Enhancing Nutrition: A Review of Provitamin A Carotenoid Cassava Breeding Initiatives in East Africa
Abstract
Biofortification of staple food stands as one of the most reliable methods of alleviating vitamin A deficiency (VAD). Evidence suggests that introducing provitamin A carotenoid (pVAC) cassava into the diets of preschool and primary school children in East Africa has led to improvements in their retinol levels. Notably, Kenya and Uganda have initiated cassava biofortification programs in the region. These efforts involve the assembly and characterization of pVAC cassava germplasm, alongside the development of essential tools such as genomic prediction (GP) models and molecular markers for accelerating genetic gains in the biofortification programs. However, several challenges have emerged, including a negative correlation between carotenoid content and dry matter content in cassava roots, diseases, the absence of affordable high-throughput phenotyping methods, poor cassava flowering, poor pollen viability, low capacity in bioinformatics analyses, degradation of carotenoids during processing, and inadequate germplasm conservation facilities. To address these hurdles, cassava breeding programs in the region require enhanced infrastructure and human capacity to optimize efficiency in cassava biofortification with pVACs.
1. Background
Cassava (Manihot esculenta Crantz) is a starchy crop majorly grown in tropical regions of Africa, Asia, and Latin America for food and industrial use [1]. Globally, cassava ranks fifth in terms of production after maize, rice, wheat, and potato, respectively [2]. About 800 million people in the world depend on cassava as a daily staple, and out of these, 500 million are in sub-Saharan Africa [3]. Cassava is drought-tolerant, requires fewer inputs for production, and can grow in marginal land where other crops cannot survive [4].
In East Africa, the average food security of cassava is higher (>10 months per year) than most crops [5]. This means that for more than 10 months, households in the region have no problem or anxiety with consistent access to adequate cassava meals per year. Cassava root is rich in starch but deficient in other body required nutrients such as vitamins, iron, zinc, lipids, and proteins [6]. Among the vitamins that are deficient in cassava roots are provitamin A (beta-carotene), vitamin C (ascorbic acid), thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), folate (vitamin B9), vitamin E, and vitamin K [7, 8].
People who depend on cassava as their staple are vulnerable to vitamin A deficiency (VAD) [9]. Vitamin A is important for enhanced vision in humans, cell proliferation, and apoptosis [10]. Other benefits of vitamin A are enhancement of immunity and reduction of the risk of diseases such as cancer, anemia, and cardiovascular diseases [11, 12]. In Kenya, 89% of the children in cassava consuming communities have inadequate vitamin A intake [13]. Compared to fortification, supplementation, and dietary diversification, biofortification is the most sustainable means of alleviating VAD [14]. Biofortified crops are self-sustaining because farmers can grow them throughout without incurring continuous costs such as those associated with other methods of addressing VAD [15]. In addition, biofortified food can reach rural populations that highly depend on local staples and cannot reach and/or afford diverse food to achieve a balanced diet, fortified foods, and supplements [16].
Global efforts are underway to biofortify cassava with high levels of bioavailable provitamin A carotenoids (pVACs) [17]. Studies showed that daily feeding of preschool and primary school children with pVAC cassava improved their VAD status [18, 19]. Consumption of biofortified cassava is a sustainable homegrown treatment against VAD among cassava consumers. Studies have shown that biofortified cassava with pVAC increases cassava root shelf life by delaying postharvest physiological deterioration (PPD) for about 5 days [20–22]. Furthermore, pVAC cassava enhances the quality of starch for the food processing industry by reducing starch pasting temperature and peak time [23]. pVAC cassava is readily accepted for consumption by communities in East Africa [24, 25].
An ambitious initiative to biofortify cassava with pVAC in East Africa is currently underway. Through this groundbreaking effort, cassava biofortification programs have been initiated in Kenya and Uganda. Indeed, field trials in Uganda have shown promising results with some biofortified cassava genotypes showing good levels of genetic gains [23, 26, 27]. This review aims to provide information on pVAC cassava biofortification progress, challenges, innovations, and prospects in East Africa.
2. Cassava Genetic Resources in East Africa
Cassava genetic resources are conserved in the national gene banks. The pVAC cassava genetic resources in East Africa consist of genotypes assembled from local landraces, the International Institute of Tropical Agriculture (IITA), and the International Centre for Tropical Agriculture (CIAT) improved through hybridization [26, 28]. This collection has created diversity in the region that need to be conserved for the sustainable development of new cassava varieties.
Conservation methods for cassava plant genetic resources comprise in situ and ex situ methods [29]. In in situ conservation, genetic resources are conserved in their natural habitat, and the species maintained in their original place. Traditionally, cassava is conserved in the field plots where it can be conserved for many years without regeneration [30]. This conservation has an advantage of technical simplicity and quick availability of materials for breeding nurseries and evaluation. However, conservation in the field has problems such as lodging due to excessive growth and accumulation of diseases and pests [31]. Furthermore, conservation of cassava in the field experiences adaptation problem when the edaphoclimatic conditions of the gene bank location are different from the collection site. Generally, in situ conservation method is prone to natural calamities and adverse effects of climate change, and thus, it has to be complimented with ex situ method [31].
Ex situ conservation is the conservation of biodiversity outside their natural habitat which is reliable but expensive [32]. This conservation strategy for cassava includes methods such as in vitro and cryopreservation. In vitro conservation allows conservation of rooted cassava plantlets. The plantlets are derived through several ways, but due to phytosanitary reasons, it is recommended to generate them from meristem tips [33]. Meristem tips are recommended because the majority of systemic pathogens do not advance to new and rapidly growing meristematic tissues. Furthermore, chemotherapy can be employed as extra precautions to reduce contamination chances [34] The meristem tips are cultured in nutrient agar inside the test tubes or transparent jars that are maintained under controlled temperature (20°C day temperature and 15°C night temperature) and light (500 to 1000 Lx illumination) [30]. The cultured plantlets are subjected to minimum growth condition that enables them to be conserved for 12–18 months, and thereafter, they are renewed. To renew the in vitro plantlets, their stems are planted into new sterile growth media.
Cryopreservation is a technique that is used to preserve biological constructs such as organelles, cells, and tissues by cooling samples to a very low temperature (up to −196°C) [35]. Biological and chemical activities in living cells are reduced under very low temperatures. This phenomenon leads to the long-term preservation of samples. The different types of cryopreservation are (i) slow freezing, (ii) vitrification (freezing samples with rapid cooling so that ice crystals never forms), (iii) nonfreezing subzero preservation, and (iv) dry state preservation [36]. It has been reported that meristem cryopreservation is highly efficient in the conservation of cassava genetic resources with an average recovery of 79% [37]. Other similar reports indicated that cryopreservation is a suitable procedure for long-term preservation of embryogenic cultures of cassava genetic resources [38].
In East Africa, the majority of pVAC cassava plant genetic resources are conserved using in situ method [39]. Conservation and maintenance are carried out in research institutes and farmers’ fields [29]. This conservation is common because it is cheap to implement and maintain. Conversely, ex situ conservation methods are not often used in East Africa due to high cost of infrastructure and maintenance associated with it. Therefore, in the region, in situ conservation is often not complemented by ex situ conservation; making genetic resources face a threat of natural calamities. While the majority of East African research institutes are planning to expand the cassava conservation strategy, the National Crops Resources Research Institute (NaCRRI) in Uganda has established an in situ cassava conservation facility.
3. Breeding Progress of pVAC Cassava in East Africa
3.1. Genetic Diversity of pVAC Cassava Population
Genetic gain for any trait is a function of selection accuracy, selection intensity, genetic diversity, and cycle time [40]. Thus, one of the requirements to achieve genetic gains in breeding programs is the availability of significant genetic diversity. A few studies have been undertaken to quantify the genetic diversity of pVAC cassava in East Africa; notable is the research work done by Esuma et al. [26]. In this study, genetic diversity was assessed for 64 pVAC cassava accessions and white root cassava accessions (including Ugandan landraces) that clustered into three distinct subpopulations. The entire study groups showed high diversity with average heterozygosity of 0.5583 ± 0.0182.
Currently, the cassava program at Kenya Agricultural and Livestock Research Organization (KALRO) has initiated pVAC cassava breeding. This program has accessed 200 pVAC cassava genotypes, which are undergoing hybridization with local varieties. Effort to genetically characterize this population is underway. Reports on genetic diversity of cassava germplasm in Burundi indicate that their cassava germplasm has a few genotypes whose root flesh color is yellow. This indicates that part of their germplasm has pVAC cassava genotypes that can be used to initiate a biofortification program [41].
In East Africa, cassava biofortification with pVAC is in the initial stages. Presently, only Kenya and Uganda have assembled germplasm and characterized its diversity for use by breeders. At this early stage, there is less information, and thus, it is anticipated that more information about genetic diversity of pVAC cassava in the region will be available in future.
3.2. Breeding Scheme for Cassava Biofortification With pVACs
Cassava breeding programs use phenotypic recurrent selection developed by CIAT and IITA. This follows a long cassava breeding scheme where a breeding cycle takes a minimum of 6 years [42].The cassava breeding scheme stages include seedling evaluation trial, single-row trial (SRT) also known as clonal evaluation trial (CET), preliminary yield trial (PYT), advanced yield trial (AYT), and lastly, the uniform yield trial (UYT).
This breeding scheme starts with acquiring botanical seeds that are germinated in a screen house and later transplanted into a seedling evaluation trial (Table 1). Selection of the seedling at harvest is only based on the capacity to produce more than six cuttings, and this takes place at 9–10 months after transplanting. The seedling evaluation trial is followed by SRT/CET, which is the first stage where selection takes place based on pVAC, harvest index (HI), plant type, and cassava mosaic disease (CMD). Genotypes are planted in unreplicated single-row plots of six or more plants in one location. In PYT, genotypes are planted in one location using randomized complete block design (RCBD) in three replications, with each genotype represented by two-row plots, each with five plants. At this stage, evaluation is carried out for pVAC, HI, DMC, and cassava brown streak disease (CBSD). Genotypes in AYT are planted in one location using RCBD in three replications where each genotype is represented by four to six row plots each with five plants. Evaluation at this stage is based on pVAC, DMC, HI, CBSD, cyanogenic potential (HCNp), and fresh root yield. The last evaluation stage is UYT whose experimental design, plot size, and evaluation is the same as that of AYT. However, UYTs are planted for 2 consecutive years in more than five locations. At AYT and UYT, farmers are invited to participate in selection. Varieties that meet national program criteria are then released.
| Year | Trial | Activity | Reps | Locations |
|---|---|---|---|---|
| 1 | Population development | • Selection of parents and hybridization | — | 1 |
| 2 | Seedling evaluation | • Selection of plants that have the capacity to produce >5 cuttings | — | — |
| 3 | Single-row trial SRT/CET | • Selection based on agronomic performance from 6 plant plots (single row) | — | 1 |
| 4 | PYT | • Selection based on agronomic performance from 10 plant plots (2 rows of 5 plants each) | 3 | 1 |
| 5 | AYT | • Selection based on agronomic performance from 20 to 25 plant plots (4−5 rows of 5 plants each) | 3 | 1 |
| 6–7 | UYT |
|
3 | 5–10 |
- Abbreviations: AYT, advanced yield trial; CET, clonal evaluation trial; CIAT, International Center for Tropical Agriculture; PYT, preliminary yield trial; SRT, single-row trial; UYT, uniform yield trial.
East Africa cassava breeding programs modified the CIAT cassava breeding scheme to reduce the costs and increase local adoption of varieties [43]. This scheme is characterized with (i) omission of CIAT AYT, (ii) modification of PYT where selected genotypes are planted in more locations instead of one location and farmers are invited to participate in the evaluation, and (iii) modification of UYT where instead of using the same genotypes across the locations, different genotypes are planted in different locations based on the requirements on each location.
Indeed, the cassava breeding scheme takes a long time as indicated above. However, a new and quicker selection scheme for pVAC called rapid cycling recurrent selection where a selection cycle takes 3 years has been developed by CIAT, Columbia breeding program (Figure 1) [44]. This breeding scheme takes advantage of the high heritability (h2 = 72%) of carotenoids in cassava roots [45], and the finding is supported by the fact that carotenoids in cassava are not strongly influenced by environmental effects [27]. Therefore, high heritability and minimum environmental effects enable the selection of pVAC rich clones in one location.
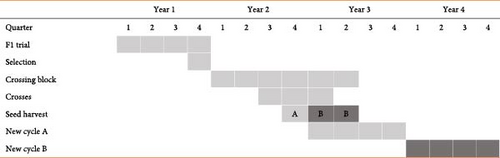
In this scheme, selection is solely based on high carotenoid content in cassava roots [44]. Selection of parental genotypes (F1) takes place at the seedling evaluation trial which is unreplicated and established in one location in the first year (Year 1) during harvesting time (10th−12th month). In Year 2, the selected genotypes are planted in a crossing block that is maintained for 18 months. Parents are hybridized after flowering at 7th−15th months after planting (MAP). Seeds harvested at 10–12 MAP of Year 2 are planted in Year 3 as new cycle A. From this cycle, genotypes with high carotenoid content are selected as potential varieties. Since the crossing block is extended to 18 months, more seeds can be harvested in Year 3 and planted in Year 4 and new cycle B. The rapid cycling recurrent selection is currently being implemented in East Africa’s cassava biofortification programs.
The use of genomic selection in combination with the rapid cycling recurrent selection scheme could reduce the selection cycle from 2 to 3 years to about 1–2 years (Figure 2). In this case, the selection of parents is carried out at the second quarter of Year 1 through genomic selection. The selected parents are crossed in the third quarter, and the resultant seed is harvested in the fourth quarter of Year 1 while a new cycle starts in Year 2. This new breeding scheme improves the rate of genetic gains for pVAC in cassava biofortification programs.

3.3. Achievement in Breeding for pVAC Cassava
The region has achieved limited success in pVAC cassava biofortification. In Uganda, the cassava biofortification program reported that the initial base germplasm population has carotenoid content ranging from 1.2 to 14.2 μg/100 g [26]. Current reports indicate that from this germplasm, genetic gains for pVAC cassava having carotenoid content of 9.81–11.94 µg/g (also expressed as 981–1194 μg/100 g) have been achieved [23, 27]. According to National Institutes of Health [46], this amount of pVAC is equivalent to 81.75–99.5 μg retinol activity equivalent (RAE) which is about 11% of the recommended amount for an adult. The average daily vitamin A intake that can meet the nutritional requirements of a healthy individual is known as the recommended dietary allowance (RDA) which is given as RAE in Table 2 [46, 47].
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| 0–6 months | 400 μg RAE | 400 μg RAE | — | — |
| 7–12 months | 500 μg RAE | 500 μg RAE | — | — |
| 1–3 years | 300 μg RAE | 300 μg RAE | — | — |
| 4–8 years | 400 μg RAE | 400 μg RAE | — | — |
| 9–13 years | 600 μg RAE | 600 μg RAE | — | — |
| 14–18 years | 900 μg RAE | 700 μg RAE | 750 μg RAE | 1200 μg RAE |
| 19–50 years | 900 μg RAE | 700 μg RAE | 770 μg RAE | 1300 μg RAE |
| >50 years | 900 μg RAE | 700 μg RAE | — | — |
- Note: Source: National Institutes of Health [46].
- Abbreviations: RAE, retinol activity equivalent; RDA, recommended dietary allowance.
To determine RAE in a food per sample weight (such as a 100 g sample), the quantity of pVAC in that sample is divided by a conversion factor of 6 or 12 depending on the region [48]. According to Institute of Medicine Food and Nutrition [49], 1 µg of RAE for β-carotene, α-carotene, and β-cryptoxanthin is 12 μg, 24 μg, and 24 μg, respectively. Since our pVAC is mainly β-carotene, a conversion of 12 was applied in this review article.
In Kenya, reports indicate that the cassava biofortification program in 2012 had pVAC cassava germplasm with a mean carotenoid content ranging from 1105.85 µg/100 g to 1736.96 µg/100 g (92.15 RAE–144.74 RAE) [28]. This germplasm comprised 129 pVAC genotypes derived from IITA. It is reported that these genotypes were hybridized with Kenyan local cassava varieties, widening up the pVAC cassava germplasm base to 324 genotypes [50]. However, due to the challenges of in situ conservation, this germplasm succumbed to natural disasters. Nevertheless, KALRO has reinitiated this program, and currently, it has assembled new pVAC cassava germplasm. Efforts to characterize this germplasm and incorporate the use of molecular breeding tools into the biofortification program are underway.
Despite having a scarcity of high-throughput genotyping and genome sequencing laboratories in the region, scientists are developing molecular tools to use in cassava biofortification. However, the capacity in molecular analysis and bioinformatics is not sufficient for the efficient use of molecular tools in cassava breeding. Limited reports have indicated success in molecular characterization of the region’s pVAC cassava population and incorporation of genomic prediction (GP) in the cassava biofortification programs. Research done by Esuma et al. [51] reported a GP accuracy of 0.58 for total carotenoid content (TCC) in cassava roots. Kompetitive Allele Specific PCR (KASP) markers to use in cassava biofortification with pVAC have been validated [52]. This marker type provides a cost-effective strategy for implementing marker-assisted breeding in cassava biofortification with pVAC.
Determination of social perception on the consumption of pVAC cassava in the region has been achieved [25]. This was revealed through a survey that was conducted in Busia, an eastern part of Uganda, which is in the border of the western part of Kenya. Results from the survey showed that at least 85% of men, women, and youth actively cultivate pVAC cassava landrace. The respondents pointed out the attributes that influenced their preference to this landrace to be high yields and early maturity. Interestingly, the respondents had poor knowledge on the nutritional benefits of consuming pVAC cassava. This report is an indication that pVAC cassava is socially accepted in the region and varieties improved through participatory breeding could be adopted at large.
Starch pasting properties of pVAC cassava population in Uganda was reported to be desirable for the food processing industry [23]. The reports indicated that carotenoid content correlated negatively with starch pasting temperature and peak time, signifying a reduction in energy requirement for food processing compared to white-rooted cassava varieties. Findings from this research show that effort is directed toward genetic improvement of pVAC cassava for direct consumption and use in the food processing industry.
4. Challenges of Cassava Biofortification With pVAC in East Africa
4.1. Long Growth Period and Negative Correlation Between Root Dry Matter Content and Carotenoid Content
Cassava has a long growing period taking 12 months to mature. Cassava breeding initiatives face many difficulties associated with the plant’s long breeding cycle. In contrast to annual crops that have brief reproductive cycles, the breeding cycle of cassava is lengthy and usually lasts for more than 8 years [53]. This prolonged cycle slows the rate of genetic gains and delays the release of improved cultivars to farmers. Furthermore, because of the long breeding cycle, cassava breeding is vulnerable to environmental changes or new diseases. After years of development, breeders may discover that a variety they spent years breeding is vulnerable to a new disease or any other new problem. East Africa cassava biofortification programs are addressing this challenge through the use of marker-assisted breeding and genomic selection for rapid genetic improvement of the crop [51, 52].
Among the major traits that influence cassava adoption by farmers is DMC [54]. In African cassava germplasm, DMC and carotenoid content in cassava roots have a negative correlation ranging from r = −0.42 to r = −0.45 [55, 56]. This implies that as one trait is enhanced, the other one is reduced. Current reports indicate that this negative correlation is possibly due to genetic linkage which is also known as linkage disequilibrium (LD) and not pleiotropy [57]. LD is a nonrandom association of alleles between loci. It is the tendency of genes located in close loci being inherited together after failing to undergo independent assortment during meiosis [58]. Consequently, cassava breeders have to take more time hybridizing their germplasm to break the genetic linkage. As of now, researchers at KALRO are investigating the molecular basis of the negative correlation between pVAC and DMC through transcriptomic and gene expression analysis in the carotenoid biosynthesis pathway. Gene editing techniques such as CRISPR/Cas9 can be employed to introduce specific changes that can quickly break this linkage. In general, the negative correlation between the traits constrains cassava breeders’ efforts of biofortifying cassava with pVAC.
4.2. Cassava Diseases
The major constraint of cassava production in East Africa is diseases. Diseases have a significant impact on cassava biofortification with pVAC because they cause root rotting. Infected cassava roots have low concentration of pVAC leading to reduced nutritional value and quality. In addition, rotten cassava roots cannot provide a true representative sample for evaluation. These undermine efforts to improve the nutritional composition of cassava through biofortification.
CBSD is one of the most devastating diseases affecting cassava in East Africa [59]. This disease is only found in Eastern Africa and may spread to other parts if it is not contained. It is caused by two distinct viruses: cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) of the Potyviridae family and Ipomovirus genus [60, 61]. CBSD can cause 100% loss of cassava roots (Figure 3) making it difficult for breeders to get data from root samples. These viruses cause characteristic brown streaks on the tuberous roots, making them unmarketable. Infected plants also show symptoms such as leaf yellowing, stem necrosis, and reduced root size, leading to significant yield losses


Similarly, CMD affects cassava plant architecture and equally compromise cassava yields [63, 64]. This disease is caused by various strains of the cassava mosaic virus (CMV) in Geminiviridae family and Begomovirus genus [65]. CMD leads to characteristic mosaic patterns on leaves, stunted growth, and reduced root yield. It can spread rapidly through infected plant materials and whiteflies, making it challenging to control.
Other cassava diseases include cassava bacterial blight (CBB) and cassava anthracnose disease. CBB is caused by the bacterium Xanthomonas axonopodis pv. Manihotis [66]. This disease manifests as angular leaf spots, stem cankers, and wilting, eventually leading to defoliation and reduced root yield. It can be spread through contaminated planting materials and tools [67]. Cassava anthracnose disease is caused by the fungus Colletotrichum gloeosporioides. It results in dark, sunken lesions on stems and leaves, reducing photosynthesis and leading to yield losses [68]. The disease is more prevalent in areas with high rainfall and humidity. These diseases can be reliably controlled through the use of disease-resistant varieties [69]. These diseases can cause huge loss once they infect pVAC cassava varieties that are not resistant consequently constraining biofortification efforts. Researchers in the region are carrying out a continuous improvement of cassava against these diseases through breeding. Similar efforts have been put into place, resulting to Kenyan government approving the release of genetically engineered CBSD resistance cassava variety [70]
4.3. Lack of Efficient Cassava Root Phenotyping Methods
High phenotyping costs of carotenoids in cassava roots limit the evaluation of large populations [71]. Available options for carotenoid phenotyping in cassava include high-performance liquid chromatography (HPLC), UV spectrophotometer, iCheck carotene, chromameter, qualitative color charts, and near-infrared spectroscopy (NIRS). The use of HPLC and UV spectrophotometer is reliable but costly and inefficient when analyzing more than 10 samples per day at approximately $20 per sample in East Africa [71, 72].
Proxy methods such as iCheck carotene and chromameter are relatively cheap but slow. Qualitative color charts are subjective to a person’s perception of color and, thus, cannot be reliably used with many people on the same population. NIRS provides a quick, versatile cost-effective, and reliable carotenoid phenotyping method. However, purchasing NIRS is expensive and requires calibrations and periodical improvements of the models, and this requires technical knowledge, which is limited in the region. Phenotyping of carotenoids content in cassava roots is a major challenge since carotenoids degrade quickly when exposed to light through isomerization of trans β-carotene to cis β-carotene [73].
Currently, East Africa’s national agricultural research institutes such as KALRO and NaCRRI have invested in benchtop NIRS devices. Unlike other portable NIRS devices, the benchtop devices are not portable, and therefore, they cannot be directly used in the field. Since carotenoids degrade quickly once exposed to light, there is a need for highly portable and high-throughput phenotyping methods directly applicable in the fields [74]. Nevertheless, efforts to calibrate the benchtop NIRS and acquire more portable device for pVAC phenotyping in cassava roots are underway.
4.4. Insufficient Flowers for Crossing
Cassava improvement with pVAC and other traits is constrained by few flowers available for crossing, male sterility, unsynchronized flowering, and abortion of fruits before they mature into seed [75, 76]. Inherently, cassava has poor flowering and poor pollen viability. This poses a challenge in its genetic improvement. Cassava flowering has been reported to be influenced by environmental factors [77]. In East Africa, cassava population is reported to have significantly fewer female flowers with male flowers opening 30 days after female flowers [76]. Nevertheless, cassava programs in the region have established a few interventions to address the cassava flowering issue. Notably, the use of red light to extend photoperiod (RLE) has been studied and found to enhance cassava flowering synergistically with pruning and plant growth regulators (PGRs) [78]. Indeed, these authors noted a significant improvement in the flowering ability of all 20 genotypes evaluated for flowering and fruit set under RLE and PGR.
4.5. Lack of Sufficient Cassava Germplasm Conservation Facilities
In East Africa, cassava germplasm is conserved through on-farm conservation in research institutes and farmer fields [29]. In this conservation method, diverse cassava varieties are cultivated in the fields, where the genetic diversity is preserved. This method permits the continuous adaptation of cassava to the local environment and farming systems [79]. However, in situ conservation method presents a threat of diseases and pests that affect the crop. In addition, this method faces risks associated with climate change leading to extreme weather conditions affecting the conserved germplasm [31].
There is inadequate modern ex situ germplasm conservation facility specific for cassava, to complement in situ conservation [80]. Inadequate conservation facilities for complementing in situ conservation have led to the loss of the region’s pVAC cassava diversity. Low diversity narrows the germplasm base making it impossible for plant breeders to get novel alleles to use in genetic improvement of crops against emerging challenges. Despite these obstacles, efforts are being made to enhance conservation strategies and improve collaboration between researchers and government institutions to ensure the long-term conservation of cassava germplasm in East Africa.
4.6. Degradation of Carotenoids During Processing
When cassava root samples containing pVAC are exposed to light, pVAC degrades by undergoing oxidation followed by isomerization. This creates a constraint in cassava biofortification because during sampling, degradation of pVAC makes it difficult to get a true value of a sample. Similarly, the degradation of pVAC leads to the loss of nutrients required by cassava food consumers.
Exposing carotenoid samples to light induces the rearrangement of double bonds in the carotenoid molecule, leading to the formation of different isomeric forms. Since carotenoid molecule is rich in electrons and highly reactive, it quickly undergoes isomerization and oxidation during food processing and storage [73]. The process of degradation of carotenoid content is shown in Figure 4.
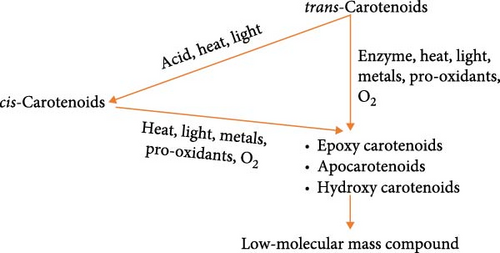
The major pVACs in cassava are typically β-carotene and its isomers. Isomerization of β-carotene leads to conversion of trans-β-carotene to cis-β-carotene. Both trans-β-carotene and cis-β-carotene are oxidized to epoxy carotenoids, apocarotenoids, and hydroxyl carotenoids [81]. This is followed by fragmentation leading to low-molecular mass compounds that have neither color nor vitamin A activity. Generally, cis-β-carotene has lower vitamin A activity compared to trans-β-carotene, and therefore, isomerization reduces the nutritional value and bioavailability of pVAC in cassava root [73]. Isomerization of β-carotene can be initiated during cassava root processing activities such as peeling, heat treatment, slicing, pulping, or juicing. Its magnitude depends on the amount of carotenoids, oxygen, temperature, the wavelength of light, and the presence of enzymes, antioxidants, and metals [81]. Therefore, proper handling and storage of cassava root samples, minimizing light exposure, can help preserve the nutritional quality of the root and maintain the levels of pVAC. In East Africa, studies to address pVAC degradation are yet to be reported.
5. Innovations to Support Cassava Biofortification With pVAC in East Africa
5.1. Genomics
To support cassava biofortification with pVAC in East Africa, various innovations in genomics have been deployed. The use of GP in pVAC cassava breeding is employed to address the challenge associated with the crop’s long breeding cycle. The effectiveness of GP on cassava biofortification with pVAC has been studied for use, using part of East African cassava germplasm [51]. In that study, an initial GP model with an accuracy of 58% for pVAC was achieved. This is a great milestone toward the utility of this tool for rapid genetic improvement of pVAC in the region.
To effectively deploy marker-assisted selection (MAS) in the region, KASP markers associated with pVAC and root DMC have been validated [52]. These markers were developed from SNPs identified from genome-wide association studies (GWAS). Indeed, the genetic architecture of pVAC cassava unraveled through GWAS revealed four SNPs in chromosome one associated with TCC [56]. Further analysis revealed that these SNPs are in close proximity with phytoene synthase loci that is responsible for the accumulation of carotenoid content in cassava root
5.2. Phenomics
East African cassava biofortification programs are developing cheap, accurate, and high-throughput methods for phenotyping pVAC in cassava roots. These methods include the use of NIRS which is available as desktop and portable options that can be used directly in the field [71]. Currently, there is a highly portable NIRS device called SCIO, which can fit into the trousers’ pocket [82]. NIRS is a cheap, versatile, accurate, highly portable, and able to quickly phenotype hundreds of samples per day [83]. The use of NIRS requires the development of prediction model that associates spectra and phenotype. Recent reports shows that NIRS models developed using simple sampling reference alternatives are reliable to use for pVAC and DMC phenotyping in cassava [74]. Despite the efforts placed to acquire devices and develop high-throughput phenotyping methods such as NIRS in the region, least has been achieved on the development of pVAC prediction models. This is partly attributed to the high cost associated with acquiring of reference data for pVAC modeling.
5.3. Grafting and PGRs
Grafting has been reported to promote the development of flowers in nonflowering cassava genotypes [84]. Flowering is initiated by a flowering signal called FT (florigen) stimulus [85]. Molecular studies have reported that the overexpression of FT gene in cassava accelerates the rate of flowering [86]. The production of florigen is induced by day length and translocated from leaves to apical meristem where the flower bud is produced [84]. Since florigen moves from leaves to apical meristem, grafting in cassava is carried out on a flowering genotype stock and a nonflowering genotype scion (Figure 5). Two or more pairs of cassava leaves are left on the understock at a height of 12 cm [88].
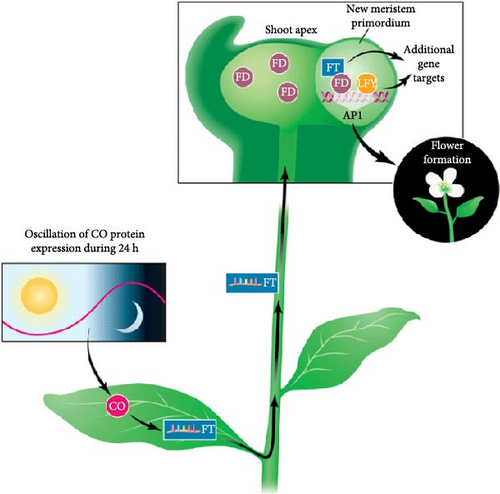
Endogenous (hormones) and environmental (temperature and photoperiod) signals influence a plant’s transition from vegetative to reproductive phases [89]. This transition involves differentiation of apical meristem causing cellular change that leads to flower bud development. Apical meristem’s change to the reproductive phase can be induced by the exogenous application of PGRs such as auxins, abscisic acid, ethylene, and gibberellins [88]. In East Africa, studies on the application of grafting and PGR on pVAC cassava population are yet to be reported. Nevertheless, recent studies in the region revealed that the use of RLE enhances flowering [78].
5.4. Embryo Rescue
Embryo rescue is a tissue culture technique where a developing embryo is cultured before it is aborted [90]. The majority of pollinated flowers in cassava abort early before they develop into fruits [91]. High rate of cassava fruit abortion occurs at about 10–14 days after pollination [92]. This period presents a reliable time to use embryo rescue and develop seedlings before cassava fruits abort. Application of embryo rescue technique provides a fundamental intervention against cassava fruit abortion. Studies on cassava fruit abortion and effectiveness of embryo rescue in East Africa have been reported [93]. However, its application on pVAC cassava population across other East African research institutes is yet to be achieved. This could be attributed to insufficient personnel and a lack of advanced laboratories to implement this technology in the region. Nevertheless, in other regions, researchers at CIAT, Colombia, have successfully developed a protocol for cassava embryo rescue in the first 2 weeks after anthesis [94]. The use of cassava embryo rescue at CIAT, Colombia, has been implemented, and reports show that this technology resulted in embryo culture seed germination percentage of 66%, plantlet field establishment of 98.89%, and high yield-related traits compared to results from previous experiments [95].
5.5. Genome Editing
Genome editing is a technology that is used to make changes in the genome by accurately targeting a specific DNA sequence. This technology uses site-directed nucleases to cut DNA strand with high precision and causing changes without introducing new genome to the organism [96]. CBSD causes up to 100% root damage which affects cassava biofortification efforts, because damaged roots cannot be evaluated for their carotenoids content. Genome editing provides a quick and effective strategy to protect pVAC cassava against CBSD [97]. Currently, research on gene editing use to protect cassava against diseases such as CBSD is underway in East Africa. Research has shown that CRISPR-/Cas9-mediated genome editing is highly efficient in modifying M. esculenta Phytoene desaturase (MePDS), which codes for enzymes involved in carotenoid biosynthesis in cassava [98]. Similar studies have successfully used genome editing to disrupt cytochrome P450 genes to eliminate poisonous cyanide levels in cassava roots and leaves [97]. This demonstrates that genome editing provides an efficient means of leveraging cassava biofortification.
5.6. Genetic Engineering
Genetic engineering also known as genetic modification (GM) is a laboratory technology that modifies the genetic makeup of an organism. This modification can be the changing of a single base pair, deleting a DNA segment, or adding a foreign gene from another species to produce the desired trait [99]. Many successful reports on the use of GM technology to improve crops have been reported [100]. This makes GM a promising tool for enhancing food and nutritional security in Africa through improvement of staple crops such as cassava. In East Africa, Kenya has set a great precedence by approving the environmental release of CBSD-resistant GM cassava varieties developed by KALRO [70]. This provides a gateway on the use of genetic engineering for cassava biofortification in the region. Indeed, transgenic pVAC cassava has been successfully developed by BioCassava Plus project at the University of Nebraska, USA, and the expression of the transgene was evaluated in Kenya [101]. This research indicates that the transgenic cassava was effectively biofortified using GM technology. Reports have indicated that transgenic pVAC cassava has limited PPD, and this has led to extended root shelf life [21].
6. Conclusion and Future Prospects
In this review, we note that cassava-consuming communities in East Africa are vulnerable to VAD. Biofortification of staple food is the most reliable means to dispense vitamin A to malnourished communities. Cassava biofortification with pVAC in East Africa is in the initial stages of breeding where only Kenya and Uganda have initiated this effort. Cassava breeders in this region are constrained with (1) negative association between carotenoids and dry matter content; (2) diseases that affect both cassava roots and leaves; (3) lack of cheap, portable, and high-throughput phenotyping methods; (4) poor cassava flowering, low pollen viability, and high cassava fruit abortion; (5) lack of cassava sufficient germplasm conservation infrastructure; and (6) long cassava growing period that slows the rate of genetic gains.
Despite the current efforts in the region to develop breeding tools and characterize germplasm, more resources are needed to upscale research and infrastructure. In the future, most research institutes in East Africa will have to establish cassava biofortification programs to tackle the vulnerability of VAD in the region. Novel interventions have to be deployed to support cassava genetic improvement with pVAC. Further research is needed to provide a solution to pVAC cassava flowering challenges through grafting a flowering stock with a nonflowering scion. Studies on the use of PGRs to enhance cassava flowering need to be carried out, protocols developed, and optimized. In addition, there is a need to carry out studies to validate more proxy high-throughput and portable phenotyping methods for carotenoids in cassava roots. High-throughput genotyping facilities need to be established for breeding programs in the region. In addition, there is a need for capacity development in bioinformatics and genomic analysis to effectively implement marker-assisted breeding and GP. More in situ and ex situ conservation facilities need to be established to for reliable cassava germplasm conservation in the region.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No external funding was received for this review.
Open Research
Data Availability Statement
The insights presented in this review are derived from prior studies, all of which have been appropriately referenced within the manuscript.




