Effect of Gelling Agent and Plant Growth Regulators on In Vitro Shooting and Rooting of Stevia rebaudiana Bertoni
Abstract
Stevia rebaudiana Bertoni is an economically important medicinal plant producing zero-calorie diterpenes glycosides in its leaves. This herb is a new source of natural sugar substitute, which makes it a valuable crop for the world. The growth and development of Stevia in vitro were strongly influenced by genotype, exogenous plant growth regulators (PGRs), and type of gelling agent. This study aimed to evaluate the effects of two different gelling agents (agar and gelrite) and PGRs on plant growth and multiplication of stevia. Nodal explants were placed on Murashig and Skoog (1969) (MS) medium supplemented with kinetin (KIN), indole-3-acetic acid (IAA), and 6-benzylaminopurine (BAP) and gelled with agar or gelrite for shoot proliferation. After 4 weeks, in vitro shoot segments were cultured on MS medium without any PGRs and gelled with 7 g/L of agar or 3 g/L of gélrite for rooting. Direct shoot formations were highly effective on MS medium gelled with agar. The highest number of shoots was reported for plants propagated on MS medium fortified with 0.5 mg/L of BAP + 0.5 mg/L of IAA. MS medium without any PGRs (control) produced a long shoot with the highest number of internodes. Rooting of stevia was best on an agar-gelled medium.
1. Introduction
Stevia rebaudiana Bertoni is a perennial herb native to Paraguay and belongs to the Asteraceae family [1]; it contains sweet compounds named diterpene glycosides, which are estimated to be 300 times sweeter than sucrose with zero calories [2, 3]. Most artificial sweeteners are known for their neurological side effects in renal. On the contrary, sweeteners from stevia have no side effects and play a vital role in reducing blood sugar levels (recommended for diabetics) [4]. This plant contains more than 60 steviol glycosides, shares the same molecular structure (steviol), and with an aglycone steviol backbone conjugated with β- and α-glycosidic bonds to different sugar moieties [5], in sweating intensity, and their proportion in the leaves [6]. Among them, stevioside is the most abundant compound and constitutes (60%–70%) of all sweeteners, while Rebaudioside A represents only (30%–40%) of all diterpenes glycosides but is characterized by the absence of bitter aftertaste [7, 8].
Stevia seeds are tiny, lose viability during storage, and have low fertility. Therefore, a high level of variation is usually found among seed-propagated plants with regard to sweetening levels and composition [9, 10]. Vegetative propagation by stem cutting is limited by the low number of individuals obtained from a single plant, the unavailability of sufficient plant material, and the extremely slow growth of cuttings, which requires good hygiene to prevent disease infection [11–13]. Therefore, it is necessary to develop an alternative approach for the successful reproduction of stevia, in a short time, with less somaclonal variation, and a high success rate to respond to the growing demands for this powerful medicinal plant and to help its conservation [14–16].
Tissue culture in regulated growth conditions guarantees the large-scale production of high-quality planting materials, with effective sweetening properties, in all seasons and different environmental conditions [17]. In vitro, micropropagation by shoot tip and leaf is the only rapid process for the propagation of stevia [18]. This method can provide large-scale production progeny, true-to-type, homogenous, and pathogens-free plants of stevia with high quality in a short time [19]. Shoot proliferation is based on the exploitation of pre-existing meristem of the explant, which leads to the multiplication of shoots in a short time. Technically, successful in vitro development of stevia plants is influenced by several factors, including the type of explant, medium composition, type and concentrations of plant growth regulators (PGRs) as well as gelling agent [20, 21]. Nodal segments and axillary buds [22] are widely used for in vitro multiplication of stevia on Murashig and Skoog [23] (MS) medium fortified with different combinations of auxin and cytokinin [21, 24]. In vitro culture additionally depends significantly on genotype. Different plant growth can be produced by the same type of explant in different plant genotypes [25].
The type and concentration of the gelling agent is one of the most crucial elements influencing the chemical and physical properties of the culture medium in vitro. Gelling agents make the medium firm wish to influence the diffusion characteristics of the medium. Thus, the morphogenetic response, growth, and development of tissue-cultured plant material can be greatly influenced by gelling agents [26]. Solidifying agents offer the best strength to sustain tissue responses; as a result, properly prepared and solidified artificial media offer ideal circumstances for the development of plant cells and tissues [27]. Also, cellular differentiation and cytokinin-sensitivity of in vitro plant cell cultures are strongly influenced by gelling agents [28]. Further, the choice of an appropriate gelling agent decreases vitrification and provides healthy, vigorous plants with higher chlorophyll levels [29]. The two most commonly used gelling agents are agar (agarose) extracted from the algae Laminaria and gellan gum, also known as Gelrite or Phytagel, an extracellular polysaccharide obtained from bacterial (Pseudomonas elodea [30]). Agar is the most popular gelling agent; used for its desirable qualities such as clarity, stability, and inertness [31]. Gelrite is purer and clearer than agar and is utilized in low concentrations to obtain the same consistency [32].
Studies on the impact of gelling agents, which are frequently employed in micropropagation, on the growth and development of stevia plants are limited. The purpose of this work is to study the effect of two gelling agents (agar and gelrite) in combination with PGRs (6-benzylaminopurine [BAP], kinetin [KIN], indole-3-acetic acid [IAA]) on the multiplication and in vitro growth of nodal segments of S. rebaudiana Bertoni.
2. Materials and Methods
2.1. Plant Material and Culture Conditions
Nodal explants of two genotypes, G1 and G2, were collected from a greenhouse conditioned at the National Institute for Agricultural Research (INRA), Kenitra, and used as plant material. Nodal stem segments of 2 cm length were excised, disinfected in a solution of 15% sodium hypochlorite for 15 min, and then rinsed with sterilized tap water before culture.
MS medium supplemented with 3% sucrose was used as a basal medium. Different concentrations and combinations of BAP, KIN, and IAA were added to the culture media before adjusting the pH to 5.8. Two gelling agents (3 g/L gelrite and 7 g/L agar) and four combinations were tested (Table 1). The explants (with a single axillary bud) were placed down vertically in the culture tubes containing the different culture media incubated in a culture room in a 16 h light: 8 h dark photoperiod at a relative humidity of 55%–60% and 27°C ± 2°C temperature.
| Concentration of PGRs | Gelling agent | |
|---|---|---|
| Agar (7 g/L) | Gelrite (3 g/L) | |
| M0 (Control) | M0′ (Control) | |
| BAP (1.5 mg/L + KIN (0.25 mg/L) | M1 | M1′ |
| IAA (0.5 mg/L) | M2 | M2′ |
| KIN (0.5 mg/L) | M3 | M3′ |
- Abbreviations: BAP, 6-benzylaminopurine; IAA, indole-3-acetic acid; KIN, kinetin; MS, Murashig and Skoog; PGRs, plant growth regulators.
2.2. Multiplication Phase
After 4 weeks of incubation, multiple shoots obtained from the initiation stage were separated into pieces (1 node/piece) and then transferred to test tubes containing new media for multiplication. Subculture was achieved every 4 weeks four times (four subcultures).
2.3. Rooting Phase
For rooting, individual vitropousses were transferred to an MS-free medium (control) gelled with 3 g/L gelrite or 7 g/L agar.
2.4. Statistical Analysis
Thirty cultures were raised for each treatment. The data obtained were processed using the “SAS” software. The comparison of the means relating to shoots and root induction was carried out using the Duncan test for all the treatments. The significance level of 5% is accepted for all analyses.
3. Results
3.1. Multiplication Phase
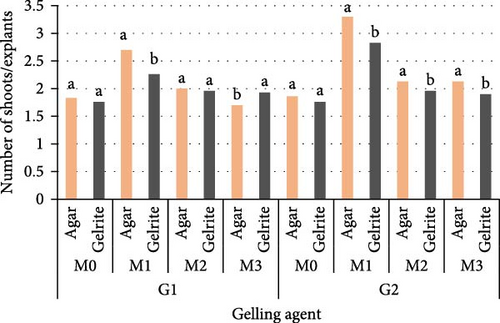
In this study, multiple shoots emerged directly from nodal explant after 5 days of culture on M0 (control) gelled with agar or gelrite. While shoot, induction was observed in mediums with PGRs after 7 days of culture. There was a significant effect between media, genotype, and type of gelling agent. The interaction between the three parameters for the number of shoots/explant shows no significant effect (p = 0.082). In the agar experiment, the percentage of shoot induction was found to be very high (mean 92%), while for gelrite, only 80% of shoot induction was detected. For both gelling agents, the maximum number of initial shoots has occurred on MS media fortified with 1.5 mg/L BAP and 0.25 mg/L KIN (Figure 1). Explants cultured on M1 gelled with agar have generated 2.7 and 3.3 shoots/explants for G1 and G2, respectively, and the same media gelled with gelrite have developed 2.26 and 2.83 shoots/explants for G1 and G2, respectively. The lowest number of shoots was obtained in control for both genotypes.
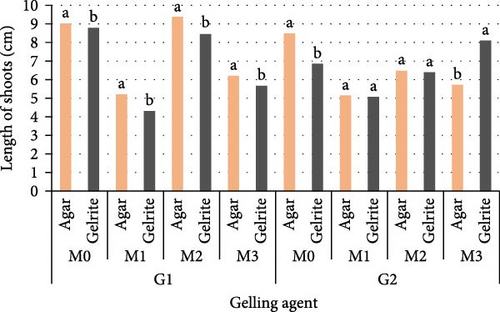
The results show that the differences between PGRs combinations, genotype, and type of gelling agent and their interaction factors had a very significant effect on the average mean length of shoots of stevia (p = 0.0017). For both genotypes and both gelling agents, the longest shoots mean was (9.02 and 8.79 cm, respectively, for G1 and 8.48 and 6.86 cm for G2) more stimulated by controls M0 and M0′. In addition, both Media M2 and M2′ have regenerated shoots past 7 cm in length. The shortest shoots length varied between 4.31 and 5.21 cm with M1 and M1′ (Figure 2).
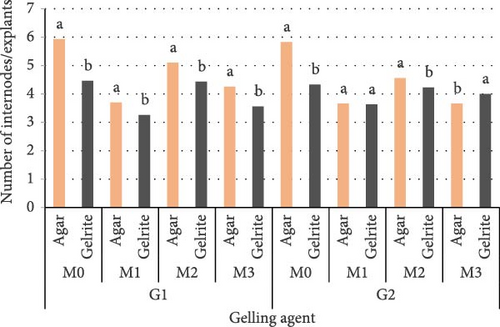
ANOVA showed that the number of internodes/explants was significantly affected by the type of gelling agent and by media compositions. These parameters have not been affected by genotype. For both genotypes, Control has regenerated the highest number of internodes/explants M0 (5.93/G1) and M0′ (4.46/G1), M0 (5.83/G2) and M0′ (4.33 /G2). The lowest number of internodes has been observed on M1 and M1′ (Figure 3).
The type of gelling agent has remarkably influenced the appearance of shoots. Gelrite-solidified mediums induced relatively short shoots with yellow, green, rolled leaves, with compact greenish callus in their bases and brownish-green stems (Figure 4). These shoots were lost during hardening. On agar media, plants showed a very good growth and development with green leaves and vigorous stems.

3.2. Subculture
From the results of statistical analysis, it is clear that the number of shoots obtained after subculture was significantly affected by the hormonal composition of the media and the type of gelling agent. After four subcultures, the shoot multiplication rate was high on M3 (1450 shoots/explant) and M3′ (1024 shoots/explant), followed by M2 (1089 shoots/explant) and M2′ (1376 shoots/explant) who regenerated some roots. Subcultures on M1 and M1′ have formed callus that stopped the development of shoots. Consequently, subculture was not achieved in this medium. In this experiment, agar-solidified media stimulated the regeneration and development of vigorous shoots. On the contrary, most of the shoots obtained from gelrite-gelled medium appeared to be vitrified, abnormal, with a glassy appearance.
3.3. Rooting Phase
The results of data analysis indicated a significant difference between both gelling agents for the number of roots (p = 0.0146). Agar-gelled media showing a high shoot capacity in 3 days with the highest number of roots (5.56 roots/shoots) (Figure 5). The number of roots was significantly different with Gelrite-solidified media, which recorded only 3.54 roots/shoots after 5 days of culture (Table 2). The type of gelling agent has significantly affected the length of roots. The longest roots (2.99 cm) were developed on an agar-gelled medium with a high percentage of root induction (97.22%).

| Roots induction (%) | Days for the emergence of roots | Means number of roots/shoots | Roots lent (cm) | ||
|---|---|---|---|---|---|
| G1 | Agar | 97.22 | 3 | 5.56a | 2.99a |
| Gelrite | 84 | 5 | 3.54b | 1.75b | |
| G2 | Agar | 92 | 3 | 4.53a | 2.09a |
| Gelrite | 80 | 7 | 3.35b | 1.07b | |
- Note: Means followed by the different letters are significantly differed from each other within the single variant or there interactions at 5% level according to Duncan’s multiple range test.
4. Discussion
In this study, the association between BAP and KIN (1.5 mg/L BAP + 0.25 mg/L KIN) was effective in inducing the shooting of stevia. These shoots were short and tiny, with very small and abnormal leaves, and forming green compact callus in their base that stopped the growth of shoots in the subcultures. Cytokinin, like BAP, was thought to promote cell division in plant tissue culture. Furthermore, cytokinin controls the production of proteins associated to plant growth that are necessary for mitosis [33]. When cytokinin is added to media, it stimulates the development of stevia plants’ aboveground portions; as a result, there are usually more shoots and leaves [34]. Jadid et al. [35] have noticed that the type and concentration of the cytokinin influence shoot proliferation of the plant. It was mentioned that the addition of both cytokinin (BAP) and KIN is necessary to obtain the best multiplication of Stevia in vitro [18]. On the other hand, Karunarathna et al. [4] and Aneela and Shaista [28] concluded that shoots grown on MS medium containing BAP have developed green compact callus at the base retarded the development of shoots. Yesmin [15] also reported that the subculture of shoots on MS medium containing BAP produced stunted and bushy shoots.
The hormone-free MS medium gave the lowest number of shoots. This medium was better in terms of shoot elongation with a high number of internodes for both gelling agents. This may be explaining by the abundance of endogenous auxin in explant [36]. However, auxin is known for its stimulating action on stem elongation [37]. Several authors reported that the maximum shoot length was obtained on a hormone-free MS medium in comparison with that supplemented with BAP [38–40]. Based on the results obtained, KIN was a growth regulator that supported the propagation of shoots by continuous subculture and obtained the highest multiplication rate. This was in line with the research of Das and Mandal [22], who emphasized the use of KIN in stevia multiplication compared to BAP.
The two genotypes studied showed a difference in all parameters (shoot number, shoot height, nodes number, root number, and root length). This variation between genotypes may be due to the differences in the composition of the exogenous hormones added into the medium [41] and their interactions with endogenous hormones. Clonal micropropagation of different genotypes with the use of lower concentrations of PGRs is an important technique to cultivate and preserve the Stevia plant [42]. Also, it is necessary for studies to develop a protocol of propagation for the genetic material that is available for specific regions [43].
The type of gelling agent affected the multiplication of stevia shoots. Agar-solidified medium performed better than the gelrite-gelled medium in all growth parameters (number of shoots, length of shoots, and number of internodes). Abnormal morphology and vitrified stems and leaves with yellow-green color and glassy appearance were developed during the culture period of shoots. This study confirmed the findings of Aneela and Shaista [28], who mentioned that gelrite is not effective for the multiplication of stevia in vitro. Also, Several researchers proved that hyperhydricity affected as much as 84% of the newly formed shoots on media gelled with gelrite In closed culture vessels [31].
The differential properties of various gelling agents depend on the degree of clarity, polymerizability, and water retention capacity, which in turn affect the availability of minerals and carbohydrates [27]. This may explain the differential response between agar and Gelrite in our shooting and rooting experiment. The gelling agents influence the tissue-culture process by controlling the water and nutrients accessibility to the explant [26]. Gelling agents enhance growth by providing plants with direct physical touch with nutrients [44]. Agar is a gelling agent metabolically inert and has been used for a long time for its stability, clarity, low adhesivity, and good diffusion characteristics [26]. Hence, in the present study, agar seems to have good nutrient uptake, which promotes to shooting and rooting of S. rebaudiana.
Gelrite is an extracellular polysaccharide that contains fewer organic impurities and fewer free minerals than agar [45] and is known by better availability of water and facilitates the diffusion of inhibitory molecules such as phenols [46]. The superiority of agar over Gelrite products may be attributed to the fact that gelling with gelrite involves Ca2+ ions, thus affecting the availability of Ca2+ in the medium after solidification [30]. This can lead to excessive hydration of tissues, which results in poor regeneration of normal and mature plants and the formation of enlarged, thick, translucent, and brittle shoots. This physiological malformation is associated with a deficiency in chlorophyll and poor lignification [31]. In the present study, Gelrite increases vitrification in stevia vitroplant. For this, agar was found to be the ideal gelling agent to overcome the problem of vitrification of stevia shoots caused by gelrite [47].
5. Conclusion
The results of this study demonstrate a rapid, efficient, and simple protocol for the direct regeneration of multiple shoots from nodal explants of selected S. rebaudiana genotypes by repeated subculture in a short time. This protocol could be commercially effective to produce uniform plants of stevia in a relatively short period and with a high multiplication rate.
The type of gelling agent used mostly influenced the in vitro growth of S. rebaudiana nodal segment, resulting in variations in the chemical and physical characteristics of the cultivated nodal segment. The MS medium gelled with 7 g/L is most suitable for in vitro induction of Stevia shooting and rooting with the aim to obtain healthy cultures. Considering all the aspects (number of shoots, number of internodes, shoots length, number of roots, and root length), agar was found to be a suitable gelling agent for Stevia propagation and to overcome the vitrification of shoots. Gelrite at 3 g/L was not appropriate for in vitro multiplication of Stevia. Agar is frequently utilized as a gelling agent in tissue culture because of its practical gelling qualities. Nevertheless, agar is expensive, and studies conducted to found different gelling agents as low-cost alternatives to agar are necessary.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ghizlane Bouaaza: concept and design, data acquisition. Ouiam chetto: drafting manuscript, data analysis/interpretation. Lhou Beniken: statistical analysis. Laila Dziri: experimental design, revised the manuscript. M’hamed Majji: reviewed the prepared manuscript for submission. Rachid Benkiran: critical revision of manuscript, supervision. Hamid Benyahia: supervising, final approval.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Acknowledgments
The support received from the COSUMAR group and Dr. Abdelmjid Zouahri, coordinator of the Research Unit of Environment and Conservation of Natural Resources INRA, CRRA of Rabat, for providing all research facilities and encouragement for this work is gratefully appreciated.
Open Research
Data Availability Statement
The data used to support the findings of this study are included within the article.




