Grapevine Berry Growth and Sugar Loading During Ripening Are Differentially Impacted by Environment X Management Interactions
Abstract
Background and Aims: Grapevine berry ripening is driven by water and sugar loading from the phloem, beginning with berry softening and ending at physiological maturity. This study aimed to characterise the kinetics of sugar and water accumulation at the berry population level under different climatic, vigour and crop management conditions.
Methods and Results: The experiments were conducted in a commercial vineyard of the ‘Tannat’ cultivar in Uruguay, for 8 years. Two zones with high (H) and low (L) vigour were delimited. In two contrasted rainy and dry years, specific treatments (nitrogen, water availability and leaf/fruit ratio) were carried out. The sugar and water accumulation kinetics were studied on populations of berries, which were both synchronised from the onset of ripening to maximum sugar loading and normalised by their maximum volume. A bilinear model between berry growth and sugar accumulation was fitted, indicating an apparent uncoupling between water and sugar loading. Wet and high-vigour conditions accentuated this apparent uncoupling compared to dry and low-vigour conditions, with maximum berry growth reached earlier than the maximum sugar content. In addition, management practices favouring plant water and carbon status positively influenced the duration of ripening (up or down).
Conclusions and Significance: This study provides a deeper understanding of how management practices can be harnessed to improve grape production. Water management was a key lever to counteract the climatic and/or vigour impact both on the apparent uncoupling between berry growth and sugar loading at the population level and the asynchrony of berry development within the population.
1. Introduction
Berry growth follows a sigmoidal curve pattern divided into three stages: a first stage of rapid green growth, a lag phase and a final ripening phase [1–3]. Ripening begins with berry softening (also called veraison), and it is marked by chlorophyll degradation [4, 5], sugar and water accumulations, organic acid degradation and anthocyanin synthesis [6, 7]. The potential for berry growth and composition varies among genotypes [8]. Within a given genotype, the dynamics of primary and secondary metabolite accumulation are influenced by environmental conditions and cultural practices [9, 10]. Vine vigour is a key factor affecting berry development and biochemical composition, primarily through its influence on the leaf:fruit ratio, which determines carbohydrate availability and the berry microclimate. High-vigour vines typically produce larger berries [11], lower sugar concentration and higher pH compared to less vigorous vines [12]. In contrast, low-vigour vines lead to smaller berries, lower yields and greater cluster exposure to solar radiation. Conversely, a few authors have reported negative correlations between increased vigour and phenolic compound accumulation [11]. Water availability (WA) also plays a critical role in berry development and grape composition. Notably, moderate to severe water deficits imposed from flowering to harvest have been shown to reduce berry size by limiting cell expansion during the early stages of berry development, as well as to decrease at physiological maturity both the total sugar content per berry and, under severe stress, the berry sugar concentration [13, 14]. Nitrogen availability generally has a limited effect on primary berry metabolism [15], despite its role in promoting vine vigour [16]. However, nitrogen has been shown to influence secondary metabolism, particularly by enhancing anthocyanin metabolism [17].
Berry water dynamics largely determine final berry size, as water constitutes 75%–85% of berry weight at harvest [18]. Water flow also drives sugar import in the berry [19]. Water enters the berry predominantly via xylem during the green stage and via phloem during ripening [20]. From the onset of ripening onwards, the xylem remains functional, intact and unobstructed [21, 22]. Although controversial, some studies suggest that xylem backflow to the plant may occur once phloem unloading ceases [23]. During the phloem unloading period, berry sugar accumulation is primarily sourced from leaf photosynthesis and, when photosynthetic activity is insufficient, from carbohydrate reserves stored in perennial organs [24]. Sugar concentration in the berry increases proportionally with berry growth [25], reaching at physiological maturity 1.1 M with a glucose/fructose ratio of 1 [26]. Once this threshold is reached, both sugar loading and berry expansion cease. Sugar concentration generally increases after this stage due to berry water loss mainly through cuticular conductance, as stomata become occluded with wax during the early stages of berry development [27, 28].
The ripening process is individually regulated in each berry and does not occur simultaneously across all berries within a cluster [6]. Berry development within a bunch has been reported to be highly asynchronous [29, 30]. Due to the asynchrony, berry populations require 40–50 days to reach a hexose concentration of 1.1 M, whereas an individual berry reaches this threshold in approximately 26 days [4, 31, 32]. Additionally, because of variability in berry development, sugar concentration is only weakly correlated with berry weight [32]. One approach to synchronising berry development is to sort individual berries by sugar content and normalise their size relative to the maximum reached at the cessation of phloem unloading [32]. Although this method is highly relevant for physiological studies, it is too labour-intensive for large-scale field trials. Field trials typically rely on sampling berry populations without accounting for interberry heterogeneity and asynchrony. However, similar to individual berries, berry populations can be synchronised and homogenised. Based on commonly used field refractometer measurements, the baseline soluble solid concentration at softening is approximately 6°Brix [33, 34]. The sugar-loading plateau reached in individual berries at maximum volume and 1.1 M hexoses [32, 35, 36] corresponds to ca. 18°–20° Brix at the population level. Synchronising the developmental stages of the average berry within the range of 6° to 20° Brix and normalising average berry weight based on the maximum weight reached at 20° Brix can improve our understanding of the effects of abiotic constraints and vineyard management on the berry development kinetics at the population level.
This study aimed first to characterise the kinetics of sugar and water accumulation in berry populations under different climatic conditions, vine vigour levels and crop management practices (nitrogen supply, WA and leaf/fruit ratio). The developmental stage of berry population samples was synchronised based on their Brix (6–20°Brix), and their volume was normalised to their maximum to eliminate scaling effects in the analysis of sugar and water accumulation. Subsequently, the influence of weather conditions and plant physiological variables on the parameters governing water and sugar accumulation kinetics was examined. Finally, the effect of crop management treatments on the variability of berry sugar content and berry weight within the berry population was assessed.
2. Materials and Methods
2.1. Study Site and Experiments
The experiments were carried out in a commercial vineyard in Canelones, Uruguay (34° 36 S, 56° 14 W). The vineyard consisted of 1.1 ha of Vitis vinifera L. cv. Tannat, grafted on SO4 rootstock and planted in 1998. The site was located on a gradual north–south slope of 1%–2%. Vines within rows were oriented north–south, with vine density of 3333 plant ha−1, spaced 2.5 m between rows and 1.2 m between vines within rows. Vines were pruned using a double guyot system (12 buds per plant), and shoots were trained to a vertical shoot position (VSP) system. The vineyard exhibited high spatial variability in vine vigour, particularly from east to west. Ferrer et al. [37] identified three distinct vigour zones within the vineyard, classified as high (H), medium (M) and low (L) vigour (Figure S1). These zones were delineated based on aerial surveys conducted at veraison (January in the Southern Hemisphere) over three consecutive years (2015, 2016 and 2017). High-resolution multispectral images (0.2-m ground resolution) were acquired from aircraft flights at an altitude of 620 m, and vine vigour was assessed using the Normalised Difference Vegetation Index (NDVI). NDVI values ranged from 0.57 to 0.61 for H, 0.55 to 0.57 for M and 0.48 to 0.55 for L (Figure S1). These classifications remained consistent over the years, with the same vineyard areas, showing stable three classes of NDVI were consistently located in the same parts of the vineyard every year [16]. Management practices (hereafter referred to as the ‘control treatment’) were uniform across the entire vineyard, regardless of vigour zone. The vineyard was not irrigated. Mineral nutrition was provided through soil application of urea (46%N) at preflowering and postharvest, at a rate of 140 kg ha−1.
The first experiment aimed to assess the effect of vigour differences (H vs. L) under contrasting climatic conditions in terms of WA (rainy vs. dry years). This experiment was conducted over eight consecutive years (2014–2021). For this purpose, only the most contrasting vigour zones of the vineyard were selected. Control treatments (H and L) were arranged in a random block design with three replicates and 21 plants per block, i.e., 63 plants per vigour condition (Figure S1).
The second experiment, conducted in two climatically contrasted years (2019 and 2020), evaluated the impact of different water and nitrogen availability treatments and canopy management strategies on berry growth and composition. These treatments were applied within each vigour zone (H and L) and compared to the corresponding control treatments (described above). In total, 63 plants per treatment were selected, distributed across three blocks (21 plants per block, Figure S1). In H zones, three treatments were applied:reduced water supply (H-W), reduced nitrogen supply (H-N) and leaf removal to improve bunch zone microclimate (H-L). Specifically, the reduced water supply treatment (H-W) was implemented from the onset of ripening (later on called veraison) to harvest by covering the soil with polyethylene (white surface on both sides, 220 μm thick, with ultraviolet protection, Tashiro Takata, Uruguay). The nitrogen restriction treatment (H-N) received no nitrogen fertilisation (0 N units in the season). The leaf removal treatment (H-L) involved removing approximately 60% of the leaves in the bunch zone before flowering. In L zones, treatments aimed to increase water (L + W) and nitrogen supply (L + N). The supplemental nitrogen treatment (L + N) received 210-kg urea per hectare, with 70 kg applied before flowering in addition to the 140 kg mentioned above. The additional irrigation treatment (L + W) aimed to maintain the values of leaf predawn water potential (Ψp) above −0.35 MPa throughout the whole seasons, corresponding to a mild water deficit [38]. For more detailed information, see Pereyra et al. [16].
2.2. Weather and Microclimate Measurements
Rainfall was recorded in the vineyard. The reference evapotranspiration (ETo) was obtained from a weather station owned by INIA (National Institute of Agricultural Research; 34°40′S, 56°20′W), located 10 km from the experimental site, and operated in compliance with World Meteorological Organization (WMO) standards (Table S1). WA was calculated as the sum of rainfall and supplemental irrigation for the L + W treatment. For the H-W treatment, it was assumed that rainfall did not infiltrate the soil from veraison to harvest. The vapour pressure deficit (VPD) was calculated following FAO guidelines, using microclimatic temperature and relative humidity data. The microclimatic conditions, including minimal (Tmin) and maximum temperature (Tmax) temperatures, were recorded hourly using HOBO sensors (HOBO U23 ProV2 loggers, USA). Three sensors were placed in the bunch zone of three plants per treatment from flowering to harvest (2014–2021). Mean temperature (Tm) was calculated based on Tmin and Tmax. The mean values of the VDP, Tm, cumulative rainfall (RR) and WA were calculated by dividing the growing season into three distinct phases: from bud break to bloom (BB_BL; 1st September to 15th November), from bloom to the onset of ripening (or veraison) (BL_V; 15th November to 15th January) and from the onset of ripening (or veraison) to harvest (V_H; 15th January to 15th March).
2.3. Plant Measurements
2.3.1. Nitrogen, Carbon and Water Status
The SPAD index, a measure of relative chlorophyll content, can serve as a proxy for nitrogen content. SPAD measurements (MC-100, Apogee Instruments Inc. Logan, UT, USA) were taken weekly from flowering to harvest on ten sun-exposed and fully expanded leaves, selected from ten plants per block (30 leaves per treatment).
During the winter of the two contrasting meteorological years (August 2019 and 2020), trunk diameter (TD) was evaluated using a digital calliper (Neiko 01407 ± 0.2 mm) at 10 cm above the grafting point. Wood samples were collected from the trunks of four plants per block (12 plants per treatment). Samples were extracted from 20 cm above the grafting point using a 4.0-mm drill bit to a depth of approximately half the TD. The samples were dried for 48 h at 60°C and then ground through a mesh smaller than 0.1 mm. A 20-mg trunk subsample was used for starch extraction (starch in trunk, ST). The extraction was carried out with an ethanol solution (80% v/v). The starch content was subsequently quantified enzymatically using spectrophotometry at 340 nm.
Leaf photosynthetic activity (Photo) was recorded during the 2019 and 2020 growing seasons using an infrared gas analyser (Licor-6400, LI-COR Biosciences, Inc., Lincoln Nebraska). Measurements were taken between 9:00 AM and 11:00 AM on nine healthy, sun-exposed, mature leaves per treatment, at four different phenological stages: flowering, bunch closure, onset of ripening and preharvest. The photosynthetically active radiation within the Licor chamber was set at 950 μmol photons m−2 s−1. From flowering to harvest, leaf predawn water potential (Ψp) was measured using a pressure chamber (SoilMoisture equipment, Santa Barbara, CA, USA). Nine healthy, fully expanded leaves were collected from each treatment (three leaves per block). Measurements were conducted between 2:00 AM and 4:00 AM immediately after detaching the leaf from the plant. A threshold of −0.35 MPa was used to indicate mild water deficit conditions [38]. A water stress index (WSI) was calculated as the sum of all water potential values recorded throughout the seasons [39]. WSI is expressed in absolute values (MPa day−1).
2.3.2. Vegetative Growth, Yield and Berry Sample
Exposed leaf area (ELA, in m2/ha) was assessed annually from 2014 to 2021 at the onset of ripening on nine plants per treatment, following the method described by OIV [40]. Canopy width and height were measured to calculate ELA. At harvest, yield per plant (Y, kg plant−1) was determined for 63 plants per treatment (21 plants for each block). The leaf-to-fruit ratio was then calculated as the ratio of ELA to Y.
Berry sampling was carried out weekly after berry softening to determine the evolution of berry weight and composition at the population level. For each treatment, 300 berries (100 berries per block) were sampled, with 10 bunches per replicate (one bunch per plant). Berries were collected from the central zone of bunch (excluding wings and shoulders) on 21 plants per block. The sample was then crushed using a juicer (Phillips HR2290, Phillips, Netherlands). Sugar content (total soluble solids, TSS, g L−1) was estimated from Brix values determined with a refractometer (Atago, Japan), using the equation: TSS (g L−1) = °Brix × 0.59 × 17 [41]. Additionally, the TSS per berry (TSS, mg berry−1) was determined at each sampling date.
Final bunch harvest was scheduled when berry pH reached 3.3 to 3.4, a threshold chosen to ensure the stability of must and wine quality during vinification. In years when these pH values were not attained, harvest was advanced to avoid excessive berry dehydration and weight loss while still maintaining suitable conditions for winemaking.
2.4. Data Analysis
Berry development at the population level was analysed within the 6–20°Brix range. The lower limit (6°Brix) was assumed to correspond to the onset of sugar loading, coinciding with berry softening, while the upper limit was considered to represent the cessation of phloem-mediated water and sugar transport (1.1 M, equivalent to 18–20°Brix) [26]. To track sugar accumulation dynamics, Brix values outside this range were excluded from the analysis, as values above 20°Brix primarily reflect concentration effects due to berry dehydration rather than additional sugar loading. On each sampling date, berry volume was estimated as the ratio of berry weight to berry density (D, g L−1). Berry density was determined following the methodology described by Vila et al. [42]. The normalised berry volume (NBvn) was calculated as the ratio between berry volume at each sampling date (Bvn) and the maximum volume reached at the end of berry development (Bvmax). This calculation was performed for each block in each evaluated year.
Statistical analyses were conducted using Origin PRO 9.1 (Origin Lab Corporation, Northampton, MA, USA) and R (2023). A bilinear model was applied to each block to describe the relationship between NBv and TSS concentration (g L−1). Five parameters were derived from this model: the slope, break point (BP), duration of Phase I, duration of Phase II and total duration of both phases (TDP). Phase I corresponded to the period of simultaneous sugar and water accumulation, during which berry volume increased. The slope corresponded to the rate of growth to sugar concentration during this first phase, and BP to the TSS value reached at the end of the phase. Phase II represented the period of sugar accumulation at a constant volume, with no further water uptake. The model intercept was also estimated from the linear equation fitted for Phase I. Then, two-way analysis of variance (ANOVA) was performed, followed by Fisher’s test for mean comparisons. The effect of vigour differences (H vs. L) on grape ripening dynamics was evaluated over the 2014–2021 period. To this end, relationships between meteorological variables (WA BB_Bl, WA Bl_V, WA V_H, Tm Bl_V, Tm V_H, VPD Bl_V and VPD V_H), plant/berry variables (WSI_Bl_V, WSI_V_H, leaf/fruit ratio and Bw) and model parameters (slope, BP and TDP) were assessed using principal component analysis (PCA). Additionally, for the 2019 and 2020 seasons, the analysis incorporated the effects of different treatments (water, nitrogen and leaf area). Ultimately, Pearson’s correlation coefficients were then used to examine relationships between microclimatic variables (WA Bl_V, WA V_H, VPD BB_Bl, VPD Bl_V and VPD V_H), plant/berry variables (WSI Bl_V, WSI V_H, TD, ST, Photo and Bw) and the parameters of the bilinear model (slope, BP and TDP).
3. Results
3.1. Berry Development Response to Climate and Vigour Condition
The water supply showed strong interannual and intraseasonal variability (Figure 1). Based on total rainfall during the growing period (bud break to harvest), the years were classified into three groups. Group 1 (2014, 2015 and 2019) was characterised as wet, with total precipitation exceeding 550 mm. However, rainfall distribution differed within this group: in 2014%, 60% of the rainfall was concentrated between veraison and harvest (V_H), while in 2015, the majority occurred before flowering (BB_Bl), and in 2019, rain was more evenly distributed across the three phenological stages. Group 2 (2016, 2018 and 2020) included dry years, with total rainfall below 450 mm. Within this group, the highest rainfall occurred during the Bl_V period in 2016 (54%) and during the BB_Bl period in both 2018 (51%) and 2020 (49%). Group 3 (2017 and 2021) was classified as intermediate, with total rainfall ranging between 450 and 550 mm. In these years, rainfall was proportionally highest during the V_H period (37% in 2017 and 48% in 2021). However, given the relatively low annual total rainfall and the distribution pattern, 2021 could arguably be considered closer to a dry year than to an intermediate one.
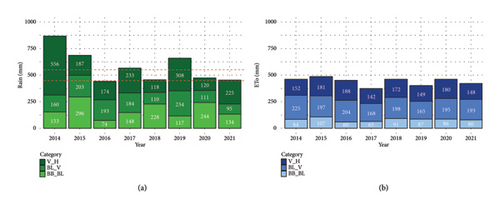
Additionally, the comparison of rainfall with the seasonal crop evapotranspiration (ETo) revealed that in most years, total rainfall did not fully cover the vineyard water demand, particularly in dry years such as 2016, 2018 and 2020. This mismatch suggests the vines likely experienced varying levels of water stress during the season, especially from fruit set to harvest (BL_V and V_H periods). The climatic balance (difference between the cumulated rainfall and ETo) provides a more accurate picture of the water status and stress conditions experienced by the vines throughout the different years (Table S1).
Yields varied between years and between vigour conditions (Table 1). The H zone consistently showed higher yields (ranging 6.7–8.3 kg plant−1) compared to the L zone (ranging from 3.9 to 6.8 kg plant−1) (p value < 0.05), except in 2014. In that year, yield in the H zone was reduced by the incidence of bunch rot, which predominantly affected high-vigour vines (data not shown). Over the eight seasons, mean leaf area per vine ranged from approximately 1.05 m2 in 2018 to 2.2 m2 in 2016. In both dry and intermediate seasons, high-vigour vines developed a significantly larger leaf area compared to low-vigour vines (p ≤ 0.05). Rainy years were characterised by larger berry weight (Bw) and higher sugar content per berry (mean Bw: 1.56 g; TSS: 310 mg berry−1), followed by the intermediate years (Bw: 1.38 g; TSS per berry: 251 mg berry−1) and finally, the dry years, which showed the lowest values (Bw: 1.16 g; TSS per berry: 244 mg berry−1). Bw and TSS were generally higher in the H zone compared to L across all years, except for Bw during rainy years (2014 and 2015). Conversely, no significant differences were observed between vigour zones for Brix or sugar concentration (TSS, g L−1).
| Year | Vigour | Yield (kg plant−1) | Leaf area (m2 vine−1) | Bw (g) | TSS (g L−1) | TSS per berry (mg berry−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rainy years | ||||||||||
| 2014 | High | 6.70 | 1.5 | 1.62 | 190 | 301 | ∗ | |||
| Low | 5.70 | 1.2 | 1.50 | 180 | 255 | |||||
| 2015 | High | 8.30 | ∗ | 2.0 | 1.54 | ∗ | 223 | 343 | ∗ | |
| Low | 6.80 | 1.5 | 1.46 | 201 | 309 | |||||
| 2019 | High | 6.90 | ∗ | 1.9 | ∗ | 1.69 | ∗ | 221 | 348 | ∗ |
| Low | 5.30 | 1.1 | 1.59 | 211 | 306 | |||||
| Dry years | ||||||||||
| 2016 | High | 7.50 | ∗ | 2.3 | ∗ | 1.40 | ∗ | 209 | 266 | ∗ |
| Low | 5.80 | 2.1 | 1.08 | 201 | 208 | |||||
| 2018 | High | 7.00 | ∗ | 1.2 | ∗ | 1.24 | ∗ | 211 | 293 | ∗ |
| Low | 5.40 | 0.9 | 0.95 | 190 | 231 | |||||
| 2020 | High | 6.70 | ∗ | 1.5 | ∗ | 1.29 | ∗ | 227 | 279 | ∗ |
| Low | 4.70 | 1.3 | 1.02 | 203 | 189 | |||||
| Intermediate years | ||||||||||
| 2017 | High | 7.20 | ∗ | 1.8 | ∗ | 1.62 | ∗ | 225 | 323 | ∗ |
| Low | 5.40 | 1.2 | 1.25 | 201 | 239 | |||||
| 2021 | High | 7.10 | ∗ | 1.9 | ∗ | 1.45 | ∗ | 192 | 230 | ∗ |
| Low | 3.90 | 0.9 | 1.20 | 202 | 212 | |||||
| Significance | ||||||||||
| Rainy vs dry | † | ns | † | ns | † | |||||
| Rainy vs intermediate | ns | ns | † | ns | ns | |||||
| Dry vs intermediate | ns | ns | † | ns | ns | |||||
- Note: Bw: maximum berry weight; mean values. Yield (n = 63), Bw: berry weight (n = 3). TSS: total soluble solids (n = 3). The bold values are used to highlight the most relevant results, which correspond to those showing statistically significant differences.
- ∗Significantly differences between vigour conditions for each year at p ≤ 0.05, according to Fisher’s LSD test.
- †Statistical differences between year groups for each comparison at p ≤ 0.05, according to Fisher’s LSD test.
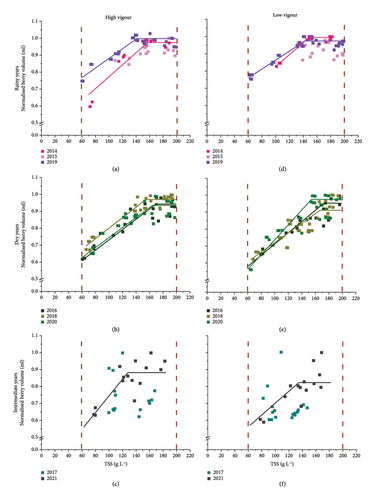
Each point represents a sample of 100 berries. The bilinear adjustment represents the average adjustment for each year and vigour condition (n = 3).
Changes in NBv as a function of sugar accumulation allowed to distinguish two clear phases in a bilinear model (Figure 2, Table 2). Phase I was characterised by an increase in sugar concentration and berry volume, while during the second stage, sugar content kept increasing at constant berry volume.
| Year | Vigour | Slope (mL/g L−1) | R2 | Breaking point | Phases duration (days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSS (g L−1) | I | II | TDP | ||||||||
| Rainy years | |||||||||||
| 2014 | High | 0.0034a | 0.82 | 158a | ∗ | 27a | 11b | 38 | |||
| Low | 0.0040a | 0.97 | 147a | 24a | 13b | 37 | |||||
| 2015 | High | n.d | n.d | n.d | n.d | n.d | n.d | ||||
| Low | n.d | n.d | n.d | n.d | n.d | n.d | |||||
| 2019 | High | 0.0029b | 0.92 | 140b | 21b | 27a | ∗ | 48 | |||
| Low | 0.0026b | 0.96 | 142b | 24b | 23a | 47 | |||||
| Dry years | |||||||||||
| 2016 | High | 0.0030 | 0.87 | 169a | 45a | ∗ | 10b | ∗ | 55a | ||
| Low | 0.0033 | 0.96 | 173a | 51a | 9b | 60a | |||||
| 2018 | High | 0.0031 | 0.95 | 155b | ∗ | 39b | ∗ | 13a | ∗ | 52a | |
| Low | 0.0028 | 0.83 | 168b | 42b | 16a | 58a | |||||
| 2020 | High | 0.0029 | ∗ | 0.91 | 165b | ∗ | 38a | 11ab | ∗ | 49b | |
| Low | 0.0041 | 0.97 | 157b | 38a | 9ab | 47b | |||||
| Significance | |||||||||||
| Rainy vs dry years | n.s | — | < 0.0001 | < 0.0001 | 0.0004 | 0.013 | |||||
- Note: Letters indicate differences between years of the same climate group (Fisher LSD test, p ≤ 0.05). Slope: growth/sugar concentration ratio. Phase I: accumulation of sugars simultaneously with the accumulation of sugars, from 60 g L−1TSS to BP. Phase II: sugar accumulation at constant volume, from BP to 200 g L−1 TSS. TDP: total duration phases, sugar accumulation over the range 60–200 g L−1TSS. R2: adjusted R-square. The bold values are used to highlight the most relevant results.
- ∗Indicates significant differences between vigour zones according to Fisher’s LSD test (p ≤ 0.05).
However, for the 2015 (rainy) and 2017 (intermediate) seasons, the bilinear fit was of poor quality. In 2015, the late onset of sampling likely failed to capture the initial phase of sugar accumulation, limiting the model’s performance. In 2017, the high variability was likely driven by uneven ripening within and between bunches, possibly caused by irregular flowering and fruit set under fluctuating environmental conditions. Consequently, the subsequent analysis focuses on comparing the most contrasting years (rainy vs. dry years).
Globally, all model parameters except the slope varied among the rainy and dry years (Table 2). The slope averaged about 0.0032 mL/g L−1. However, it should be noted that berry volume at 6°Brix (initial point of the slope) represented on average 75% of the maximum berry volume for rainy years vs 60% for dry years. This seasonal contrast was even more pronounced under low-vigour conditions. The BP at the end of berry volume increase (end of Phase I) was reached at lower TSS (146 mL/g L−1) under rainy years compared to dry years (164 mL/g L−1). Lastly, the TDP I and II (from 60 to 200 g L−1) was lower for rainy years (45 days) compared to dry years (56 days), mainly due to the lower duration of Phase I, as Phase II tended in contrast to be longer for rainy years.
The parameters of the model also differentially varied between H and L and between years, depending on the group of years. For the rainy years (Figures 2(a) and 2(d), Table 2, Table S2), there were no differences between the vigour zones for any of the parameters. However, differences were observed between the years in this group: 2014 had a higher slope, BP and longer duration of Phase I compared to 2019. In dry years (Figure 2(b), Table 2), the slope was higher for L than H in 2020. BP was also higher for L compared to H in 2018, while it was the opposite in 2020. The total duration of sugar accumulation did not differ between the two vigour zones, although the duration of each phase showed differences between the vigours. The H zone displayed a longer Phase I and shorter Phase II than the L zone in 2016 and 2018. In contrast, Phase II tended to be longer for the H zone in 2020. BP and durations of Phases I and II also varied among years in this group. BP was higher in 2016 compared to 2018 and 2020. TDP was higher in 2016 and 2020 compared to 2018, mainly due to a longer Phase I.
An exploratory analysis of the results (PCA) is presented in Figure 3(a). The two first axes of the PCA accounted for 63.9% of the variability (PC1: 41.3% and PC2: 22.6%). The scores of PC1 grouped the years in the same way as described above, forming three distinct clusters. Rainy years (2014, 2015 and 2019) were associated with high PC1 scores, while dry years (2016, 2018 and 2020) showed low PC1 scores. Intermediate years (2017 and 2021) were positioned between these two groups on PC1. The loadings indicated that rainy years were associated with high WA during fruit set to the onset of ripening, i.e., veraison (WA BL_V) and during ripening (WA V_H), high atmospheric demand from bud break to flowering (VPD BB_Bl) and high berry weight (Bw). Conversely, dry years were associated with higher mean temperature during ripening (Tm V_H), higher water deficit from fruit set to veraison (WSI BL_V) and during ripening (WSI V_H), higher VPD (VPD V_H), later BP and longer total degree-days period. On PC2, the loadings showed that WA from bud break to flowering (WA BB_Bl) was opposed to the leaf:fruit ratio. Years with higher preflowering WA (WA BB_Bl) had positive PC2 scores (2015, 2018, 2020 and 2021) and tended to display lower leaf:fruit ratios, while the years 2014, 2016, 2017 and 2019 showed the opposite trend with negative PC2 scores.
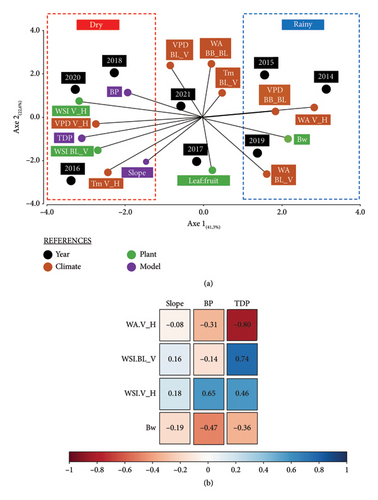
The relationships between model parameters (slope, BP and TDP) and weather, plant and berry variables were evaluated using Pearson’s correlation coefficient and the corresponding p values for all years at global level (Figure 3(b)). Only statistically significant correlations (p < 0.05) with an absolute r-value close to or exceeding 0.5 are reported. The slope showed only weak and nonsignificant correlations with all examined variables (|r| < 0.19, p value > 0.05). In contrast, the TDP I and II was significantly and negatively correlated with WA during ripening (WA V_H) and positively correlated with the WSI both from fruit set to veraison (WSI BL_V) and during ripening (WSI V_H). Likewise, the BP showed a positive correlation with WSI V_H and a negative correlation with maximum berry weight (Bw).
3.2. Berry Development Response to Nitrogen, Water and Leaf Removal Treatments
For two contrasting years in terms of water supply (rainy year: 2019/dry year: 2020), different water supply/restriction, nitrogen and leaf removal treatments were applied according to vigour conditions (Figure S1). As observed previously, yield was higher for the rainy year and high vigour (Table 3). Yield also varied among the treatments for each year and vigour conditions. In the H zone, leaf removal (H-L) had the lowest yield in both years, while it displayed higher berry weight. The H-N maintained yield in the rainy year but reduced yield in the dry year compared to H. Water restriction (H-W) did not change yield. The L + N treatment increased yield (+16%) compared to L in 2019. The treatment with additional water (L + W) applied during the dry year resulted in an 80% higher yield per vine than the control, due at least partly, to higher berry weight. Water restriction (H-W) during the rainy year or water supply (L + W) during the dry year increased sugar concentration compared to the controls. However, water or nitrogen restriction (H-W, H-N) during the dry year had the reverse effect leading to lower TSS values.
| Treatments | Yield (kg plant−1) | Leaf area (m2 vine−1) | Bw (g) | TSS (g L−1) | TSS per berry (mg berry−1) |
|---|---|---|---|---|---|
| 2019 (rainy) | |||||
| H | 7.0a | 1.5a | 1.72b | 221b | 348b |
| H-W | 7.0a | 1.3ab | 1.70b | 232a | 362ab |
| H-N | 6.9a | 1.3ab | 1.71b | 222b | 345b |
| H-L | 4.9b | 1.1b | 1.85a | 226b | 387a |
| L | 5.3b | 1.1 | 1.57 | 211 | 306 |
| L + W | n.d. | n.d. | n.d. | n.d. | n.d. |
| L + N | 6.3a | 1.2 | 1.60 | 212 | 320 |
| 2020 (dry) | |||||
| H | 6.70a | 1.4a | 1.34b | 227a | 279b |
| H-W | 6.50a | 1.4a | 1.33b | 210b | 256b |
| H-N | 5.60b | 1.2ab | 1.18c | 208b | 227c |
| H-L | 5.20b | 1.1b | 1.44a | 224a | 312a |
| L | 4.70b | 1.1b | 1.02b | 203b | 189b |
| L + W | 8.40a | 1.5a | 1.53a | 223a | 311a |
| L + N | 4.30b | 0.9b | 0.95b | 201b | 176b |
- Note: Bw: maximum berry weight; H: high vigour; H-W: water restriction; H-N: nitrogen restriction; H-L: leaf removal, L: low vigour; L + W: water supply; L + N: nitrogen supply. Contrasting years: 2019 (rainy) vs 2020 (dry). Average values for samples of 300 berries. Different letters indicate significant differences between treatments considering each year and vigour condition separately according to a Fisher’s LSD test (p ≤ 0.05). The bold values are used to highlight the most relevant results.
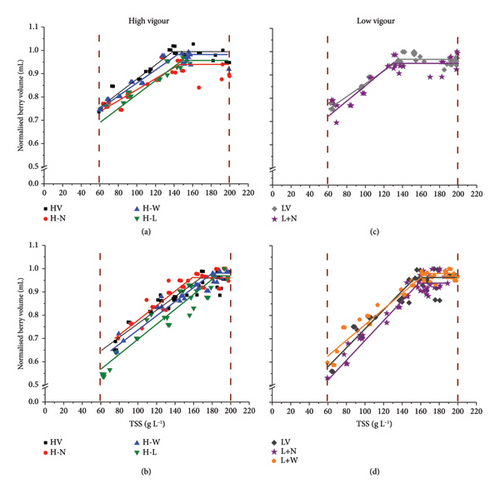
For the rainy year (2019), the average growth/sugar concentration ratio (Slope) was 0.0028 mL/g L−1, with no differences between treatments of high and low-vigour zones, except for the H-N treatment (Figure 4(a); Table 4, Table S3). The BP varied by about 8 g L−1 between the treatments: it was higher for the defoliated treatment (H-L) in the H zone, while it was lower for the N supply treatment (L + N) in the L zone. The TDP I and II decreased compared to the control in the H zone, both for H-W treatment (−5 days) and for H-L treatment (−2 days), mainly due to the shortening of Phase II. Despite no difference in TDP was observed for other treatments in H (H-N) and L (L + N) zones compared to the controls, the durations of Phases I and II were different, with a more prolonged Phase I (and shorter Phase II) for H-N and a more prolonged Phase II (and shorter Phase I) for L + N.
| Treatments | Slope (mL/g L−1) | R2 | Breaking point | Phases duration (days) | ||
|---|---|---|---|---|---|---|
| TSS (g L−1) | I | II | TDP | |||
| 2019 (rainy) | ||||||
| H | 0.0029a | 0.92 | 140b | 21b | 27a | 48a |
| H-W | 0.0028a | 0.96 | 142b | 22b | 21c | 43c |
| H-N | 0.0023b | 0.83 | 143b | 25a | 24b | 49a |
| H-L | 0.0030a | 0.92 | 150a | 22b | 24b | 46b |
| L | 0.0026 | 0.96 | 142a | 24a | 23b | 47 |
| L + W | n.d | n.d | n.d | n.d | n.d | n.d |
| L + N | 0.0032 | 0.83 | 130b | 21b | 26a | 47 |
| 2020 (dry) | ||||||
| H | 0.0029 | 0.91 | 165b | 38a | 11b | 49a |
| H-W | 0.0030 | 0.95 | 181a | 39a | 6c | 45b |
| H-N | 0.0033 | 0.85 | 158c | 36b | 17a | 53a |
| H-L | 0.0033 | 0.93 | 185a | 39a | 10b | 49a |
| L | 0.0041a | 0.91 | 157b | 38a | 9b | 47b |
| L + W | 0.0033b | 0.94 | 167a | 33b | 17a | 50a |
| L + N | 0.0042a | 0.96 | 159b | 36a | 9b | 45b |
- Note: H: high vigour; H-W: water restriction; H-N: nitrogen restriction; H-L: leaf removal, L: low vigour; L + W: water supply; L + N: nitrogen supply. Slope: growth/sugar concentration ratio. Phase I: accumulation of sugars simultaneously with the accumulation of sugars, from 60 g L−1. TSS to BP. Phase II: sugar accumulation at constant volume, from BP to 200 g L−1. TSS. TDP: total duration phases, sugar accumulation over the range 60–200 mg.NBvn−1 TSS. R2: adjusted R-square. Different letters indicate significant differences between treatments for each year and each vigour condition according to Fisher’s LSD test (p ≤ 0.05). The bold values are used to highlight the most relevant results.
For the dry year (2020), the slope ranged from 0.0029 mL/g L−1 in the H zone to 0.041 mL/g L−1 in the L zone for the control treatments, suggesting a higher water flow or lower sugar loading in berries in the L zone (Figure 4-BD; Table 4). While the slope did not vary between the treatments in the H zone, it decreased when the water supply was increased (L + W) in the L zone. The BP differentially varied between treatments compared to the controls for both zones. It increased by ca 18 g L−1 for H-L and H-W, while it decreased by 10–25 g L−1, respectively, for L + W and H-N (Table 4). Lastly, the TDP I and II varied among the treatments for the two zones. In the H zone, TDP was shorter for H-W (ca. −5 days), due to the shortening of Phase II. In the L zone, TDP increased (ca. 4 days) when the water supply was increased (L + W treatment). This increase was explained by a longer Phase II that was not compensated by the shortening of Phase I. As observed in 2019, TDP for H-N was similar to the control, in spite of variations of the durations of the two phases (shorter Phase I and longer Phase II).
The first two principal components of the PCA (Figure 5(a)), performed on all treatments, weather, microclimatic, plant and berry variables for the two contrasting years (2019 and 2020), explained 66.2% of the total variance. Axis 1 accounted for 45.7% and was mostly driven by the loadings of WA (WA BL_V, WA V_H), VPD, plant water stress during ripening (WSI V_H) and trunk starch reserves at budburst (ST). The model parameters TDP I and II and BP were projected on the negative side of Axis 1, along with water deficit-related variables (WSI V_H, VPD) and ST. Axis 2 (20.5% of the variance) was structured by an opposition between water stress before veraison (WSI BL_V) and photosynthesis (Photo). The year scores clearly separated 2019 (rainy) and 2020 (dry) along Axis 1, highlighting that differences were more pronounced under dry conditions, where the effect of vigour level was amplified. Within each year, treatment scores spread along Axis 2. In both years, treatments from the low-vigour zone (L) were positioned closer to water stress before veraison (WSI BL_V), except for the L + W treatment in 2020, which was associated with higher photosynthetic activity, similar to most high-vigour treatments (H-W and H-L).
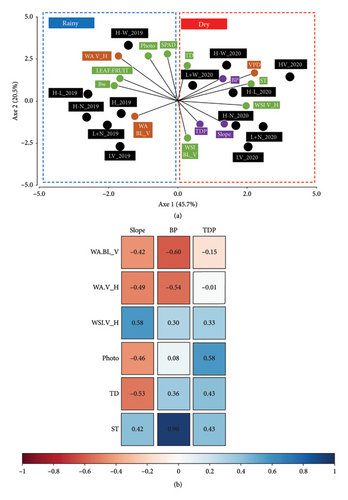
As nitrogen status at harvest (SPAD), leaf:fruit ratio and VPD did not significantly correlate with the model parameters, they were not shown in the table. High correlations (r > 0.5) were observed between the parameters of the bilinear model and the other weather/microclimatic and plant variables. Indeed, the growth/sugar concentration ratio (Slope) was negatively correlated with TD and leaf/fruit ratio and positively with WSI V_H. BP decreased when WA before or after veraison (WA BL_V, WA V_H) increased. TDP and BP increased as the carbohydrate-related variables were higher (TS, Photo).
4. Discussion
High WA and plant vigour were shown in the present study to favour the uncoupling between berry growth and sugar-loading dynamics.
The potential size of the berry is primarily established during the early stages of fruit development, when cell division and enlargement take place [13, 43], making it highly sensitive to abiotic constraints such as water deficit. Under our growing conditions, high WA from flowering to the onset of ripening (known as veraison) (WA Bl_V) during rainy years (2014, 2015 and 2019; Figure S2) promoted berry growth, with berries reaching 60% of their maximum weight by veraison (Figure 1, Table S2). In contrast, during dry years (2016, 2018 and 2020), berries reached only 40% of their maximum size by veraison. The reduced WA Bl_V may have affected the turgor of mesocarp cells, leading to decreased water import from the xylem [3, 44]. From the onset of ripening onwards, the functionality of vascular tissues and water balance in the berry also conditions berry size and sugar content [45]. After reaching maximum sugar accumulation (1.1 M), phloem unloading ceases while berry water loss may continue, leading to berry shrivelling and rise of metabolite concentrations [32]. Normalising berry volume and synchronising the onset of sugar accumulation for a population of berries allowed the comparison of berry ripening processes (water vs. sugar flows) for the two vigour zones over the 8 years of experiment. The relationship between the NBv and sugar concentration displayed a bilinear pattern (Figure 1), which has never been reported in previous studies to our knowledge. Yet, to a classical first phase (Phase I) of simultaneous accumulation of water and sugars, we followed a second phase (Phase II) with additional sugar accumulation at constant berry volume. The maximum berry volume (Phase I) was reached 18 days and 11 days earlier than maximum sugar loading (Phase II), respectively, for rainy and dry years (Table 2).
Higher water deficit from veraison to harvest (WSI V_H) lowered the apparent decoupling between water and sugar flows, illustrated by the higher BP and shorter Phase II (Figure 1, Table 2). The climatic and edaphic conditions of Uruguay could explain these results, which differ from those reported in the literature. The soil where the trial was developed is classified as Vertic argiudoll fine, smectitic and thermal, and is characterised by a high content of organic matter in all horizons and a 2:1 dominance of clays [46], which determines a high WA. In addition, the interannual variability of rainfall is high and the monthly distribution of rainfall is not homogeneous (0 mm–300 mm per month), generating periods of water deficit or excess during grape ripening (Figure 1). Finally, the total duration of sugar loading (TDP, Phase I + II) ranged from 37 to 58 days, depending on the years, with high variability among rainy and dry years (Table 2). Those values are in the range 45 ± 5 days to reach a hexose concentration of 1.1 M reported elsewhere [4, 31]. The vigour differences present in the plot were associated with edaphic differences that determined changes in root exploration and WA among contrasting vigour levels [46]. Therefore, even in dry years, vines in the high-vigour zone likely remained less stressed compared to low-vigour plants. In addition, the microclimate in the cluster zone was also modified for H due to higher leaf area [46]. Ultimately, the higher soil water supply, together with lower VPD in the bunch zone in H plants, may have favoured berry water accumulation, leading to a higher apparent uncoupling between water and sugar loading, as observed during rainy years. Water losses in the berries during late ripening stages have been reported to be due to reduced water supply from phloem [47], while transpiration continues [35]. Under our growing conditions, no loss of berry volume was observed during the late ripening phases (except in 2017), suggesting a generally favourable water balance associated with a low rate of berry transpiration (Figure S2).
In spite of apparent uncoupling of water and sugar loading, which was exacerbated for rainy years and high vigour, the ratio of water to sugar accumulation during Phase I (Slope) was rather stable, regardless of climatic conditions reaching on average 0.0032 mL/g L−1. The changes observed (Figure 2) in the slope relied on the berry volume at the beginning of ripening and on the sugar concentration reached at maximum berry volume (BP). Dry years had a lower berry volume at the onset of ripening (40% of the maximum volume) and needed to accumulate +18 g L−1 to reach the maximum berry volume (Table 1) compared to rainy years (60% of the maximum volume at the onset of ripening). Accordingly, the rate of water and sugar accumulation in the berry seems to be more determined by genetic factors than by vigour or climatic conditions. For this reason, in the next sections of this paper, more emphasis will be placed on discussing the influence of cultivation techniques, vigour and climate on BP and TDP.
Management practices differentially influence berry growth and sugar-loading dynamics though their impacts on water and carbon availability. In our case, water restriction (H-W), as well as increased water supply (L + W), altered the relationship between the NBv and sugar content (Figures 3 and 5, Table 4). Specifically, when compared to the controls (H, L) reducing water supply in the H zone (H-W) shortened the duration of Phase II and total ripening duration (TDP, up to −5 days), whereas water supply in the L zone (L + W) prolonged Phase II and TDP (+3 days). These findings highlight water management as a key lever to mitigate the apparent uncoupling between berry growth and sugar loading together with the total ripening length. Interestingly, higher BP was observed in both H-W and L + W compared to their respective controls. This increase in BP may be attributed to an improved carbon status, as evidenced by higher photosynthetic activity and/or greater trunk starch reserves in those treatments (Figure 5, Table S4). In agreement with those results, a positive effect of ripening induction was observed under moderate water deficit conditions, favouring the plant’s carbon balance [48–50].
Preflowering leaf removal (H-L) in this trial increased the exposure of the bunch and reduced the incidence of bunch diseases during the rainy year (2019), as reported in several studies [37, 51, 52]. The removal of photosynthetically active leaves at this stage is also expected to promote fruit abscission [48, 49, 51] and ultimately to favour greater and faster accumulation of sugars in the remaining berries [53]. Yet, during the rainy year (2019), the higher VPD in the cluster zones due to higher exposure, together with lower yield and rather high photosynthetic activity (Table S4), lowered the apparent uncoupling between water and sugar loading (higher BP, shorter Phase II) and shortened the ripening duration (TDP reduced by −2 days) for H-L compared to the control (H).
The nitrogen restriction treatment (H-N) influenced the parameters of the berry development model, decreasing the slope of water and sugar accumulation in the rainy year, while in the dry year less sugar was needed to reach the maximum volume (Table 4). The lower slope for this treatment indicated a lower water flow or higher sugar loading in berries compared to other treatments. The nitrogen restriction treatment (H-N) influenced the parameters of the berry development model, decreasing the slope of water and sugar accumulation in the rainy year, while in the dry year, less sugar was needed to reach the maximum volume (Table 4). The lower slope for this treatment indicated a lower water flow or higher sugar loading in berries compared to other treatments. The role of nitrogen in sugar accumulation is controversial. NO3− and NH4+ assimilation is regulated by internal factors (C metabolism) and external factors, such as WA [54]. Adverse environmental conditions (drought) can restrict photosynthetic activity and reduce N assimilation, and thus amino acid synthesis [55].
Finally, when considering all treatments in those 2 years, our results indicate that sugar accumulation and berry growth dynamics were primarily driven by WA (WA Bl_V, WA V_H and WSI V_H) and carbon metabolism (photosynthesis, trunk diameter [TD] and starch content [ST]) (Figure 5). Surprisingly, increased carbon metabolism extended the berry ripening period (TDP) and reduced the apparent asynchrony between water loss and sugar accumulation (as reflected by BP), similar to the effect observed under water deficit conditions.
As in other studies, we recently reported that site-specific management according to plant vigour influences grape production and composition parameters [37, 56]. The H-L, H-W and L + W treatments were the ones that allowed obtaining better oenological and productive characteristics, also favouring intracluster homogenisation (Table 3) [16]. Leaf removal in zone H was the most efficient treatment to improve productivity and quality [37]. In addition, water restriction improved grape quality, especially in rainy years. The performance of H-L and H-W in the high-vigour zone resulted in a shorter total ripening period (TDP) due to a reduction of Phase II. On the other hand, it was demonstrated that the deficit irrigation strategy applied in the L zone increased vegetative growth, yield, improved grape anthocyanin content [37] and increased TDP due to a longer duration of Phase II but with a reduction of Phase I (Table 4). The longer ripening duration with the application of L + W irrigation, and particularly the lengthening of Phase II, could favour the accumulation of secondary metabolites under cooler climatic conditions. The changes in ripening times reported by this work could be accentuated or aggravated by climate change. Phenological and ripening processes have been reported to advance by up to 2 to 3 weeks [54, 57], causing ripening under cooler conditions with impacts on grape composition [58]. The climate outlook (2010–2070) for Uruguay and the region indicates an increase in the average temperature of between 1.5°C and 4.0°C, with an increase in heatwave phenomena and greater variability of precipitation [59]. Given these perspectives, it is necessary to continue advancing in the study of the dynamics of grape ripening, and particularly the relationship between water flows, primary and secondary grape metabolism, aspects that will be deepened in future works. Controlled irrigation (L + W) could also maintain the plants with an adequate water status to reduce heat damage during critical ripening periods. The weight loss observed in some years, especially in 2017 (Figure S2), could be related to an acceleration of cell death favoured by high temperature and water deficit conditions [60]. Yet, it has been reported that wilting and cell death could be reduced by supplying irrigation by avoiding excessive heating [56].
5. Conclusions
This study addressed the dynamics of berry growth and sugar accumulation as a function of climatic conditions and cultivation techniques. A bilinear model between berry growth and sugar accumulation was fitted, indicating an apparent uncoupling between water and sugar loading at the berry population level under Uruguayan climate, which has never been reported. Indeed, after a first phase of simultaneous water and sugar accumulation (Phase I, up to BP), a second phase of additional sugar accumulation at constant volume (Phase II) was observed. The slope of water to sugar accumulation (Phase I) was rather stable. However, rainy years, and to a lower extent H zone, accentuated the apparent uncoupling between water and sugar flows compared to dry years and shortened the total ripening duration. Among the different treatments applied for two extreme rainy/dry years, water management accentuated the tendencies mentioned above, shortening (H-W, −5 days) or extending (L + W, +8 days) Phase II. Thus, water management was shown to be a key lever to counteract the climatic and/or vigour impact on the apparent asynchrony between berry growth and sugar loading at the population level. This study provides a deeper understanding of how management practices can be harnessed to improve grape production under different climatic and vigour conditions. Further progress is required to incorporate the dynamics of accumulation of other primary and secondary metabolites into this type of approach.
Disclosure
This work was part of my PhD studies finalised in September 2023. The PhD was carried out in cotutelle mode between the Universidad de la República (Uruguay) and Montpellier SupAgro (France).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Agencia Nacional de Investigación e Innovación (ANNI—Uruguay) and Comisión Sectorial de Investigación Científica (CSIC—Uruguay; INI2019_216).
Acknowledgements
The authors extend their heartfelt thanks to the students from the Viticulture team at UdelaR (Uruguay) for their invaluable assistance in gathering field data. The authors also appreciate the support of the ‘Establecimiento Juanico’ winery for facilitating our experimental work.
Supporting Information
Figure S1: NDVI vigour map and plot location of water, nitrogen and leaf removal treatments in the high (H) and low (L) vigour zones. Table S1: Pre-bud break rainfall and vineyard water balance over the growing season. Figure S2: Evolution of berry weight and total soluble sugar per berry as a function of days after softening (DAS). Table S2: Model parameters for the bilinear fit for the 2014–2021 data series. Table S3: Model parameters for the bilinear fit for the two contrasting climatic years (2019–2020). Table S4: Climatic, productive and physiological variables for the two contrasting climatic years (2019–2020).
Open Research
Data Availability Statement
The data presented in this study are available upon request from the corresponding author (pending privacy and ethical considerations).




