wood chips are used, making them a less suitable option for achieving the full benefits of traditional barrel ageing [12].
Typically, wood chips undergo a toasting process. During this procedure, volatile compounds such as furfural, 5-hydroxymethylfurfural, and 5-methylfurfural are formed through the thermal degradation of cellulose and hemicellulose in the wood. These compounds are responsible for aromas such as caramel, roasted almonds, and bitter almonds [17–21]. During toasting, compounds such as vanillin are formed through the thermal degradation of lignin, while oak lactones, such as cis- and trans-β-methyl-γ-octalactone, originate from glycosidic precursors. These substances enhance the wine with the characteristic aromas of oak wood and impart complex sensory properties [9, 10, 22]. Additionally, phenolic compounds such as guaiacol, eugenol, and p-ethylphenol, and benzene compounds play a significant role, as they can substantially influence both the structure and flavour of the wine [15, 23, 24].
There has been limited research on the application of alternative wood species such as blackcurrant (Ribes nigrum L.), vineyard peach (Prunus persica), grapevine (Vitis vinifera), and common hazel (Corylus avellana) in oenology. However, wood types such as black locust (R. pseudoacacia) and sweet chestnut (Castanea sativa) have been investigated in a few studies. For example, Tavares et al. [25] studied the development of phenolic compounds in a Portuguese red wine that was in contact with Robinia wood chips for 90 days. The authors found that Robinia wood extracts had a lower phenol concentration compared with other wood extracts such as black mulberry (Morus nigra L. and Morus alba L.) [26]. This suggests that Robinia wood might have specific sensory properties and potentially impact wine quality, warranting further investigation. Sweet chestnut wood has also been explored in several studies [27–30]. Fernández de Simón et al. [11] observed that wines aged in chestnut wood barrels achieved moderate scores across all olfactory descriptors, reflecting a balanced but less distinctive aromatic profile. In comparison, wines aged in oak barrels received the highest scores in global wine valuation, which likely reflects overall wine quality and consumer preference, particularly for descriptors such as toasty, almond, caramel, vanilla, and smoky. Thus, further research and experience with the use of sweet chestnut wood in oenology are indeed worthwhile.
The main objective of this study was to investigate the analytical profile of alternative wood species that have been only marginally investigated until now. These species include blackcurrant (R. nigrum), vineyard peach (P. persica), grapevine (V. vinifera), R. pseudoacacia, common hazel (C. avellana), and sweet chestnut (C. sativa), compared with the commonly used oak (Quercus spp.). To achieve this objective, the aforementioned wood species were introduced to the experimental wine made from the Grüner Veltliner grape variety in the form of toasted and untoasted wood chips. Additionally, a control sample (base wine) with no wood chips added was included for comparison. This experimental setup aimed to enable a differentiated analysis of the effects of each wood species on the analytical properties of the wines.
2. Materials and Methods
2.1. Grüner Veltliner
The base wine used for the experiment was produced from the Grüner Veltliner grape variety (vintage 2021) and originated from the Enology Department of the Federal College and Research Institute for Viticulture and Pomology in Klosterneuburg, Austria, where it was vinified according to a standardised protocol. The Grüner Veltliner grapes were harvested on 4 October 2021 with a total soluble solids concentration of 22.9°Bx. Fermentation was carried out in a 1000-L stainless steel tank at 17°C using the yeast Oenoferm Veltliner (Erbslöh, Geisenheim, Germany), and the wine did not undergo malolactic fermentation. Following fermentation, the wine was filtered using a cross-flow filtration system and stored at 15°C until the start of the experiment. The base wine was produced in a dry style, with an alcohol content of 13.3%vol. and a total acidity of 5.2 g/L (calculated as tartaric acid). The basic parameters are presented in Table 1.
| Grüner Veltliner | |
|---|---|
| Alcohol present (%vol.) | 13.3 |
| Glucose (g/L) | n.d. |
| Fructose (g/L) | n.d. |
| pH | 3.34 |
| Tartaric acid (g/L) | 2.0 |
| Malic acid (g/L) | 1.6 |
| Lactic acid (g/L) | n.d. |
| Volatile acid (g/L) | n.d. |
- Abbreviation: n.d. = not detected.
2.2. Wood Chips
The wood chips used in this study were provided by Fassbinderei Schön GmbH (Sitzenberg-Reidling, Austria) in standardised batches of 250 g per wood species. Following a 3-year seasoning period (open-air drying), the wood was processed into uniform chips using a woodchipper. To ensure consistency and to minimise variations in extraction behaviour, the chips were produced with precise and uniform dimensions. In addition, 100 g of each wood species was toasted at 180°C for 20 min in an oven, without replication, ensuring consistent conditions for all samples.
After layer filtration, 1-L bottles were filled with the Grüner Veltliner base wine. Precisely, 3.0 g of the respective wood chips was added to each bottle, which was then sealed with a screw cap. For each wood species, both toasted and untoasted variants were prepared, with five 1-L bottles for each variant. The bottles were stored horizontally in wire mesh boxes at 12°C for 98 days. After this contact period, the wine from each 1-L bottle was separated from the wood chips and transferred into three 250-mL glass bottles, resulting in 15 replicates (3 × 250 mL per 1-L bottle) for each variant. The samples were labelled and stored at 12°C until chromatographic analysis.
2.3. Analytics
2.3.1. Replicates
As described above, the five 1-L bottles were subdivided into three 250-mL bottles following the contact period with the wood chips. For the control sample, as well as both blackcurrant variants, all five biological replicates were subjected to chemical analysis, with each replicate represented by one of the 250-mL bottles derived from the original 1-L bottles. For both variants of the remaining wood species, including oak, vineyard peach, grapevine, black locust, common hazel, and sweet chestnut, four out of the five biological replicates were selected for chemical analysis. The experimental procedure is illustrated in Figure S1 to enhance comprehension.
2.3.2. Basic Parameters
The experimental Grüner Veltliner wines were analysed for their basic parameters both before and after the treatments, in accordance with OIV/OENO Resolution 390/2010, using the FOSS-WineScan (FT 120 Reference Manual; Foss, Hamburg, Germany). The treatments did not lead to significant changes in the basic parameters of the base wine [31].
2.3.3. Spectrophotometric Colour Measurement
A spectrophotometer (model 8453; Agilent Technologies, Santa Clara, CA, USA) was employed to determine the colour intensity of the wines. The measurements were conducted following the OIV-MA-AS2-11 method at one characteristic wavelength (420 nm) [32] in a 10-mm cuvette. Subsequently, the colour intensity was calculated as follows: colour intensity (CI) = absorbance at 420 nm (A420).
2.4. High-Performance Liquid Chromatography (HPLC)
2.4.1. Monohydroxybenzoic and Hydroxycinnamic Acids
The levels of monohydroxybenzoic hydroxycinnamic acids in the experimental wines were determined using HPLC (model 1200; Agilent Technologies) employing an RP-C18 column (Poroshell 120 SB-C18 2.1 × 150 mm, 2.7 μm; Agilent Technologies) and a diode array detector (model DAD SL; Agilent Technologies). Gallic acid, catechin, procyanidin B1, procyanidin B2, ethyl gallate, and epicatechin were quantified at 280 nm, while caftaric acid, caffeic acid, p-coumaric acid, and ferulic acid were quantified at 320 nm. Note that cis- and trans-coutaric acids were calculated as caftaric acid. The method is based on a procedure by Vrhovsek et al. [33] and was last adapted by Philipp et al. [34].
2.4.2. Ellagitannins
HPLC was used to quantify specific ellagitannins in the experimental wines. This approach used a model 1260 instrument (Agilent Technologies) with an RP-C18 column (Poroshell 120 SB-C18 2.1 × 150 mm; Agilent Technologies). Detection was carried out using both a diode array detector (model DAD SL; Agilent Technologies) and a fluorescence detector (model 1260; Agilent Technologies). Quantification was performed at 260 and 280 nm and using fluorescence detection at excitation wavelengths of 345 and 460 nm for the substances vescalagin, castalagin, syringic acid, scopoletin, and ellagic acid. The method is based on a procedure by Philipp et al. [3] and was last modified by Philipp et al. [35].
2.5. Solid-Phase Extraction–Gas Chromatography–Selected Ion Monitoring–Mass Spectrometry (SPE-GC-SIM-MS)
2.5.1. Carbonyl Compounds, Lactones and Volatile Phenols
SPE-GC-SIM-MS was conducted to quantify carbonyl compounds, lactones, and volatile phenols. The applied method was based on an adapted procedure outlined by Philipp et al. [35]. For this purpose, 10 mL of the sample was mixed in a volumetric flask with 50 μL of internal standard (3,4-dimethylanisole, final concentration 10 μg/L) and vortexed. Sample clean-up was performed using SPE cartridges (LiChrolut EN, 200 mg; Merck, Darmstadt, Germany), which were preconditioned by rinsing twice with a dichloromethane–chloroform mixture (2:1, v/v) followed by methanol. Prior to application, the cartridges were conditioned with 1 mL of buffered synthetic wine prepared according to Philipp et al. [36].
The samples were applied to the cartridges under gravity, followed by drying the cartridges under vacuum for 15 min. Then, the analytes were eluted with two portions of 0.75 mL of dichloromethane–chloroform (2:1, v/v). The eluate was dried over sodium sulphate, concentrated under a stream of air to approximately 0.3 mL, and transferred into microvials (200 μL). A volume of 1 μL was injected in split mode (5:1) at 260°C. Helium was used as the carrier gas at a flow rate of 1 mL/min.
The gas chromatograph temperature programme started with a hold at 50°C for 5 min, followed by an increase of 4°C/minute to 120°C, then 3°C/min to 180°C, and finally 20°C/min to 265°C, where it was held for 9.25 min. The total runtime was 55 min, and the transfer line temperature to the mass spectrometer was set to 270°C. Analyses were carried out in a single-ion mode (EI+, 70 eV).
The calibration and validation of the method are documented in Table S1. The five-level calibration was performed in buffered synthetic wine, while the validation was conducted using a selected Pinot Blanc wine.
2.6. Statistical Evaluation
SPSS Statistics Version 29.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Initially, the data were checked for a normal distribution using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. Subsequently, a one-way analysis of variance (ANOVA) was conducted, with a significance level set at p < 0.05. Additionally, a principal component analysis (PCA) and a two-way ANOVA were conducted using XLSTAT 2023.3.1 (Lumivero, Denver, CO, USA) [37, 38]. To control the error rate, the Benjamini–Hochberg correction was applied. Furthermore, for better visualisation, a heatmap was created using SR-Plot [39].
3. Results and Discussion
3.1. Colour Intensity (420 nm)
Table 2 provides an overview of the colour intensity [420 nm] of the base wine and the wines treated with selected toasted and untoasted alternative wood species. Highly significant differences (p < 0.001) were observed, indicated by different letters within the rows. The two-way ANOVA results, analysing the effects of wood species, toasting and their interaction, clearly demonstrate the complex influence of these factors.
| Colour intensity (420 nm) | |
|---|---|
| Wood species | ∗∗ |
| Toasting | ∗∗ |
| Wood species × toasting | ∗∗ |
| Base wine | 0.05a ± 0.00 |
| Sweet chestnut, toasted | 0.13h ± 0.00 |
| Sweet chestnut, untoasted | 0.09def ± 0.00 |
| Oak, toasted | 0.09de ± 0.01 |
| Oak, untoasted | 0.08cd ± 0.00 |
| Common hazel, toasted | 0.07b ± 0.00 |
| Common hazel, untoasted | 0.06a ± 0.00 |
| Robinia, toasted | 0.11fg ± 0.00 |
| Robinia, untoasted | 0.10ef ± 0.01 |
| Blackcurrant, toasted | 0.12g ± 0.00 |
| Blackcurrant, untoasted | 0.07bc ± 0.00 |
| Vineyard peach, toasted | 0.09de ± 0.01 |
| Vineyard peach, untoasted | 0.10ef ± 0.02 |
| Grapevine, toasted | 0.08cd ± 0.00 |
| Grapevine, untoasted | 0.07bc ± 0.01 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified colour intensity (mean ± standard deviation) for the base wine and the variants is shown. Significant differences are indicated by different letters.
- ∗∗p < 0.01.
Notably, toasted chestnut exhibited the highest colour intensity, significantly exceeding that of both the base wine and the untoasted chestnut variant. Similarly, toasted blackcurrant wood resulted in a pronounced increase in colour intensity. Both toasted and untoasted Robinia, as well as hazelnut, induced a moderate yet measurable enhancement. Vine and vineyard peach woods showed comparable values, regardless of toasting, slightly surpassing those of the base wine.
Overall, the findings highlight that both the choice of wood species and the toasting process influence colour development. Toasted chestnut and blackcurrant woods were particularly effective, producing the most substantial impact. This effect can be attributed to the enhanced release of phenolic compounds from the wood during toasting, especially ellagitannins and phenolic acids, which exhibit antioxidant properties and may inhibit oxidative degradation of colour-active compounds in the wine [40–42].
Furthermore, contact with wood – especially toasted variants – promotes oxidative reactions as well as condensation and polymerisation of phenolic compounds. These processes can lead to the formation of colour-active structures, enhancing absorption along the visible spectrum and consequently increasing both the intensity and the stability of colour in white wine [43–46].
Woods such as chestnut are particularly effective in enhancing colour intensity due to their high content of hydrolysable tannins and gallic acid [47, 48]. These compounds, owing to their antioxidant properties, can mitigate oxidative reactions within the wine, thereby reducing the degradation of colour-contributing substances. Additionally, they promote the formation of yellowish pigments or polymeric structures that, particularly in white wine, enhance absorption within the visible range and thus contribute to increased colour intensity [12, 49].
Interestingly, certain alternatives, such as untoasted vineyard peach and toasted Robinia, surpassed even traditional oak in their influence on colour intensity. This finding underscores the potential for selective utilisation of alternative woods to modulate colour-enhancing effects, a feature gaining importance in the context of tailoring wine profiles [50, 51].
Moreover, the natural oxygen permeability of wood chips further promotes oxidative and polymerisation processes of phenolic compounds such as flavanols, leading to the development of colour-active structures. This not only enhances colour intensity but may also positively influence astringency and the overall sensory balance of the white wine [52, 53].
3.2. Monohydroxybenzoic and Hydroxycinnamic Acids
Table 3 presents the concentrations of monohydroxybenzoic and hydroxycinnamic acids, as well as their total values, in the base wine and wines treated with selected toasted and untoasted wood species. The two-way ANOVA results revealed significant differences (p < 0.001), influenced by wood type, toasting and their interaction.
| (mg/L) | Gallic acid | Protocatechuic acid | Caftaric acid | Tyrosol | cis-Coutaric acid | trans-Coutaric acid | Vanillic acid | Catechin | Caffeic acid | Procyanidin B1 | Fertaric acid | p-Coumaric acid | Procyanidin B2 | Ethyl gallate | Epicatechin | Ferulic acid | Σ Monohydroxybenzoic and hydroxycinnamic acids |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wood species | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | n.s. | ∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | ∗∗ | n.s. | ∗∗∗ | ∗∗∗ | ∗∗ | n.s. | — |
| Toasting | ∗∗∗ | ∗∗∗ | n.s. | ∗∗ | n.s. | n.s. | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | n.s. | n.s. | ∗∗ | — |
| Wood species × toasting | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | n.s. | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | n.s. | ∗∗ | ∗∗∗ | n.s. | ∗∗∗ | — |
| Base wine | 0.7b ± 0.0 | 0.4ab ± 0.0 | 3.0ef ± 0.1 | 14.0abc ± 0.3 | 0.8cd ± 0.0 | 0.3ab ± 0.0 | 0.2a ± 0.0 | 2.1a ± 0.1 | 1.8ab ± 0.0 | 0.9ab ± 0.0 | 2.5a ± 0.4 | 1.1bcd ± 0.0 | n.d. (a) | n.d. (a) | 1.6a ± 0.2 | 0.9b ± 0.0 | 30.3a ± 0.5 |
| Sweet chestnut, toasted | 7.1e ± 0.1 | 1.3e ± 0.0 | 2.8de ± 0.0 | 14.4cde ± 0.1 | 0.9d ± 0.1 | 0.3a ± 0.0 | 0.5a ± 0.0 | 1.6a ± 0.3 | 1.8ab ± 0.0 | n.d. (a) | 3.8b ± 0.1 | 1.1ab ± 0.0 | n.d. (a) | 0.2b ± 0.1 | 1.7abc ± 0.2 | 0.9b ± 0.0 | 38.4a ± 0.3 |
| Sweet chestnut, untoasted | n.d. (a) | 0.5c ± 0.0 | 2.7bcd ± 0.1 | 15.7h ± 0.2 | 0.8bcd ± 0.0 | 0.4b ± 0.0 | 0.2a ± 0.0 | 2.0a ± 0.1 | 1.8ab ± 0.0 | 1.0ab ± 0.1 | 2.7a ± 0.0 | 1.1abc ± 0.0 | n.d. (a) | 1.2d ± 0.1 | 1.8abc ± 0.1 | 0.9b ± 0.0 | 32.8a ± 0.3 |
| Oak, toasted | 2.7d ± 0.9 | 0.8d ± 0.0 | 2.6abc ± 0.0 | 14.9def ± 0.3 | 0.6ab ± 0.1 | 0.3ab ± 0.0 | 0.3a ± 0.0 | 2.0a ± 0.0 | 1.8ab ± 0.0 | 1.0ab ± 0.1 | 3.0a ± 0.5 | 1.1a ± 0.1 | n.d. (a) | 0.6c ± 0.1 | 1.6ab ± 0.1 | 0.9b ± 0.0 | 34.1a ± 1.5 |
| Oak, untoasted | 2.2cd ± 0.3 | 0.5bc ± 0.0 | 3.1f ± 0.1 | 13.9ab ± 0.1 | 0.9cd ± 0.0 | 0.3ab ± 0.0 | 0.2a ± 0.0 | 2.0a ± 0.1 | 1.8ab ± 0.0 | 0.9ab ± 0.1 | 2.8a ± 0.0 | 1.1abc ± 0.0 | n.d. (a) | n.d. (a) | 1.5a ± 0.0 | 0.9b ± 0.0 | 32.1a ± 0.4 |
| Common hazel, toasted | 0.9b ± 0.0 | 1.4f ± 0.0 | 2.7bcd ± 0.0 | 14.4cde ± 0.4 | 0.9d ± 0.0 | 0.4b ± 0.0 | 0.3a ± 0.0 | 1.7a ± 0.2 | 1.8ab ± 0.0 | 0.6ab ± 0.4 | 3.0a ± 0.0 | 1.1abc ± 0.0 | n.d. (a) | n.d. (a) | 1.7abc ± 0.1 | 0.9b ± 0.0 | 31.8a ± 0.8 |
| Common hazel, untoasted | 0.8b ± 0.1 | 0.4a ± 0.0 | 2.5abc ± 0.1 | 14.5def ± 0.3 | 0.8bcd ± 0.0 | 0.4b ± 0.0 | 0.2a ± 0.0 | 2.2a ± 0.2 | 1.8ab ± 0.0 | 1.0ab ± 0.2 | 2.8a ± 0.5 | 1.1bcd ± 0.0 | n.d. (a) | n.d. (a) | 1.6ab ± 0.1 | 0.9b ± 0.0 | 31.1a ± 0.6 |
| Robinia, toasted | 1.1b ± 0.0 | 1.2e ± 0.0 | 2.7cd ± 0.1 | 15.0f ± 0.4 | 0.9cd ± 0.1 | 0.4b ± 0.0 | 12.9b ± 3.5 | 183.0b ± 30.4 | 1.8b ± 0.0 | n.d. (a) | 2.8a ± 0.1 | 1.1abc ± 0.0 | 19.4c ± 1.8 | 0.2b ± 0.0 | 2.1cd ± 0.1 | 1.7c ± 0.2 | 246.4b ± 33.9 |
| Robinia, untoasted | 1.0b ± 0.0 | 0.4a ± 0.0 | 2.6bcd ± 0.1 | 14.7def ± 0.2 | 0.8bcd ± 0.0 | 0.3ab ± 0.0 | n.d. (a) | 265.5c ± 20.6 | 1.8ab ± 0.0 | n.d. (a) | 2.7a ± 0.0 | 1.1abc ± 0.0 | 21.5d ± 0.3 | n.d. (a) | 2.0bcd ± 0.1 | n.d. (a) | 314.5c ± 20.7 |
| Blackcurrant, toasted | 2.7d ± 0.5 | 0.6c ± 0.0 | 2.5a ± 0.0 | 14.9ef ± 0.1 | 0.7abc ± 0.0 | 0.3ab ± 0.0 | 0.3a ± 0.0 | 2.0a ± 0.1 | 1.8ab ± 0.0 | 0.8ab ± 0.0 | 2.8a ± 0.0 | 1.1ab ± 0.0 | n.d. (a) | 0.2b ± 0.0 | 1.7abc ± 0.2 | 0.9 ± 0.0 | 33.2a ± 0.6 |
| Blackcurrant, untoasted | 1.9c ± 0.3 | 0.5ab ± 0.0 | 2.7bcd ± 0.2 | 13.8a ± 0.3 | 0.9cd ± 0.0 | 0.3ab ± 0.0 | 0.3a ± 0.0 | 2.3a ± 0.1 | 1.8ab ± 0.0 | 0.8ab ± 0.1 | 2.9a ± 0.1 | 1.2cd ± 0.1 | 2.1b ± 1.4 | 0.3b ± 0.0 | 1.7abc ± 0.1 | 0.7 ± 0.4 | 34.1a ± 1.8 |
| Vineyard peach, toasted | 0.7b ± 0.0 | 0.6c ± 0.1 | 2.5a ± 0.1 | 14.9ef ± 0.1 | 0.7abc ± 0.1 | 0.3ab ± 0.0 | 0.5a ± 0.1 | 5.1a ± 1.3 | 1.8ab ± 0.0 | 2.4c ± 0.9 | 2.7a ± 0.0 | 1.1ab ± 0.0 | n.d. (a) | 0.2b ± 0.1 | 2.3d ± 0.2 | 0.9b ± 0.0 | 36.7a ± 2.4 |
| Vineyard peach, untoasted | 0.8b ± 0.0 | 0.5c ± 0.0 | 2.5ab ± 0.1 | 14.0abc ± 0.3 | 0.8bcd ± 0.0 | 0.4b ± 0.0 | 0.4a ± 0.0 | 9.7a ± 2.2 | 1.9c ± 0.1 | 6.2d ± 1.3 | 2.7a ± 0.4 | 1.2d ± 0.0 | 0.9ab ± 0.0 | 0.2b ± 0.1 | 2.0bcd ± 0.1 | 1.1b ± 0.1 | 45.4a ± 3.8 |
| Grapevine, toasted | 1.2b ± 0.2 | 0.5bc ± 0.0 | 2.5a ± 0.0 | 14.7def ± 0.2 | 0.6a ± 0.1 | 0.4b ± 0.0 | 0.7a ± 1.0 | 2.3a ± 0.0 | 1.8a ± 0.0 | 1.1b ± 0.1 | 2.7a ± 0.0 | 1.1ab ± 0.0 | 0.3a ± 0.5 | 0.2b ± 0.0 | 2.0bcd ± 0.3 | 0.9b ± 0.0 | 32.8a ± 1.0 |
| Grapevine, untoasted | 1.2b ± 0.2 | 0.4ab ± 0.0 | 2.7bcd ± 0.0 | 14.3bcd ± 0.2 | 0.8bcd ± 0.2 | 0.4b ± 0.0 | 0.2a ± 0.0 | 2.8a ± 0.5 | 1.8ab ± 0.0 | 1.5b ± 0.4 | 2.7a ± 0.0 | 1.1abc ± 0.0 | 0.9ab ± 0.1 | n.d. (a) | 2.1cd ± 0.5 | 0.9b ± 0.0 | 33.7a ± 1.5 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviations: n.d. = not detectable; n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
The most pronounced effect of toasting was observed in the release of gallic acid: toasted chestnut exhibited the highest concentrations, whereas the untoasted variant contained no detectable amounts, clearly emphasising the thermally induced release mechanism. Similar trends were noted for Robinia and blackcurrant woods. These findings confirm that the thermal breakdown of native wood-derived phenolic acids and their derivatives plays a pivotal role in their release [47, 48].
Vanillic acid levels were also significantly affected. Toasted Robinia produced the highest concentrations, indicative of lignin degradation during toasting [54]. The increases observed in toasted chestnut and vineyard peach further underline the general contribution of toasting to the formation of this compound, which, although not directly aroma-active, can influence sensory perception through its antioxidant and structure-enhancing properties [19, 55].
Notably, catechin concentrations were the highest in untoasted Robinia, followed by untoasted vineyard peach, both significantly surpassing those in oak. This highlights the substantial presence of condensed tannins and flavonoids in alternative woods, which are partially degraded during toasting [53, 56]. The reductions observed in toasted variants suggest polymerisation or oxidative reactions during thermal processing, processes also relevant in white wine maturation [57].
Procyanidin B2 was detected in untoasted Robinia and, to a lesser extent, in blackcurrant, while it was undetectable in oak. This finding underscores the unique composition of alternative woods, particularly regarding their content of condensed tannins and procyanidins, which can contribute significantly to wine texture and astringency [58].
The total concentrations of monohydroxybenzoic and hydroxycinnamic acids demonstrated that both wood type and toasting induce substantial variations. While oak and toasted chestnut exhibited comparable values, Robinia and vineyard peach – particularly in their untoasted forms – displayed markedly higher levels. This can be attributed to the naturally higher phenolic acid content of these wood species and their derivatives [48, 59].
Overall, these results highlight the significant role of wood species in the release of phenolic compounds. Alternative woods such as chestnut or Robinia can substantially increase the concentrations of specific phenolic acids, thereby influencing both the antioxidant capacity and potentially the sensory properties of the wine. Gallic and vanillic acids, in particular, play key roles in this context [60, 61].
Consistent with previous studies, the extraction of phenolic compounds is heavily dependent on wood species, porosity, oxygen permeability, and thermal treatment [20, 62–64]. During maturation in contact with wood, the accumulation of these compounds can enhance the antioxidant potential of white wine while also contributing to the development of texture, specifically body and astringency [58, 65].
3.3. Ellagitannins
Table 4 presents the concentrations of ellagitannins (vescalagin and castalagin), syringic acid, scopoletin, and ellagic acid in the base wine and in wines treated with alternative wood species. Two-way ANOVA revealed significant differences (p < 0.001), with wood species, toasting, and their interaction, all acting as key influencing factors.
| (mg/L) | Vescalagin | Castalagin | Syringic acid | Scopoletin | Ellagic acid | Σ Ellagitannins |
|---|---|---|---|---|---|---|
| Wood species | ∗∗∗ | ∗∗∗ | ∗∗∗ | — | ∗∗∗ | — |
| Toasting | ∗∗∗ | ∗∗ | n.s. | — | n.s. | — |
| Wood species × toasting | ∗∗∗ | ∗∗∗ | n.s. | — | n.s. | — |
| Base wine | 2.5a ± 0.0 | n.d. (a) | 0.1a ± 0.0 | n.d. | n.d. (a) | 2.6a ± 0.0 |
| Sweet chestnut, toasted | 13.8c ± 0.7 | 33.2c ± 1.4 | 0.6a ± 0.0 | n.q. | 1.0c ± 0.1 | 48.5c ± 2.0 |
| Sweet chestnut, untoasted | 84.8d ± 1.5 | 74.9d ± 4.9 | 0.2a ± 0.0 | n.d. | 1.0c ± 0.1 | 160.8d ± 6.1 |
| Oak, toasted | 6.7b ± 3.5 | 13.4b ± 10.3 | 0.2a ± 0.0 | n.q. | 0.7b ± 0.2 | 21.0b ± 14.0 |
| Oak, untoasted | 6.6b ± 1.8 | 11.2b ± 4.6 | 0.2a ± 0.0 | n.q. | 0.9c ± 0.1 | 18.9b ± 6.3 |
| Common hazel, toasted | n.d. (a) | 0.7a ± 0.0 | 0.4a ± 0.0 | n.d. | 0.1a ± 0.0 | 1.2a ± 0.0 |
| Common hazel, untoasted | n.d. (a) | 0.7a ± 0.0 | 0.2a ± 0.1 | n.d. | 0.1a ± 0.0 | 1.0a ± 0.1 |
| Robinia, toasted | 1.0a ± 0.0 | 0.6a ± 0.0 | 6.2b ± 0.5 | n.d. | n.d. (a) | 7.8a ± 0.5 |
| Robinia, untoasted | n.d. (a) | 0.7a ± 0.1 | 5.9b ± 0.6 | n.d. | n.d. (a) | 6.7a ± 0.6 |
| Blackcurrant, toasted | n.d. (a) | 0.7a ± 0.1 | 0.2a ± 0.0 | n.d. | 0.1a ± 0.0 | 1.0a ± 0.1 |
| Blackcurrant, untoasted | n.d. (a) | 0.8a ± 0.1 | 0.1a ± 0.0 | n.d. | 0.1a ± 0.0 | 1.0a ± 0.1 |
| Vineyard peach, toasted | n.d. (a) | 0.7a ± 0.0 | 0.2a ± 0.0 | n.d. | n.d. (a) | 0.9a ± 0.1 |
| Vineyard peach, untoasted | n.d. (a) | 0.8a ± 0.1 | 0.3a ± 0.0 | n.d. | n.d. (a) | 1.1a ± 0.1 |
| Grapevine, toasted | 1.1a ± 0.1 | 0.6a ± 0.0 | 0.1a ± 0.0 | n.d. | n.d. (a) | 1.9a ± 0.1 |
| Grapevine, untoasted | n.d. (a) | 0.7a ± 0.0 | 0.1a ± 0.0 | n.d. | n.d. (a) | 0.8a ± 0.0 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviations: n.d. = not detectable; n.q. = not quantified; n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
Vescalagin and castalagin reached their highest concentrations in untoasted chestnut – an expected characteristic of this species due to its high content of hydrolysable tannins [47, 48]. Both compounds decreased substantially following toasting, yet levels in toasted chestnut remained higher than in any other treatment. This aligns with the well-documented thermal degradation of ellagitannins during toasting, a process also described for oak [29, 40].
By contrast, vescalagin and castalagin levels in Robinia, vine, hazelnut, blackcurrant, and vineyard peach were either below detection limits or very low – an expected outcome as these wood types naturally contain little to no ellagitannins [8, 59]. This further highlights the distinct position of chestnut among alternative wood species.
Syringic acid concentrations were notably elevated in both toasted and untoasted Robinia, once again demonstrating wood-specific differences in phenolic acid composition. Chestnut released moderate amounts of syringic acid, whereas other species exhibited markedly lower levels. Given that syringic acid predominantly derives from lignin degradation, this finding emphasises the role of the wood matrix and the influence of toasting on its release [19, 47].
Ellagic acid was detected in relevant amounts exclusively in chestnut—a further hallmark of its chemical profile. Interestingly, toasting had little impact on ellagic acid concentrations, which is consistent with literature indicating that while other ellagitannins degrade under heat, ellagic acid remains relatively stable [29, 47].
When compared directly with oak, toasted chestnut emerged as the only genuine alternative in terms of vescalagin and castalagin content. Although toasting reduced their levels, the remaining concentrations in chestnut remained comparable to those found in oak, underscoring chestnut’s potential suitability as an alternative wood for oenological use. The discrepancy with Alañón et al. [60], who reported only low ellagitannin concentrations in chestnut-aged wines, could stem from differences in wood processing and toasting intensity.
Ellagitannins play a crucial role in wine maturation— exhibiting antioxidant properties, stabilising phenolic structures, and significantly influencing texture and astringency [40, 58]. Particularly in white wines, they contribute to reducing oxidative ageing phenomena and are fundamental to sensory development.
In summary, toasted chestnut proved most comparable to oak regarding ellagitannin composition, presenting a compelling alternative for targeted oenological applications in white wine production. Both wood species and toasting intensity are key determinants of ellagitannin release and their potential impact on wine structure and ageing potential.
3.4. Carbonyl Compounds
Table 5 presents the concentrations of key carbonyl compounds, including furfural, 5-methylfurfural and syringaldehyde, in the base wine and the various treated wine samples. The two-way ANOVA revealed significant differences (p < 0.001), driven by wood species, toasting and their interaction.
| (mg/L) | Furfural | 5-Methylfurfural | 3,5-Dimethoxy-4-hydroxyacetophenone | 5-Acetoxymethyl-2-furaldehyde | Syringaldehyde | Σ Carbonyl compounds |
|---|---|---|---|---|---|---|
| Wood species | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | — |
| Toasting | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. | ∗∗∗ | — |
| Wood species × toasting | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | — |
| Base wine | 8.1a ± 0.9 | 0.2a ± 0.0 | 0.1a ± 0.1 | 8.3bc ± 1.8 | 0.2a ± 0.0 | 16.9a ± 1.1 |
| Sweet chestnut, toasted | 365.6f ± 73.8 | 39.6c ± 4.2 | 55.5d ± 6.4 | 6.4ab ± 0.2 | 1826.5c ± 249.0 | 2293.6f ± 291.1 |
| Sweet chestnut, untoasted | 21.7a ± 2.2 | 1.5a ± 0.8 | 2.8ab ± 0.7 | 3.3ab ± 0.6 | 76.0a ± 6.1 | 105.3abc ± 7.1 |
| Oak, toasted | 290.9e ± 16.9 | 34.1bc ± 4.3 | 3.6ab ± 0.9 | 8.4bc ± 2.1 | 106.8a ± 12.4 | 443.7d ± 21.9 |
| Oak, untoasted | 32.6a ± 1.4 | 1.7a ± 0.3 | 0.2a ± 0.1 | 4.6ab ± 0.4 | 24.3a ± 5.7 | 63.5ab ± 5.9 |
| Common hazel, toasted | 83.0bc ± 14.6 | 35.7c ± 9.3 | 25.2c ± 6.8 | 4.3ab ± 0.9 | 623.2b ± 165.9 | 771.4e ± 187.2 |
| Common hazel, untoasted | 18.5a ± 0.5 | 1.5a ± 0.2 | 0.2a ± 0.0 | 5.0ab ± 1.1 | 0.3a ± 0.1 | 25.6a ± 1.0 |
| Robinia, toasted | 38.3ab ± 6.0 | 5.6a ± 1.5 | 2.7ab ± 0.5 | 1.7a ± 0.1 | 34.1a ± 8.7 | 82.5ab ± 14.2 |
| Robinia, untoasted | 14.4a ± 2.4 | 0.4a ± 0.1 | 1.7ab ± 0.2 | 14.1de ± 2.1 | 9.9a ± 1.5 | 40.4ab ± 3.6 |
| Blackcurrant, toasted | 107.0cd ± 15.0 | 48.2d ± 8.8 | 7.2b ± 2.0 | 32.8f ± 6.1 | 86.1a ± 25.1 | 281.2cd ± 19.0 |
| Blackcurrant, untoasted | 11.5a ± 1.5 | 0.6a ± 0.1 | 0.3a ± 0.1 | 13.0cd ± 2.1 | 22.8a ± 2.3 | 48.3ab ± 3.9 |
| Vineyard peach, toasted | 144.7d ± 42.0 | 26.4b ± 8.9 | 3.0ab ± 0.5 | 12.1cd ± 2.9 | 50.8a ± 12.7 | 237.0bc ± 59.9 |
| Vineyard peach, untoasted | 16.3a ± 2.5 | 0.3a ± 0.1 | 5.1ab ± 1.1 | 16.9de ± 2.8 | 19.6a ± 4.3 | 58.1ab ± 8.4 |
| Grapevine, toasted | 49.9ab ± 8.6 | 8.7a ± 1.7 | 2.7ab ± 0.7 | 2.8a ± 0.5 | 33.4a ± 8.4 | 97.5abc ± 16.4 |
| Grapevine, untoasted | 14.0a ± 1.1 | 0.5a ± 0.1 | 1.3a ± 0.3 | 18.5e ± 1.1 | 7.1a ± 1.4 | 41.3ab ± 3.0 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviation: n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
The highest furfural concentrations were observed in toasted chestnut, followed by toasted hazelnut and toasted vineyard peach. This result confirms the pivotal role of the thermal degradation of hemicellulose in the generation of furfural compounds [66–68]. All toasted wood variants exhibited markedly higher values than their untoasted counterparts, underscoring the critical influence of toasting. Furfural is sensorially associated with sweet notes such as caramel and roasted almond, subtly yet perceptibly shaping the aroma profile of white wines [12, 55].
Similarly, 5-methylfurfural showed its highest concentrations in toasted chestnut, with comparable increases noted for toasted hazelnut, blackcurrant, and vineyard peach. As expected, levels in untoasted woods were negligible or undetectable. This compound also derives from the thermal degradation of wood components and is known for contributing sweet, toasted aromas [27, 28].
Syringaldehyde peaked in toasted chestnut, followed by toasted hazelnut. These aldehydes primarily result from lignin breakdown during toasting and impart vanilla and spicy notes to the aromatic profile [55, 69]. Again, the dominant role of toasting as a driver of compound formation was clearly evident.
In direct comparison with oak, toasted chestnut emerged as the alternative wood species most closely resembling oak in terms of furfural and 5-methylfurfural concentrations. Both compounds appeared in chestnut at levels comparable to toasted oak, corroborating findings by Martins et al. [28] and Alañón et al. [17, 18]. These studies also confirm that traces of such compounds may be present in untoasted woods.
From a sensory perspective, furfural and 5-methylfurfural are important aroma-active compounds, contributing notes of caramel, roasted almond, and subtle smokiness – effects that are perceptible even in white wines [12]. These aromatic elements are considered hallmark indicators of wood influence and can be selectively modulated depending on the wood species and toasting regime.
Interestingly, toasted blackcurrant wood exhibited the closest similarity to toasted oak regarding the syringaldehyde content, suggesting comparable lignin degradation pathways. By contrast, both chestnut and hazelnut generated considerably higher syringaldehyde levels than oak, highlighting their distinct chemical profiles.
In conclusion, this analysis reinforces the significance of toasting as a key factor in the formation of aroma-active carbonyl compounds. Toasted chestnut most closely replicates the classic oak-associated furfural and 5-methylfurfural concentrations, while other woods such as hazelnut or blackcurrant offer unique profiles with the potential for targeted modulation of white wine aroma.
3.5. Lactones
Table 6 presents the concentrations of key lactones in the base wine and in wines treated with alternative wood species. Two-way ANOVA revealed significant differences (p < 0.001), attributable to wood species, toasting, and their interaction.
| (μg/L) | trans-Whiskeylactone | cis-Whiskeylactone | δ-Decalactone | Σ Lactones |
|---|---|---|---|---|
| Wood species | ∗∗∗ | ∗∗∗ | ∗∗ | — |
| Toasting | n.s. | n.s. | n.s. | — |
| Wood species × toasting | ∗∗ | n.s. | ∗∗ | — |
| Base wine | 0.4a ± 0.1 | 0.3a ± 0.0 | 4.6ab ± 0.5 | 5.2a ± 0.5 |
| Sweet chestnut, toasted | 1.4a ± 0.4 | 0.9a ± 0.0 | 8.5de ± 1.2 | 10.9a ± 1.6 |
| Sweet chestnut, untoasted | 1.4a ± 1.4 | 0.5a ± 0.2 | 4.4ab ± 0.7 | 6.4a ± 2.0 |
| Oak, toasted | 69.7b ± 19.4 | 76.8c ± 13.2 | 5.2abc ± 0.9 | 151.7b ± 17.7 |
| Oak, untoasted | 109.8c ± 27.0 | 67.2b ± 7.3 | 3.4a ± 0.6 | 180.4c ± 31.4 |
| Common hazel, toasted | 1.9a ± 0.4 | 1.0a ± 0.3 | 8.2de ± 2.0 | 11.0a ± 2.5 |
| Common hazel, untoasted | 1.5a ± 0.3 | 0.8a ± 0.1 | 4.1a ± 0.8 | 6.3a ± 0.6 |
| Robinia, toasted | 1.7a ± 0.1 | 0.9a ± 0.2 | 8.1de ± 1.8 | 10.7a ± 2.0 |
| Robinia, untoasted | 1.1a ± 0.2 | 0.4a ± 0.1 | 8.5de ± 1.5 | 10.0a ± 1.8 |
| Blackcurrant, toasted | 1.8a ± 0.4 | 0.8a ± 0.2 | 7.2cde ± 1.7 | 9.8a ± 1.8 |
| Blackcurrant, untoasted | 0.8a ± 0.1 | 0.5a ± 0.1 | 4.7ab ± 1.1 | 6.0a ± 1.2 |
| Vineyard peach, toasted | 1.0a ± 0.1 | 0.5a ± 0.1 | 5.9bcd ± 1.0 | 7.4a ± 1.1 |
| Vineyard peach, untoasted | 1.1a ± 0.3 | 0.6a ± 0.1 | 7.9cde ± 0.9 | 9.6a ± 1.4 |
| Grapevine, toasted | 1.6a ± 0.5 | 1.2a ± 0.2 | 7.2cde ± 2.0 | 9.9a ± 2.5 |
| Grapevine, untoasted | 1.4a ± 0.2 | 0.6a ± 0.1 | 10.0e ± 1.2 | 12.0a ± 1.2 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviation: n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
For trans-whisky lactone, toasted hazelnut exhibited the highest concentrations, followed by toasted blackcurrant and toasted Robinia, all of which substantially exceeded the levels observed in the base wine. Chestnut displayed moderate values regardless of toasting, while vineyard peach variants also showed slightly elevated levels. These findings highlight both the species-specific composition and the influence of thermal processes on the release of these compounds [55, 70].
In the case of cis-whisky lactone – the more potent isomer in sensory terms – the highest concentration was measured in toasted vine wood, with toasted hazelnut and toasted Robinia also presenting significant amounts. All toasted variants contained higher concentrations of cis-whisky lactone than their untoasted counterparts, confirming the strong dependency of lactone formation on toasting and the thermal breakdown of lignin and lipid fractions [71, 72].
The analysis of δ-decalactones revealed elevated levels in untoasted vine wood, toasted chestnut and untoasted Robinia, all surpassing those in the base wine. The formation of these compounds appears influenced by both wood species and thermal treatment, with some woods exhibiting high baseline values even without toasting.
Direct comparison with oak revealed marked differences: as expected, both toasted and untoasted oak exhibited significantly higher levels of trans- and cis-whisky lactones, with none of the alternative woods approaching oak concentrations. Toasted chestnut, hazelnut and vine wood produced the highest values among the alternatives but remained well below oak levels. These results are consistent with previous studies confirming the dominance of oak—particularly Q. alba—as the primary source of lactones and their intense sensory impact [55, 68, 73].
From a sensory perspective, both whisky lactone isomers are particularly relevant. While trans-whisky lactone contributes mild oak and coconut notes, cis-whisky lactone—due to its four- to fivefold lower sensory threshold—exerts a much stronger aromatic influence, often associated with coconut and vanilla notes [70, 74]. In white wines, these lactones can be critical in shaping the aromatic profile, enhancing sweet and creamy nuances.
The findings further support the observations of Caldeira et al. [55], who reported that chestnut wood contains only small amounts of these lactones—an outcome reflected here in the moderate concentrations observed for chestnut.
In summary, lactone contributions are largely dependent on oak species and toasting, and alternative woods do not fully reproduce the intense oak-derived aromas. Nevertheless, they provide distinct aromatic profiles that can be purposefully utilised to craft specific white wine styles.
3.6. Volatile Phenols
Tables 7 and 8 present the concentrations of volatile phenols in the base wine and in wines treated with various wood species. Two-way ANOVA revealed significant differences (p < 0.001), with wood species, toasting, and their interaction identified as major influencing factors.
| (μg/L) | o-Cresol | m- and p-Cresol | Guaiacol | 4-Methylguaiacol | 4-Ethylguaiacol | 4-Vinylguaiacol | 2,6-Dimethoxyphenol | Eugenol | 2-Methoxy-4-propylphenol | Vanillin |
|---|---|---|---|---|---|---|---|---|---|---|
| Wood species | ∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ |
| Toasting | ∗∗ | n.s. | ∗∗ | ∗∗∗ | ∗∗∗ | n.s. | ∗∗∗ | n.s. | n.s. | ∗∗∗ |
| Wood species × toasting | ∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗∗ |
| Base wine | 0.4a ± 0.1 | 0.5a ± 0.1 | 2.6a ± 0.6 | 0.2a ± 0.0 | 0.2a ± 0.0 | 816.7a ± 149.4 | n.d. (a) | 0.4a ± 0.1 | 0.2bc ± 0.1 | 2.4a ± 0.3 |
| Sweet chestnut, toasted | 0.4a ± 0.0 | 1.0bc ± 0.0 | 13.4de ± 3.4 | 29.1b ± 3.1 | 1.6bcd ± 0.3 | 1557.2bcd ± 301.4 | 22.0e ± 6.3 | 4.4b ± 0.4 | 0.3e ± 0.1 | 1066.2e ± 57.2 |
| Sweet chestnut, untoasted | 0.6a ± 0.4 | 0.9abc ± 0.5 | 4.1a ± 1.5 | 1.1a ± 1.7 | 1.5abc ± 2.3 | 923.8ab ± 93.0 | 0.4a ± 0.1 | 7.6c ± 1.8 | 0.2cd ± 0.1 | 44.3a ± 8.7 |
| Oak, toasted | 0.3a ± 0.0 | 1.0abc ± 0.1 | 5.3a ± 1.3 | 0.7a ± 0.1 | 0.5ab ± 0.1 | 1161.8ab ± 196.8 | 2.6ab ± 0.7 | 12.1d ± 2.6 | 0.2bc ± 0.0 | 99.4b ± 6.8 |
| Oak, untoasted | 0.4a ± 0.0 | 0.7ab ± 0.1 | 3.5a ± 0.6 | 0.3a ± 0.0 | 0.2a ± 0.0 | 752.9a ± 81.5 | n.d. (a) | 6.9c ± 1.2 | 0.1b ± 0.0 | 16.5a ± 2.3 |
| Common hazel, toasted | 0.9b ± 0.1 | 1.0bc ± 0.1 | 10.4bcd ± 1.9 | 1.7a ± 0.3 | 3.3d ± 0.7 | 1297.6abc ± 381.0 | 25.7e ± 7.2 | 1.3a ± 0.2 | 0.1b ± 0.0 | 355.8d ± 70.8 |
| Common hazel, untoasted | 0.4a ± 0.0 | 0.7ab ± 0.1 | 3.5a ± 0.4 | 0.2a ± 0.0 | 0.3ab ± 0.0 | 996.0ab ± 80.5 | 1.1ab ± 0.3 | 0.6a ± 0.1 | 0.3de ± 0.1 | 9.8a ± 2.3 |
| Robinia, toasted | 0.4a ± 0.0 | 0.8abc ± 0.1 | 7.4abc ± 0.9 | 0.3a ± 0.1 | 0.5ab ± 0.2 | 1632.0bcd ± 212.9 | 8.0bcd ± 1.6 | 0.8a ± 0.2 | 0.2bc ± 0.0 | 36.9a ± 9.8 |
| Robinia, untoasted | 0.4a ± 0.1 | 0.9abc ± 0.2 | 6.6abc ± 1.4 | 0.2a ± 0.0 | 0.3ab ± 0.0 | 1532.6bcd ± 232.2 | 5.9abc ± 1.3 | 0.6a ± 0.1 | 0.1b ± 0.0 | 10.5a ± 1.9 |
| Blackcurrant, toasted | 0.9b ± 0.1 | 1.9d ± 0.3 | 18.0f ± 4.9 | 1.0a ± 0.1 | 7.9e ± 1.6 | 1894.0cde ± 517.7 | 20.9e ± 5.4 | 1.5a ± 0.2 | 0.1b ± 0.0 | 160.3c ± 26.6 |
| Blackcurrant, untoasted | 0.5a ± 0.1 | 0.8abc ± 0.1 | 3.2a ± 0.5 | 0.1a ± 0.0 | 0.2a ± 0.0 | 1374.7abc ± 217.4 | n.d. (a) | 0.6a ± 0.1 | n.d. (a) | 11.2a ± 1.2 |
| Vineyard peach, toasted | 0.4a ± 0.0 | 1.3c ± 0.2 | 11.2cd ± 2.7 | 0.3a ± 0.1 | 3.2cd ± 0.8 | 1392.2abc ± 400.5 | 6.9bcd ± 1.9 | 1.1a ± 0.2 | n.d. (a) | 51.5ab ± 10.6 |
| Vineyard peach, untoasted | 0.5a ± 0.1 | 1.7d ± 0.3 | 17.2ef ± 3.4 | 0.2a ± 0.0 | 0.3ab ± 0.1 | 2338.4e ± 388.9 | 12.8d ± 3.0 | 1.8a ± 0.5 | 0.1b ± 0.0 | 12.9a ± 0.6 |
| Grapevine, toasted | 0.9b ± 0.2 | 1.1bc ± 0.2 | 5.8ab ± 1.1 | 0.4a ± 0.1 | 2.1bcd ± 0.6 | 1277.5abc ± 287.8 | 4.5abc ± 1.0 | 0.8a ± 0.2 | n.d. (a) | 49.9ab ± 4.8 |
| Grapevine, untoasted | 0.5a ± 0.0 | 1.2bc ± 0.1 | 10.2bcd ± 1.8 | 0.2a ± 0.0 | 0.2a ± 0.0 | 2158.8de ± 448.0 | 10.5cd ± 1.9 | 0.6a ± 0.1 | 0.1b ± 0.0 | 12.3a ± 1.6 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviations: n.d. = not detectable; n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
| (μg/L) | cis-Isoeugenol | trans-Isoeugenol | Ethylvanillin | Acetovanillone | Methyl vanillate | 4-Hydroxy-3-methoxyphenylacetone | Ethyl vanillate | 4-Allyl-2,6-dimethoxyphenol | Coniferyl aldehyde | Sinapinaldehyde | Σ Volatile phenols |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wood species | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗ | — |
| Toasting | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. | n.s. | — |
| Wood species × toasting | ∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | n.s. | — |
| Base wine | n.d. (a) | 0.4a ± 0.0 | 0.9a ± 0.2 | 4.6a ± 0.4 | 3.6a ± 0.2 | 7.0a ± 0.6 | 0.5a ± 0.1 | 0.3a ± 0.1 | 3.5ab ± 0.3 | 1.3abc ± 0.2 | 845.7a ± 150.5 |
| Sweet chestnut, toasted | 0.2cd ± 0.1 | 1.8f ± 0.2 | 10.7e ± 0.6 | 46.5f ± 5.3 | 9.8ef ± 0.9 | 39.6g ± 5.6 | 85.4f ± 12.4 | 7.1j ± 0.8 | 3.5 ab ± 0.4 | 1.5abc ± 0.3 | 2901.9g ± 326.8 |
| Sweet chestnut, untoasted | n.d. (a) | 1.1cde ± 0.2 | 5.3d ± 1.2 | 6.1ab ± 0.6 | 5.2ab ± 1.2 | 11.1ab ± 2.8 | 0.9a ± 0.1 | 2.8hi ± 0.5 | 4.5b ± 0.8 | 1.7bc ± 0.1 | 1023.3ab ± 111.0 |
| Oak, toasted | n.d. (a) | 1.1cde ± 0.3 | 4.2cd ± 0.7 | 9.6abc ± 0.3 | 6.5bcd ± 0.4 | 17.7bcd ± 4.5 | 7.1abc ± 1.7 | 2.3fgh ± 0.1 | 3.5ab ± 0.3 | 1.3abc ± 0.2 | 1337.1abc ± 209.1 |
| Oak, untoasted | n.d. (a) | 0.9cde ± 0.1 | 3.9c ± 0.8 | 5.8ab ± 1.0 | 3.7a ± 0.6 | 7.0a ± 1.3 | 2.4ab ± 0.6 | 1.0bcd ± 0.2 | 3.8ab ± 0.8 | 1.2ab ± 0.2 | 811.1a ± 86.6 |
| Common hazel, toasted | 0.1bc ± 0.0 | 1.3e ± 0.4 | 2.4b ± 0.5 | 24.0e ± 4.5 | 11.6f ± 2.9 | 28.9ef ± 6.7 | 33.5e ± 8.5 | 2.5gh ± 0.4 | 4.4b ± 1.2 | 1.8c ± 0.1 | 1808.4def ± 481.3 |
| Common hazel, untoasted | n.d. (a) | 0.7abc ± 0.1 | 1.3ab ± 0.2 | 6.2ab ± 0.7 | 5.9abc ± 0.3 | 12.8abc ± 3.2 | 1.0a ± 0.2 | 0.8abc ± 0.2 | 3.3ab ± 0.6 | 1.7abc ± 0.2 | 1046.6ab ± 86.8 |
| Robinia, toasted | 0.2bc ± 0.0 | 1.0cde ± 0.1 | 3.6c ± 0.6 | 10.8bcd ± 0.2 | 8.2cde ± 0.5 | 20.4cd ± 0.8 | 9.2abc ± 1.5 | 1.7def ± 0.2 | 3.5ab ± 0.3 | 1.4abc ± 0.1 | 1747.2def ± 205.8 |
| Robinia, untoasted | n.d. (a) | 0.6ab ± 0.1 | 1.2ab ± 0.2 | 11.1bcd ± 2.0 | 8.3cde ± 1.8 | 21.9de ± 4.5 | 12.6bc ± 2.6 | 2.0efg ± 0.4 | 3.1ab ± 0.7 | 1.5abc ± 0.3 | 1620.4cde ± 246.3 |
| Blackcurrant, toasted | 0.2d ± 0.1 | 2.4g ± 0.3 | 2.4b ± 0.7 | 15.1d ± 2.9 | 8.6cde ± 1.5 | 31.3f ± 5.7 | 24.6d ± 7.0 | 1.6def ± 0.4 | 3.3ab ± 0.4 | 1.5abc ± 0.4 | 2197.5def ± 526.3 |
| Blackcurrant, untoasted | n.d. (a) | 1.2de ± 0.3 | 1.9ab ± 0.3 | 8.3abc ± 2.3 | 5.9abc ± 1.4 | 16.2bcd ± 3.4 | 1.7a ± 0.2 | 0.7ab ± 0.1 | 5.8c ± 1.3 | 1.2a ± 0.2 | 1434.1abc ± 216.7 |
| Vineyard peach, toasted | 0.1b ± 0.0 | 1.1cde ± 0.1 | 2.0ab ± 0.4 | 10.0bcd ± 0.8 | 8.0cde ± 0.7 | 17.9bcd ± 1.4 | 8.9abc ± 2.1 | 1.2cde ± 0.3 | 3.4ab ± 0.1 | 1.5abc ± 0.2 | 1522.1bcd ± 401.5 |
| Vineyard peach, untoasted | n.d. (a) | 0.8bcd ± 0.2 | 1.2ab ± 0.3 | 12.2cd ± 1.0 | 9.4def ± 1.3 | 19.7cd ± 3.0 | 5.8abc ± 1.1 | 3.5i ± 0.6 | 3.3ab ± 0.3 | 1.3ab ± 0.1 | 2443.4fg ± 393.5 |
| Grapevine, toasted | 0.1bc ± 0.0 | 0.9cde ± 0.1 | 1.9ab ± 0.5 | 10.9bcd ± 2.7 | 8.0cde ± 1.9 | 19.0bcd ± 1.7 | 10.8abc ± 2.2 | 1.0bcd ± 0.3 | 3.6ab ± 0.2 | 1.3abc ± 0.2 | 1400.4abc ± 299.8 |
| Grapevine, untoasted | 0.1b ± 0.0 | 0.8bcd ± 0.1 | 0.7a ± 0.1 | 11.9cd ± 1.1 | 9.7ef ± 0.6 | 23.9def ± 1.7 | 13.4c ± 2.9 | 1.6def ± 0.2 | 2.4a ± 0.3 | 1.4abc ± 0.1 | 2260.5efg ± 453.3 |
- Note: The top three rows present the results of the two-way analysis of variance, which examined the effects of wood species, toasting and their interaction. Below, the quantified levels of each compound (mean ± standard deviation) for the base wine and the variants are shown. Significant differences are indicated by different letters.
- Abbreviations: n.d. = not detectable; n.s. = not significant.
- ∗∗p < 0.01.
- ∗∗∗p < 0.001.
Guaiacol concentrations were highest in the toasted blackcurrant variant, followed by toasted chestnut and toasted hazelnut. All wood types exhibited a clear increase in guaiacol levels following toasting – a result attributable to the thermal degradation of lignin during the toasting process [68, 69]. Guaiacol is well known for its association with smoky and spicy aromas and serves as an important marker of toasting influence [44, 75].
Interestingly, the highest eugenol content was found in untoasted chestnut, reflecting the species-specific chemical composition of the wood. Although toasting did not significantly impact eugenol levels, these results reaffirm the importance of botanical origin as the primary determinant of this compound’s presence [76]. Eugenol contributes subtle spice notes reminiscent of clove and cinnamon, adding to the complexity of white wines [77, 78].
Vanillin concentrations were the highest in toasted chestnut, exceeding those in all other wood treatments, including oak. This pronounced release is attributable to the high content of lignin- and hemicellulose-based precursors present in chestnut wood [19, 55]. Other toasted variants, such as blackcurrant and vineyard peach, also displayed elevated vanillin levels, highlighting the significant role of toasting in aroma development.
Comparative analysis with oak demonstrated that alternative wood species only partially mirrored oak’s volatile phenol profile. For guaiacol, toasted oak aligned closely with toasted vine, while toasted chestnut and toasted blackcurrant produced substantially higher concentrations, rendering them less typical of classic oak ageing [28]. As expected, oak (toasted) exhibited the highest levels of eugenol, confirming its well-documented richness in this aromatic compound [77]. Untoasted chestnut approached oak’s eugenol levels, while other woods displayed lower contents.
Regarding vanillin, toasted chestnut even surpassed oak in concentration. This observation aligns with previous studies reporting higher vanillin levels in chestnut wood compared to oak [55, 79]. Given vanillin’s strong association with vanilla and sweet notes, this compound can become a dominant aromatic contributor when using alternative woods such as chestnut.
In summary, the analysis demonstrates that toasting has a clear impact on the release of volatile phenols, while wood species and botanical composition remain the primary controlling factors. Oak continues to serve as the benchmark for its balanced contribution of guaiacol, eugenol and vanillin. However, alternative woods such as chestnut or blackcurrant provide distinct aromatic signatures that can significantly differentiate the aroma profile of white wines and be employed strategically to achieve specific stylistic objectives [12, 80].
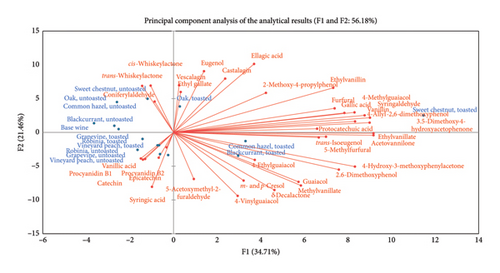
3.7. PCA
To provide a clear representation of the results, a PCA was conducted for the analytical data. The Kaiser–Meyer–Olkin (KMO) criterion for the analytical results was 56.18%, justifying the application of a PCA [37]. In addition to the alternative wood types, oak (untoasted) and oak (toasted) were included. In the PCA biplot in Figure 1, the upper left quadrant shows a cluster of sweet chestnut (untoasted), oak (untoasted), common hazel (untoasted), and blackcurrant (untoasted). There are positive correlations with substances such as cis- and trans-whiskeylactone, and coniferyl aldehyde. In the lower left quadrant, grapevine (toasted), Robinia (toasted), Robinia (untoasted), and vineyard peach (toasted and untoasted) cluster together, correlating with parameters such as vanillic acid, catechin, procyanidin B1 and B2, syringic acid, and epicatechin. The upper right quadrant includes oak (toasted) and sweet chestnut (toasted), with considerable dispersion between them. Oak (toasted) shows positive correlations with vescalagin, castalagin, and eugenol, while sweet chestnut (toasted) correlates positively with substances such as furfural, vanillin, 4-methylguaiacol, and gallic acid. Finally, in the lower right quadrant, common hazel (toasted) and Blackcurrant (toasted) cluster together, showing positive correlations with parameters such as 4-ethylguaiacol, guaiacol, and 4-vinylguaiacol.
3.8. Heatmap of the Raw Analytical Data
Hierarchical cluster and correlation matrix analyses were conducted to explore the relationships between the individual variants and their analytical profiles (Figure 2). The results demonstrated that both the type of wood and the toasting process significantly influenced the expression of certain non-volatile and volatile compounds, thereby playing a crucial role in the clustering of selected wood species. This was statistically supported by a two-way ANOVA.
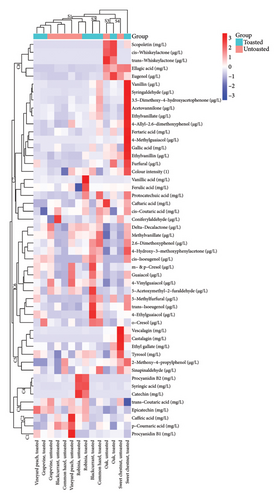
Wood species cluster S1 includes vineyard peach (toasted and untoasted), Robinia (toasted and untoasted), and grapevine (toasted and untoasted), as well as blackcurrant (untoasted) and common hazel (untoasted). These variants showed partially higher concentrations of certain non-volatile phenols. Robinia (toasted and untoasted) exhibited higher procyanidin B2, syringic acid, and catechin levels. Wood species cluster S2 includes blackcurrant (toasted) and common hazel (toasted), which primarily differ from the other wood species clusters in terms of carbonyl compounds and volatile phenols. Wood species cluster S3 includes oak (toasted and untoasted). This wood species cluster is characterised by compounds typical of oak wood, such as ellagic acid, eugenol, and cis- and trans-whiskeylactone. Wood species cluster S4 consists exclusively of sweet chestnut (untoasted). Particularly interesting is that sweet chestnut (toasted) forms a completely distinct and separate wood species cluster, mainly due to its comparatively extreme concentrations of compounds such as vanillin, syringaldehyde, acetovanillone, and ethylvanillin.
In the chemical cluster analysis, the investigated compounds were divided into eight main chemical clusters (C1–C8), with compounds of the same chemical group or similar structure tending to group within the same or nearby chemical clusters. Chemical cluster C1 contains only procyanidin B1. Chemical cluster C2 includes p-coumaric acid and caffeic acid. Chemical cluster C3 consists of trans-coutaric acid and epicatechin, while chemical cluster C4 comprises catechin, syringic acid, and procyanidin B2. Chemical cluster C5 includes tyrosol, ethyl gallate, vescalagin, castalagin, 2-methoxy-4-propylphenol, and sinapaldehyde. Chemical cluster C6 presents 5-methylfurfural, o-cresol, m- and p-cresol, guaiacol, 4-ethylguaiacol, 5-acetoxymethyl-2-furaldehyde, 4-vinylguaiacol, 2,6-dimethoxyphenol, cis-isoeugenol, trans-isoeugenol, δ-decalactone, methyl vanillate, and 4-hydroxy-3-methoxy-phenylacetone. Chemical cluster C7 encompasses gallic acid, protocatechuic acid, caftaric acid, cis-coutaric acid, vanillic acid, fertaric acid, ferulic acid, furfural, 4-methylguaiacol, vanillin, ethylvanillin, acetovanillone, ethyl vanillate, 4-allyl-2,6-dimethoxyphenol, syringaldehyde, 3,5-dimethoxy-4-hydroxyacetophenone, and coniferyl aldehyde. Finally, chemical cluster C8 includes ellagic acid, eugenol, and cis- and trans-whiskeylactone.
It should be noted that trace amounts of scopoletin (below 0.01 mg/L), falling below the quantification limit, were detected in oak (toasted and untoasted) and sweet chestnut (toasted). Because Figure 2 presents a heatmap of the raw data, this parameter was not excluded.
4. Conclusions
At present, only oak and sweet chestnut are approved by the OIV for oenological applications, with wood chips exclusively permitted from species of the genus Quercus. Based on the comprehensive analysis of the different parameters, sweet chestnut (toasted) emerged as the most suitable candidate for approval as use wood chips in oenology due to its close alignment with oak across several critical factors, including ellagitannins, carbonyl compounds, and volatile phenols. Specifically, its similarity to oak (toasted) regarding the furfural, 5-methylfurfural, vescalagin, and castalagin levels, combined with the moderate alignment in the gallic acid and catechin levels, makes it a strong alternative to oak. Other variants, such as blackcurrant (toasted), aligned with oak in specific compounds such syringaldehyde, but there was a lack consistency across the broader range of parameters. Therefore, sweet chestnut (toasted) is recommended as the best alternative to oak for use in oenology, offering similar chemical profiles. However, it is important to note that the approval of this alternative wood type for use as chips in wine production requires prior sensory analyses to ensure its suitability.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Nikolaus Schlögl: supervision, validation, data curation and writing – original draft preparation; Susanne Riepl: methodology and investigation; Mario Strauss: methodology and investigation; Harald Scheiblhofer: conceptualisation, methodology and supervision; Reinhard Eder: resources and writing – review and editing; Karin Korntheuer: investigation and formal analysis; Sezer Sari: investigation and formal analysis; Christian Philipp: conceptualisation, methodology, supervision, resources, writing – review and editing and correspondence.
Funding
No funding was received for this research.
Acknowledgements
The authors thank Walter Brandes, MA, and the team for their support during optimisation of the analysis and method.
Supporting Information
Figure S1: Experimental workflow illustrating the preparation of base wine with wood chips, a 98-day contact period, and subsequent chemical analysis via GC/MS and HPLC; Table S1: Information concerning calibration and validation of the volatile compound.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting material of this article.




