Surface Properties and Potential Immunomodulatory Effects of Candidate Probiotic Lactobacilli for Gastrointestinal Applications
Abstract
Aim: This study was aimed at evaluating the adhesive and immunomodulatory properties of indigenous lactobacilli strains isolated from fermented foods, infant feces, and human milk.
Methodology: The adhesive properties of lactobacilli strains were evaluated for hydrophobicity, coaggregation, and autoaggregation capacities. The best strains were evaluated for adhesion to HT-29 cell line in the presence of lipopolysaccharides. Further, the best performing strains were evaluated in post, pre, and coculture methods for modulation of cytokine release and their expression in cell through RT-qPCR and ELISA, respectively.
Results: The test lactobacilli strains showed strong affinity toward toluene compared to hexadecane. Seven of the strains, namely, 3ST3, 3ST7, 8BM6, 8ST7, BK5, GR27, and GR8, were strongly (> 60%) hydrophobic toward both the solvents. Strains belonging to the Lacticaseibacillus casei group (3ST5, 72%; PT1, 64.33%; and 3BM4, 60%), except Lacticaseibacillus paracasei BK5, showed promising autoaggregation. The majority of strains displayed marked coaggregation within the ranges of 50%–70%, significantly higher than probiotic Lacticaseibacillus rhamnosus GG (LGG) (33%). Qualitative adhesion revealed that strain Lactiplantibacillus pentosus 15ST2 exhibited the highest rate of adhesion (45.33%) around the edges and surface of the HT-29 cells. Most of the lactobacilli significantly (p < 0.05) inhibited Staphylococcus aureus by exclusion interference. During transcriptional studies, a significant upregulation in the expression of TLR2 and the proinflammatory marker TNF-α was observed for KN3 (14.08-fold) and the 3BM3 strain (7.76-fold) in the coculture model. While in pre- and postculture, no significant difference in the expression of TLR2, TNF-α, and IL-10 was recorded in comparison to LPS-stimulated cells. The proinflammatory cytokine IL-8 was upregulated during pre- and postculture conditions at both transcription and translation levels.
Conclusion: The indigenous lactobacilli (15ST2, 3BM3, and KN3) strains effectively adhered to intestinal cell surface and thereby have shown their potential to modulate the production of proinflammatory cytokines at both transcription and translation levels, when introduced alongside pathogens or established before bacterial infection.
1. Introduction
The group of bacteria formerly known as Lactobacillus constitute a large range of lactic acid bacteria (LAB) which are now reclassified into 25 genera (the amended genus Lactobacillus (e.g., Lactobacillus delbrueckii) and Paralactobacillus (Paralactobacillus selangorensis)), as well as the 23 new genera [1]. Among these, several species have had a long history of application, with the generally regarded as safe (GRAS) status. Lactobacilli are one of the most common groups of human-associated bacteria, present in mother’s milk, gastrointestinal tract (GIT), respiratory tract, and the genital tract [2, 3], having wide applications in food and pharmaceutical industries as starter cultures and as probiotics, because of their safety and beneficial attributes. However, the ability to aggregate, adhere to the mucus layer of the intestinal cell wall, and to modulate the immune system can be considered as beneficial probiotic characteristics [4, 5]. This attribute may allow them to survive, colonize, and exert their beneficial effects in the host GIT. Although this property does not necessarily guarantee a health benefit, it is believed to exclude pathogens from binding sites in the host cells [6, 7]. The cell surface hydrophobicity, autoaggregation, and coaggregation abilities of lactobacilli strains ensure their potential attachment to the intestinal cell surface; however, these properties do not guarantee health benefits to the body [8–10]. The ability to auto- and coaggregate enhanced their potential to build a preventive barrier for pathogen adhesion to the host cell, further making in hostile environment for biofilm formation by pathogenic bacteria [11].
Adhesion of a lactobacillus strain to the host cell might regulate cell signaling through modulating release and expression of cytokines from the host cells during, before, or after onset of disease or infection [4]. Regulation of inflammatory mediators and cytokines is a promising factor for understanding the inflammation in the intestine and thereby providing pathophysiological role of probiotic interventions. Interaction of a complex array of cells, their metabolites, and molecules of the immune system provides protection from invading pathogens via T cell mediation [12]. Although various probiotic strains have been extensively explored for their immunomodulatory properties, the evaluation of efficient indigenous probiotic strains specific to a particular geographical area is of utmost importance. More so, hostile climatic conditions affect these strains thereby enhancing their consumption, survivability, and attachment inside the host. Keeping all above in view, the present study was specifically undertaken with the objective of assessing the adhesion and immunomodulatory efficacy of potential indigenous probiotic lactobacilli isolated from fermented foods, infant feces, and human milk under different sets of conditions.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Thirty-four LAB strains were isolated from human sources (milk and infant feces) and locally fermented foods indigenous to Nigeria (Table 1). Isolates were identified by genus-specific PCR and MALDI-TOF and further characterized by 16S rRNA sequencing method [13, 14]. Reference strain Lacticaseibacillus rhamnosus GG (LGG) ATCC 53103 and one enteropathogen Staphylococcus aureus ATCC 700698 were obtained from the culture collection of the Department of Dairy Microbiology, GADVASU, India. The lactobacilli and reference cultures were maintained in de Man Rogosa Sharpe (MRS) medium supplemented with L-cysteine (Merck KGaA, Darmstadt, Germany), while S. aureus was maintained in Baird–Parker agar (BPA) medium at 37°C. All the strains were preserved at −80°C as glycerol stocks.
| S. no. | Source | Origin | Strain—lab identity | Accession numbers | Identity | Identification by |
|---|---|---|---|---|---|---|
| 1 | Human | Infant feces at 3 months | 3ST2 | KJ726660.1 | Lacticaseibacillus casei | G, M, 16S |
| 2 | Infant feces at 3 months | 3ST3 | MT515983.1 | Lacticaseibacillus casei/paracasei | G, M, 16S | |
| 3 | Infant feces at 3 months | 3ST5 | — | Lacticaseibacillus casei | G, M | |
| 4 | Infant feces at 3 months | 3ST7 | — | Lacticaseibacillus casei | G, M | |
| 5 | Human milk at 3 months | 3BM1 | MT464356.1 | Levilactobacillus brevis/plantarum | G, M, 16S | |
| 6 | Human milk at 3 months | 3BM3 | — | Lacticaseibacillus casei | G, M | |
| 7 | Human milk at 3 months | 3BM4 | — | Lacticaseibacillus paracasei | G, M | |
| 8 | Human milk at 8 months | 8BM6 | MH606197.1 | Lacticaseibacillus casei | G, M, 16S | |
| 9 | Human milk at 8 months | 8BM9 | — | Levilactobacillus brevis | G, M | |
| 10 | Infant feces at 8 months | 8ST5 | — | Lactiplantibacillus pentosus | G, M | |
| 11 | Infant feces at 8 months | 8ST7 | MT071603.1 | Lactiplantibacillus pentosus/fermentum | G, M, 16S | |
| 12 | Infant feces at 15 months | 15ST2 | MF541063.1 | Lactiplantibacillus pentosus/plantarum | G, M, 16S | |
| 13 | Fermented foods | Fura da nono | NON4 | MH704100.1 | Levilactobacillus brevis/paracasei | G, M, 16S |
| 14 | Kunu | KN3 | MT613622.1 | Lacticaseibacillus casei/paracasei | G, M, 16S | |
| 15 | Kunu | KN5 | — | Levilactobacillus brevis | G, M | |
| 16 | Kunu | KN6 | — | Lacticaseibacillus casei | G, M | |
| 17 | Kunu | KN9 | — | Levilactobacillus brevis | G, M | |
| 18 | Kunu | KN10 | Levilactobacillus brevis | G, M | ||
| 19 | Garri | GR2 | — | Levilactobacillus brevis | G, M | |
| 20 | Garri | GR4 | — | Lacticaseibacillus casei | G, M | |
| 21 | Garri | GR5 | — | Levilactobacillus brevis | G, M | |
| 22 | Garri | GR8 | — | Lacticaseibacillus casei | G, M | |
| 23 | Garri | GR11 | — | Lacticaseibacillus paracasei | G, M | |
| 24 | Garri | GR12 | MT613613.1 | Lactiplantibacillus plantarum | G, M, 16S | |
| 25 | Garri | GR13 | — | Levilactobacillus brevis | G, M | |
| 26 | Garri | GR27 | — | Lacticaseibacillus casei | G, M | |
| 27 | Garri | GR29 | — | Levilactobacillus brevis | G, M | |
| 28 | Garri | GR32 | — | Lacticaseibacillus casei | G, M | |
| 29 | Burukutu | BK4 | MN166310.1 | Lacticaseibacillus paracasei | G, M, 16S | |
| 30 | Burukutu | BK5 | — | Lacticaseibacillus paracasei | G, M | |
| 31 | Burukutu | BK8 | KT589132.1 | Levilactobacillus brevis | G, M, 16S | |
| 32 | Pito | PT1 | — | Lacticaseibacillus paracasei | G, M | |
| 33 | Fermenting akpu | AK1 | — | Lacticaseibacillus casei | G, M | |
| 34 | Fermenting akpu | AK5 | MN658813.1 | Lacticaseibacillus casei | G, M, 16S | |
| 35 | RS | Reference strain | LGG-ATCC 53103 | — | Lacticaseibacillus rhamnosus GG | G, M |
- Abbreviations: 16S, 16SrDNA sequencing; G, genus-specific PCR; M, MALDI-ToF-MS; RS, reference strain.
2.2. Cell Surface Hydrophobicity
2.3. Aggregation Assay
Autoaggregation and coaggregation assays were performed according to the protocol of Collado et al. [16] with some modifications. Lactobacilli strains cultured at 37°C for 20 h were harvested by centrifugation at 10,000g for 10 min at 4°C (Centrifuge 5804R, Eppendorf) and washed twice with sterile phosphate-buffered saline (PBS, pH 7.2) and resuspended in the same buffer. The bacterial cultures were diluted with PBS to obtain uniform absorbance at A600. Resulting bacterial suspensions were incubated at 37°C for 5 h and A600 was measured. Autoaggregation (percentage) was expressed as [1 − At/A0] × 100, where At represents absorbance at 5 h and A0 at 0 h.
2.4. Propagation and Maintenance of HT-29 Cells
Adhesion studies were carried out using the human adenocarcinoma cell line HT-29 obtained from NCCS Pune, India. The HT-29 cell line (11 passages) was maintained on Dulbecco’s modified Eagle’s medium (DMEM) high glucose (4500 mg/L), supplemented with 10% (v/v) heat-inactivated (30 min at 56°C) fetal bovine serum (FBS), 1% nonessential amino acids, 2.5% HEPES, streptomycin (100 μg/mL), and penicillin (100 U/mL) in T25 flasks (Eppendorf, United States). The cells were incubated at 37°C and 5% CO2 and fed fresh medium every alternate day [17]. On reaching 90%–100% confluency, cells were trypsinized and seeded in 12-well tissue culture plates (Eppendorf, United States) and incubated at 37°C and 5% CO2 for adhesion assay.
2.5. Adhesion Assay
2.6. Adhesion Interference
Adhesion interference was evaluated for lactobacilli strains that showed potential adherence to HT-29 cells, along with the reference strain (LGG) and the enteropathogen S. aureus using the method given by Spencer and Chesson [19]. For the competition adhesion test, each lactobacilli test strain, along with S. aureus (cell concentration 108 CFU/mL), was simultaneously added to HT-29 monolayers and incubated for 120 min at 37°C and 5% CO2. In the exclusion test, HT-29 monolayers were first treated with test strains (108 CFU/mL) and incubated for 1 h to allow lactobacilli to interact with intestinal cells, followed by 1 h of treatment with S. aureus (108 CFU/mL). For the displacement test, HT-29 monolayers were first treated with S. aureus for 1 h, followed by treatment with test strains for another 1 h. Following the above treatments, cell supernatants were aspirated, and cell monolayers were given a gentle wash to remove any nonadherent bacterial cells. After washing, cell monolayers were trypsinized and appropriate dilutions were plated over MRS and BPA medium. Percentage adhesion was determined by calculating the number of colonies in each treatment relative to counts before adhesion.
2.7. Immunomodulatory Effects of LAB Strains Under In Vitro Cell Line Conditions
From the results of the adhesion assay, three lactobacilli strains (Lacticaseibacillus casei KN3, Lactiplantibacillus pentosus 15ST2, and Lb. casei 3BM3), one each representing fermented food, infant feces, and human milk sources, alongside the reference strain LGG, were further selected for determining their immunomodulatory potential under the in vitro HT-29 cell line model induced with bacterial lipopolysaccharide (LPS). The expression of inflammatory biomarkers was investigated in the presence of Salmonella enterica serotype Typhimurium LPS (50 ng/mL, Sigma Aldrich). Prior to the experiment, HT-29 cells were preincubated in basal medium (500 μL) for 30 min at 37°C, followed by the addition of 500 μL fresh medium in each well. The HT-29 cells were challenged with LPS and lactobacilli under three different conditions: coculture, prechallenge, and postchallenge. In the coculture model, LPS and lactobacilli cultures were added simultaneously to wells and incubated at 37°C and 5% CO2 for 2 h. In the prechallenge model, HT-29 cells were first challenged with LPS for 1 h, followed by the addition of lactobacilli cultures for another 1 h. In the postchallenge model, lactobacilli cultures were first added to HT-29 cells and incubated for 1 h, followed by the addition of LPS and incubation for another 1 h. The untreated well and LPS-treated wells were taken as negative and positive controls, respectively. At the end of incubation, the supernatant from each well was aspirated, centrifuged at 5000g for 5 min, and stored at −80°C for further ELISA-based analysis for secretory proteins. The HT-29 cells from each well were harvested after trypsinization and subjected to RNA isolation using Trizol–chloroform extraction method. RNA quantification was done using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts) and used for cDNA synthesis using TAKARA Clontech First-Strand and Second-Strand cDNA Synthesis Kit protocol (Fermentas, Burlington, Canada). The quality and concentration of cDNA were verified by the NanoDrop ND-2000 spectrophotometer for ssDNA. The cDNA aliquots (50 ng/μL) were stored at −80°C for RT-qPCR–based expression analysis of target genes [20].
2.8. Quantitative Expression of Biomarkers Using RT-qPCR
The cDNA obtained from different treatments was analyzed for expression of TLR2 and anti-inflammatory and proinflammatory biomarkers, namely, IL-10, IL-8, and TNF-α. RPS18, B2M, and ACTB were included as housekeeping genes for expression normalization. The primer sequences of target genes are presented in Table 2. The reaction mixture had a total volume of 10 μL that contained 5 μL of SYBR green, 1 μL of each forward and reverse primer (2.5 pmol/μL), and 3 μL cDNA template (50 ng/μL). The thermal cycling conditions included initial denaturation of 95°C for 5 min, followed by 35 cycles of denaturation (95°C for 15 s), annealing (60.5°C for 30 s), and extension (72°C for 30 s), and melt curve. Relative quantification of mRNA expression was assessed by normalizing the cycle threshold (CT) values of the target genes to the CT values of the housekeeping genes. The results were presented as fold change using the 2−ΔΔCT method [21].
| Primer | Sequences | Reference |
|---|---|---|
| IL-8 | F-TGGCTCTCTTGGCAGCCTTC | Seagusa et al., [22] |
| R-TGCACCCAGTTTTCCTTGGG | ||
| IL-10 | F-GTGACGTACTCCATCTCACATC | Powell et al., [23] |
| R-TAGCTCTCTGGTGGCTAAGT | ||
| TNF-α | F-TCTGGGCAGGTCTACTTTGG | Barthel and Goldfeld, [24] |
| R-TGAGCCAGAAGAGGTTGAGG | ||
| TLR2 | F-GGCCAGCAAATTACCTGTGTG | Klawitter et al., [25] |
| R-CAGAGTTTCCTGCAATGGATCA | ||
| RPS18 | F-AGCTTGTTGTCCAGACCATT | Lallemant et al., [26] |
| R-TGAGGAAAGCAGACATTGAC | ||
| B2M | F-CAGCGTACTCCAAAGATTCA | Lallemant et al., [26] |
| R-GAATGCTCCACTTTTTCAAT | ||
| ACTB | F-TGGCTGGGGTGTTGAAGGTCT | Lallemant et al., [26] |
| R-AGCACGGCATCGTCACCAACT |
2.9. Quantification of Secreted Proteins by ELISA
The supernatants collected from each treatment were analyzed for anti-inflammatory (IL-10) and proinflammatory biomarkers (IL-8 and TNF-α). Secretion of each biomarker from HT-29 cells under different conditions was evaluated using commercially available ELISA kits (Weldon Biotech) according to the manufacturer’s instructions.
2.10. Statistical Analysis
Mean values generated from triplicate data were evaluated by the mixed model analysis. Significant divergences among mean values were determined with Tukey’s test at levels of significance p < 0.05, 0.01, 0.0001. All statistical analyses were performed using the IBM SPSS statistics, 25 (IBM Corporation, United States).
3. Results and Discussion
3.1. Adhesion Properties of Indigenous Lactobacilli Strains
The probiotic strain should effectively colonize the intestine to exert its beneficial effects. The measure of intestinal colonization such as its adhesion and persistence capacities of strains is evaluated through calculating cell surface hydrophobicity, aggregation, and coaggregation properties. Cell hydrophobicity is directly related to intestinal colonization; further higher adhesion to hydrocarbons (BATH) shows better cellular aggregation and coaggregation. Bacterial hydrophobicity plays an important role in adhesion and biofilm formation. The 34 lactobacilli strains along with one reference strain of LGG (Figure 1a,b and Table 3) were investigated for their cell surface hydrophobicity. Cell hydrophobicity values are categorized as hydrophilic (0%–39%), weakly hydrophobic (40%–50%), and strongly hydrophobic (≥ 60%) [27]. Among the 34 tested candidate probiotic lactobacilli, variable results were obtained toward both hydrocarbons. Isolates from fecal samples exhibited significantly (p < 0.01, p < 0.0001) higher hydrophobicity toward n-hexadecane (4.33%–94%), compared to those from human milk (2.67%–61.67%) and fermented foods (7%–82.33%). However, when isolates were assayed against toluene, strains AK5, GR4, and 3ST2 from fermented foods and fecal sample exhibited relatively higher (p < 0.05) hydrophobicity (90.00%, 89.67%) slightly above the standard strain (83.00%). Although 45.7% of isolates were hydrophilic toward n-hexadecane and only 22.9% toward toluene, no significant (p > 0.05) difference was recorded between the two hydrocarbons (refer to Figure S1a and S1b). The variations in cell surface charge observed in the present study may be attributed to the presence of acidic surface component, surface-associated proteins of bacterial strains, low electron acceptance by the cell surface, or the presence of electrostatic interactions between the bacterial cell membranes and interacting surfaces [28]. Kos et al. [29] demonstrated that the adhesion properties of Lactobacillus acidophilus M92 declined significantly upon removal of S layer protein by proteolytic treatment. [30]. Similar findings were earlier reported by De Souza et al. [31] and Krausova et al. [32]. Interestingly, when the data was screened on basis of the lactobacilli strains studied, Lb. casei/paracasei emerged to be the most hydrophobic against both hydrocarbons. The reason for this variation in cell surface hydrophobicity between different hydrocarbons may also lie in their zeta potentials. It decreases with changes in pH due to the hydrogen-accepting capacity, characteristic of the electron ring in the aromatic hydrocarbons compared to the aliphatic hydrocarbons. This property is thought to have some implications in the adhesion of bacteria to hydrophobic surfaces.
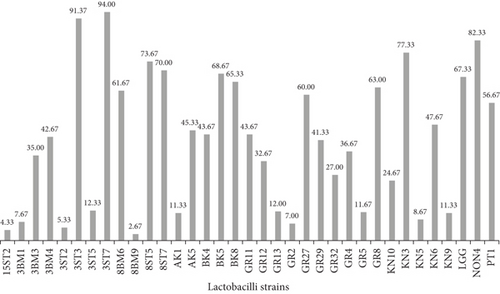
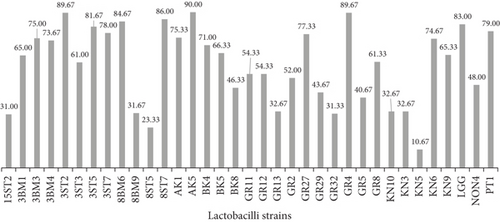
| Origin | Assay | Hydrophobicity (%) | Aggregation (%) after 5 h | Adhesion (%) | ||
|---|---|---|---|---|---|---|
| Strains | n-Hexadecane | Toluene | Autoaggregation | Coaggregation | ||
| Human sources | L. pentosus 15ST2 | 4.33 ∗ | 31.00 ∗ | 44.67 ∗ | 54.00 ∗ | 45.33 ∗ |
| L. brevis 3BM1 | 7.67 ∗ | 65.00 ∗ | 53.67 ∗ | 55.00 ∗ | 10.60 ∗ | |
| L. casei 3BM3 | 35.00 ∗ | 75.00 ∗ | 46.00 ∗ | 52.67 ∗ | 12.53 ∗ | |
| L. paracasei 3BM4 | 42.67 ∗ | 73.67 ∗ | 60.00 ∗ | 64.33 ∗ | 0.33 | |
| L. casei 3ST2 | 5.33 ∗ | 89.67 ∗ | 41.00 ∗ | 62.33 ∗ | 15.47 ∗ | |
| L. paracasei 3ST3 | 91.37 ∗ | 61.00 ∗ | 41.33 ∗ | 52.00 ∗ | 0.83 | |
| L. casei 3ST5 | 12.33 ∗ | 81.67 ∗ | 72.00 ∗ | 69.67 ∗ | 3.47 | |
| L. casei 3ST7 | 94.00 ∗ | 78.00 ∗ | 45.33 ∗ | 70.67 ∗ | 0.40 | |
| L. casei 8BM6 | 61.67 ∗ | 84.67 ∗ | 40.33 ∗ | 43.00 ∗ | 9.67 ∗ | |
| L. brevis 8BM9 | 2.67 ∗ | 31.67 ∗ | 50.67 ∗ | 61.00 ∗ | 12.73 ∗ | |
| L. pentosus 8ST5 | 73.67 ∗ | 23.33 ∗ | 45.67 ∗ | 66.33 ∗ | 1.17 | |
| L. pentosus 8ST7 | 70.00 ∗ | 86.00 ∗ | 41.67 ∗ | 53.33 ∗ | 9.13 ∗ | |
| Fermented foods | L. casei AK1 | 11.33 ∗ | 75.33 ∗ | 55.33 ∗ | 56.00 ∗ | 35.80 ∗ |
| L. casei AK5 | 45.33 ∗ | 90.00 ∗ | 44.00 ∗ | 57.67 ∗ | 0.97 | |
| L. paracasei BK4 | 43.67 ∗ | 71.00 ∗ | 47.67 ∗ | 55.00 ∗ | 6.13 ∗ | |
| L. paracasei BK5 | 68.67 ∗ | 66.33 ∗ | 45.67 ∗ | 26.00 ∗ | 7.77 ∗ | |
| L. brevis BK8 | 65.33 ∗ | 46.33 ∗ | 45.33 ∗ | 53.67 ∗ | 17.03 ∗ | |
| L. brevis GR2 | 7.00 ∗ | 52.00 ∗ | 49.33 ∗ | 50.67 ∗ | 3.3 | |
| L. casei GR4 | 36.67 ∗ | 89.67 ∗ | 39.33 ∗ | 63.33 ∗ | 0.43 | |
| L. brevis GR5 | 11.67 ∗ | 40.67 ∗ | 45.00 ∗ | 50.00 ∗ | 0.57 | |
| L. casei GR8 | 63.00 ∗ | 61.33 ∗ | 45.33 ∗ | 59.00 ∗ | 1.3 | |
| L. paracasei GR11 | 43.67 ∗ | 54.33 ∗ | 46.33 ∗ | 34.67 ∗ | 10.30 ∗ | |
| L. plantarum GR12 | 32.67 ∗ | 54.33 ∗ | 53.00 ∗ | 56.00 ∗ | 4.37 | |
| L. brevis GR13 | 12.00 ∗ | 32.67 ∗ | 42.33 ∗ | 64.67 ∗ | 2.2 | |
| L. casei GR27 | 60.00 ∗ | 77.33 ∗ | 34.67 ∗ | 62.00 ∗ | 0.93 | |
| L. brevis GR29 | 41.33 ∗ | 43.67 ∗ | 54.67 ∗ | 70.00 ∗ | 0.23 | |
| L. casei GR32 | 27.00 ∗ | 31.33 ∗ | 46.00 ∗ | 52.00 ∗ | 4.43 | |
| L. casei KN3 | 77.33 ∗ | 32.67 ∗ | 47.33 ∗ | 64.67 ∗ | 36.20 ∗ | |
| L. brevis KN5 | 8.67 ∗ | 10.67 | 56.33 ∗ | 59.67 ∗ | 2.17 | |
| L. casei KN6 | 47.67 ∗ | 74.67 ∗ | 55.67 ∗ | 60.67 ∗ | 0.53 | |
| L. brevis KN9 | 11.33 ∗ | 65.33 ∗ | 45.67 ∗ | 55.67 ∗ | 0.17 | |
| L. brevis KN10 | 24.67 ∗ | 32.67 ∗ | 42.00 ∗ | 51.67 ∗ | 0.47 | |
| L. brevis NON4 | 82.33 ∗ | 48.00 ∗ | 44.00 ∗ | 60.00 ∗ | 30.80 ∗ | |
| L. paracasei PT1 | 56.67 ∗ | 79.00 ∗ | 64.33 ∗ | 72.33 ∗ | 20.03 ∗ | |
| Reference strain | L. rhamnosus GG ATCC 53103 | 67.33 ∗ | 83.00 ∗ | 46.67 ∗ | 35.67 ∗ | 11.67 ∗ |
- Note: Values are least square means of three replicates ± SEM. Asterisk (∗) denotes high significant differences (p < 0.0001) among strains.
Autoaggregation ability of bacteria is a very important criterion for selection of potential probiotic strains as it relates to biofilm formation. Thirty-five lactobacillus strains including the standard strain LGG exhibited autoaggregations ranging from 34.7% to 72.0% (Figure S2). The highest percentages of autoaggregation were recorded after 5 h of incubation at 37°C in strains 3ST5 (72%), PT1 (64.33%), and 3BM4 (60%), while the least was GR27 (34%). Most strains showed higher aggregations than strain LGG (46.67%), as shown in Figure 2 and Table 3. No significant difference (p > 0.05) was observed between the different lactobacillus strains and the standard. Pisano et al. [33] reported that obligatory homo-fermentative lactobacilli exhibit higher autoaggregation, whereas in our investigation, high percentages of autoaggregation (≥ 40%) were recorded for facultative and obligate hetero-fermentative lactobacilli.
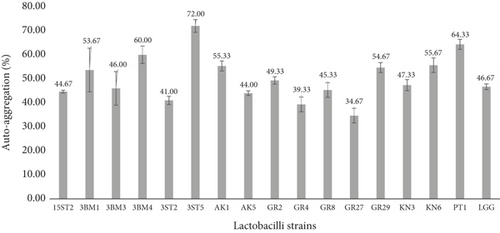
Autoaggregation ability in potential probiotic bacteria is advantageous to antagonize the presence of pathogens and also for gaining opportunity to persist longer in the GIT [16, 34]. Krausova et al. [32] reported autoaggregation of lactobacilli species ranging from 21.7% to 69.7%. The present results established that lactobacilli isolates can form excellent autoaggregation within the shortest period of incubation (5 h), consistent with reports by several other authors [10, 35, 36]. Meanwhile, a study by Pessoa et al. [37] reported very low autoaggregation abilities of Lactiplantibacillus plantarum strains within the ranges of 18.08%–20.94% after 5 h of incubation. The different results presented here may be attributed to strain-dependent or physiochemical characteristics associated with the cell surfaces.
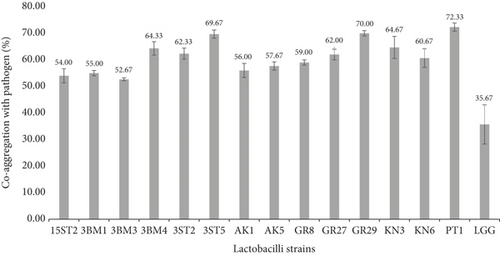
Coaggregation represents a barrier to prevent intestinal surface colonization by pathogenic microorganisms. It was suggested that coaggregation of lactobacilli may prevent colonization by pathogens [38]. Ninety-one percent of the tested strains demonstrated some coaggregation properties with S. aureus ATCC 700698 within the ranges of 50%–70% compared to the reference strain LGG, which expressed only 33% coaggregation, as shown in Figure 3 (also check Figure S3). Most of the strains (62.9%) that showed the highest coaggregation (≥ 50%) were of the Lb. casei group. The present study is in concordance with Rajoka et al. [39], who reported the highest coaggregation of Lb. rhamnosus with Escherichia coli at 49%, S. enterica at 52%, and S. aureus at 29%. Alp and Kuleasan [36] reported coaggregation of four lactobacilli strains and Weissella cibaria strain with E. coli and S. enterica within the ranges of 21.95%–83.87% after 5 h of incubation, respectively. Similarly, Arena et al. [34] observed high coaggregation (46.67% and 82.10%) among eight lactobacilli strains with different pathogens. The present study also agrees with De Souza et al. [31] and Arena et al. [34] on the hypothesis that higher coaggregation values depict good biofilm formations leading to minimal colonization by pathogens. Our results suggest that lactobacilli have the ability to promote coaggregation with pathogens, thereby competing for adhesion to the epithelial cell surface. This phenomenon is strain-dependent and might be due to the presence of specific surface molecules of lactobacilli that act as ligands binding pathogens and/or as adhesins for attachment to epithelial cells.
3.2. Adhesion of Lactobacilli to HT-29
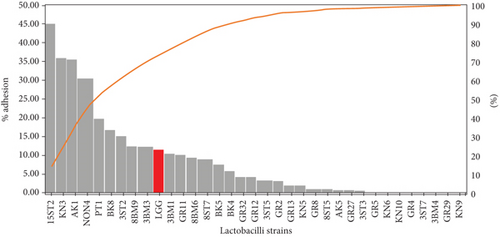
The adhesion of 35 lactobacillus strains to HT-29 cell line including the standard strain LGG was examined in vitro. The assessment of lactobacilli attachment to intestinal mucosa showed the protective effects of these strains against enteropathogens through competition for host cell binding sites. Further, assessing the adhesion ability of these strains under in vitro conditions may show their ability to interact with host cells (temporary colonization) and enhance their transit time in the gut for conferring beneficial effects. In this present study, adhesion was mostly seen around the edges of the epithelial cells which resulted in the formation of aggregates (Figure 4). High adhesion was observed for Lb. plantarum 15ST2 which adhered to the edges as well as colonized the entire cell monolayer, suggesting negligible effect of mucin on adhesion. Similar observations were made by Muryany et al. [40], who observed aggregation patterns on the surface and sides of the intestinal epithelial cells under light and scanning electron microscopes. The qualitative test which was based on microscopic examination of cells behavior in respect to adhesion was further validated quantitatively by enumerating adherent bacteria (Figure 5 and Table 3). A number of the strains showed excellent adhesion abilities, ranging from 10% to 45%. The strains, namely, Lb. plantarum 15ST2 (45.33%), Lb. casei AK1 (35.80%), Lb. casei KN3 (36.20%), Lb. brevis NON4 (30.80%), Lb. brevis BK8 (17.03%), Lb. paracasei PT1 (20.03%), Lb. casei 3ST2 (15.47%), Lb. brevis 8BM9 (12.73%), and Lb. casei 3BM3 (12.53%), showed higher percentages of adhesion, whereas the reference strain LGG showed an adhering ability of 11.67% lower than most of the strains.
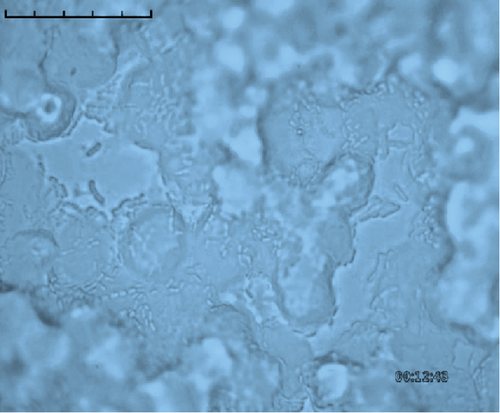
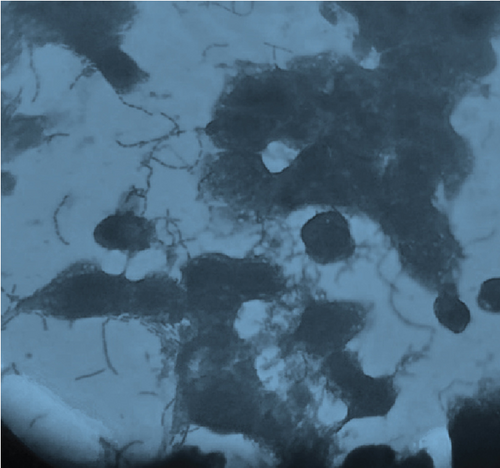
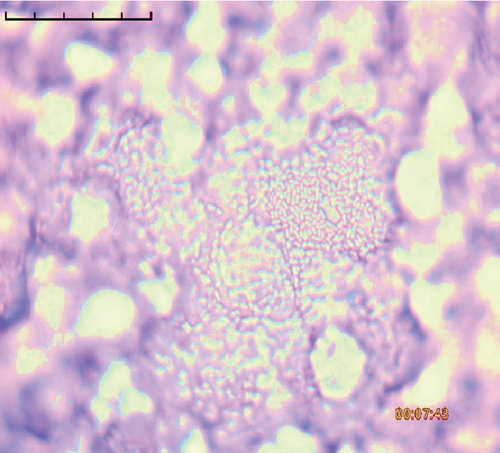
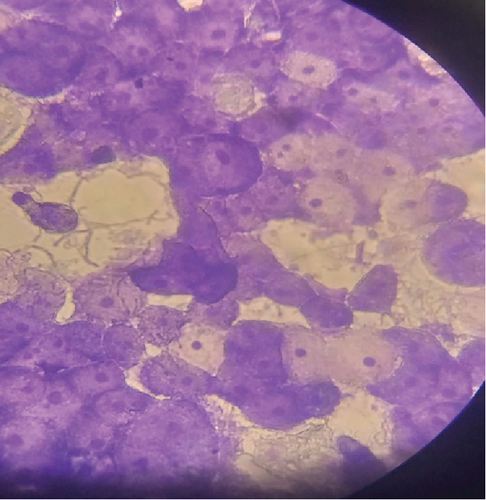
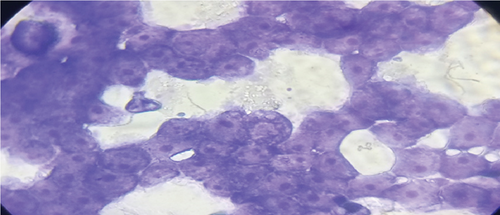
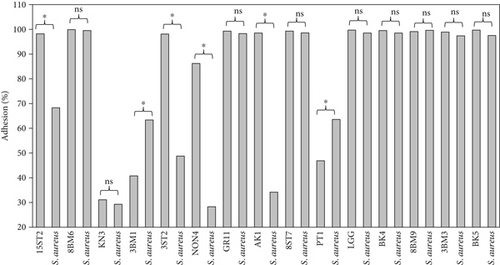
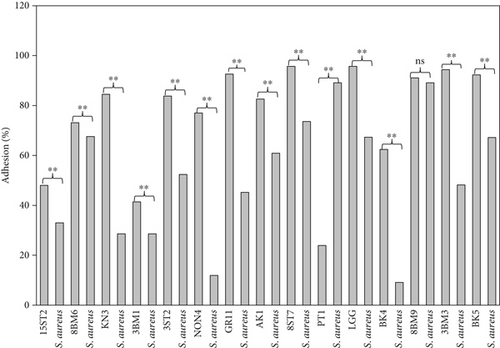
Intestinal mucosa absorbs essential nutrients from the lumen, produces mucus, and secretes protective cytokines and signaling features [41]. Therefore, it is essential that candidate probiotic strains should have good adhesive abilities to adhere and persist on the intestinal mucosa. Macromolecules associated with bacterial cell surfaces are considered to be the major determining factor in lactobacilli adhesion to the GIT. In the present study, microscopic examination showed an overall strong adhesion ability of lactobacilli strains to HT-29 cells. Ramos et al. [42] observed similar results from low adherence (0.3%) to high adherence (1.8%) with three lactobacilli strains using Caco-2 cell lines. Adhesion to mammalian epithelial cells is considered an important property in potential probiotic bacteria as it determines their ability to survive and colonize the GIT. This statement holds true for pathogenic bacteria that could cause competitive inhibition of probiotic colonization and subsequently lead to infection. So, the interference study of 15 test lactobacilli strains along with reference strain LGG and S. aureus enteropathogenic strain was conducted. In competitive interference, effective inhibition in adhesion of S. aureus with HT-29 cells was recorded with strains AK1, 3ST2, NON4, and 15ST2 with a significant (p < 0.05) margin of ≥ 50%. In the displacement interference assay, only 3ST2, PT1, 3BM3, 8BM6, 3BM1, and AK1 were able to displace S. aureus to a level of reducing the pathogen counts from 98.5% to 16.7%. The concentrations of S. aureus counted were significantly higher (p < 0.0001) among other lactobacilli strains. This indicates a noninhibitory effect against the food-borne pathogen, once established on HT-29 cells. Our results suggest that the concentration of adhering lactobacilli in the GI contents needs to be maintained at a high level before infection, so as to interfere with the pathogen adherence. Contrastingly in exclusion interference, the majority of lactobacilli strains showed high inhibition (41.4%–95.7%) of S. aureus adhesion (Figure 6). The present results were consistent with Singh et al. [43], Lau and Chye, [44], and Zawistowska et al. [38], who reported decreased (38%–90%) invasion by pathogens in exclusion interference on colon cells following pretreatment with different lactobacilli and bifidobacteria strains. In another related study, poor inhibition of pathogens was reported during displacement interference assay by Lb. plantarum strains and different enteropathogens on HCT-116 cells [45]. Nantavisai et al. [46] reported significantly reduced pathogen adhesion in exclusion study while no inhibition was observed in displacement assay of Ligilactobacillus salivarius MSMC105 on Caco-2 cells against two enteropathogens.
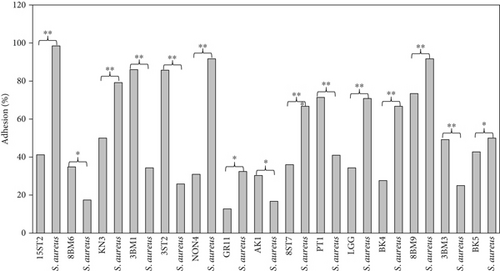
The relationship between cell surface hydrophobicity and adhesion over intestinal cells could not be positively interlinked for all the test strains. Only 47% out of the 35 lactobacilli strains screened in this study, including the reference strain LGG, showed a positive correlation between surface hydrophobicity and adhesion. In contrast to this, the remaining 53% were either potentially hydrophobic and poorly adhesive, as observed in strain Lb. casei 3ST7, or hydrophilic but strongly adhesive, as recorded by strain Lb. pentosus 15ST2. Similar results were also presented by Chaffanel et al. [6] and Krausova et al. [32], who also observed high adhesion to HT-29 cells among hydrophilic Lactobacillus, Bifidobacterium, and Streptococcus strains. However, Ehrmann et al. [47] previously reported a correlation between hydrophobicity and adhesion, while Tuo et al. [48] found no correlation among the lactobacilli strains in their study. Therefore, it can be stated here that the proportionality of surface hydrophobicity to intestinal model adhesion is strain dependent and is not always interlinked.
The ability of microbial cells to autoaggregate and coaggregate plays an important role in the formation of biofilms in several ecological niches. These two processes have been reported in relation to adhesion abilities of probiotic bacteria by several researchers [18, 49, 50]. A study by Del Re et al. [9] revealed a strong relationship between autoaggregation and adhesion. Similarly, Tuo et al. [48] reported a positive relationship between adhesion to Caco-2 cells and aggregation among the 15 lactobacilli strains screened. In this present study, a strong positive relationship was observed between aggregation abilities of 16 lactobacilli strains and their adhesion to HT-29. The formation of probiotic biofilm is beneficial to the host, which increases the persistence of beneficial bacteria in the intestinal model system, thereby preventing the colonization of pathogens [7, 51, 52]. The above results indicated that the lactobacilli strains screened in this study have ability to adhere, form biofilms, and resist the invasion of pathogens.
3.3. Expression and Secretion Analysis of Target Biomarkers
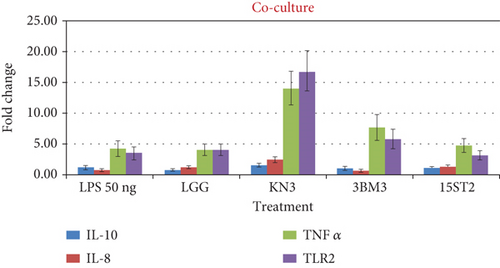
Three representative lactobacilli strains, namely, KN3, 3BM3, and 15ST2 from fermented food, human milk, and infant feces origin, respectively, were selected based on their high percentages of adhesion to HT-29 cells. In the follow-up study, HT-29 cells were challenged with Salmonella LPS and lactobacilli under coculture, prechallenge, and postchallenge models. The three lactobacillus species conferred varying effects of cytokine productions by HT-29. For instance, KN3 induced higher secretion of both TLR2 and TNF-α cytokine, followed by 3BM3 and 15ST2 which produced relatively, higher amounts of TNF-α than the TLR2. TLR2 (Toll-like receptor 2) are germline-encoded type I transmembrane receptors, expressed in a wide variety of immune cells including macrophages, dendritic cells (DCs), T lymphocytes, and intestinal epithelial cells. TLR2 are located at the cell surface, where they sense external microbial components such as lipopeptides, flagellins, and LPS. Their stimulation induces the production of proinflammatory cytokines and chemokines [53–55]. Physiologically, TNF-α is a crucial component for a normal immune response. TNF-α can activate the immune system; however, the excessive production of TNF-α can be harmful and may lead to the development of pathological complications [56–58]. In this study, large amounts TNF-α were expressed following TLR2 stimulation by Salmonella LPS during the coculture incubation model. However, under the pre- and postincubation culture conditions, a low expression of TNF-α was observed. Suggesting that, potential probiotic bacteria are capable of modulating the effects of the cytokine when introduced either before or after an infection. Our result corroborated with that of Haque et al. [59] who examined the effective inhibition of TNF-α by Lactobacillus acidophilus (LA1 or LA3) in intestinal epithelial TJ permeability in filter-grown Caco-2 monolayers over a 24-h experimental period. Similarly, Oladejo et al. [60] reported the downregulation of the of TNF-α cytokine production after LAB treatments in a rat model.
The expression of IL-8 and IL-10 was also examined at the transcriptional level. IL-8 is widely used as an inflammatory marker in intestinal cell lines among all the potential induced cytokines that HT-29 cells can release. IL-8 attracts and causes activation of neutrophils in regions of inflammation [61]. In response to IL-8 activation, neutrophils migrate to the site of inflammation, release granule enzymes, and make other intra- and extracellular changes for efficient immune response. On the other hand, IL-10 is a potential anti-inflammatory cytokine that limits the host immune response to antigens (pathogens) thereby normalizing the tissue homeostasis. In the coculture model, a nonsignificant (p > 0.05) fold change in IL-8 and IL-10 biomarker expression was recorded in comparison to LPS challenge alone. The results indicated that under coculture conditions, lactobacilli could not regulate the expression of inflammatory biomarkers.
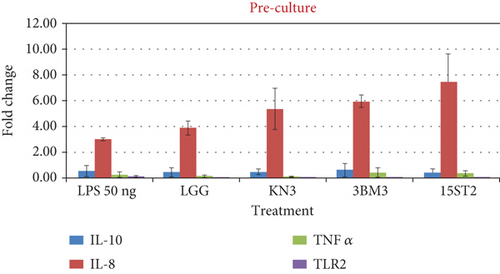
The stimulation of HT-29 cells by proinflammatory molecules like LPS leads to the production of IL-8 cytokine. During the prechallenge incubation, where LPS treatment was given prior to LAB stimulation, no significant difference (p > 0.05) in the expression of the anti-inflammatory cytokine IL-10 was recorded as observed in LPS alone and LAB-stimulated cells. In contrast, a significant upward regulation of IL-8 expression was recorded. In the postchallenge model, cells were exposed to LAB, followed by LPS exposure. There was no significant difference in the expression of IL-10 while IL-8 was variedly impacted with LAB exposure. IL-8 expression was upregulated with all the tested lactobacilli in comparison to LPS challenge (Figure 7). A similar increase in IL-8 concentrations was observed by Farkas et al. [62] when IPEC-J2 monoculture and gut–liver coculture were treated with 10 μg/mL LPS and L. plantarum culture supernatant. Our result also corroborates Rocha-Ramirez et al. [63], who recorded a significant level of IL-8 and TNF-α induction in HEK293 cells against zymosan (10 μg/mL) by all strains of Lactobacillus used in their study. LGG as a reference strain has previously been reported to suppress the proliferation of mononuclear cells [64]; but in the present study, it showed marked stimulation of IL-8 at the transcriptional level. Screening of biomarkers at the transcriptional level indicates the role of lactobacilli in modulation of immune response. However, the expression data need corroboration with the secretion profile. The transcription profile may not always match with the translation profile.
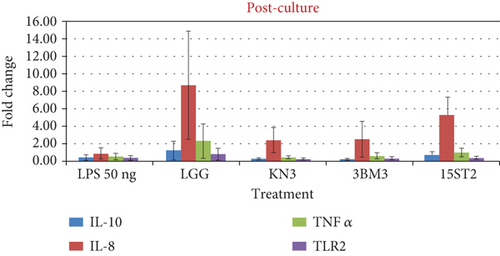
Proinflammatory indices including IL-8 and TNF-α and the anti-inflammatory index of IL-10 were also measured in soluble protein extracts of LAB-stimulated HT-29 cells using commercial human ELISA kits. Overexpression of IL-8 production was recorded for all tested LAB including the reference strain during the prechallenge incubation model. In this strategy, all the LAB strains stimulated IL-8 expression and its secretion from HT-29 cells, with maximum secretion by 15ST2 (110.81 pg/mL), followed by LGG (58.91 pg/mL), 3BM3 (52.25 pg/mL), and KN3 (46.12 pg/mL). IL-8 secretion was not impacted under coculture and postchallenge strategy (Figure 8). The results suggest that the test lactobacilli strains could regulate the immune response via mediating IL-8 expression through TLR2 pathway signaling. Garcia-Gonzalez et al. [61] credited this cross-talk between lactobacilli and immune system as an unconventional property of these beneficial microbes. Potential probiotic bacteria tend to modulate the production of proinflammatory cytokines when they are established before bacterial infection. A similar observation was previously made by Borruel et al. [65] in the intestinal mucosa of a Crohn’s disease patient cultured with lactobacilli strains. In a separate work, Al-Naijar et al. [66] observed that the supernatant from CM100 had TNF-α levels which were similar to those of LPS-stimulated cells, indicating a notable release of stimulatory cytokines like TNF-α. Enhanced modulation of the immune system by LABs against enteric infections has also been demonstrated previously [67, 68]. Haller et al. [67] demonstrated the positive influence of Lactobacillus johnsonii on TGF-β (anti-inflammatory) production by Caco-2 cells. Similar results were reported where limited correlation in mRNA and protein abundance was observed in other model systems attributing to genetic variation, transcriptional rates, mRNA stability for mRNA abundance, and to protein synthesis and degradation rates for protein abundance [69, 70]. The prime driving parameter of the mRNA expression levels and protein output may be gene-specific and may depend on the differentiation and activation status of a cell. Israelsson et al. [70] reported noncoherence in gene expression due to biological modifications of proteins, narrow detection span, requiring multiple handling and testing of the samples which may be the other cause of noncoherence. Cytokines are unstable proteins susceptible to action of serum metalloproteases and are easily degraded at room temperature and by multiple freezing/thawing.
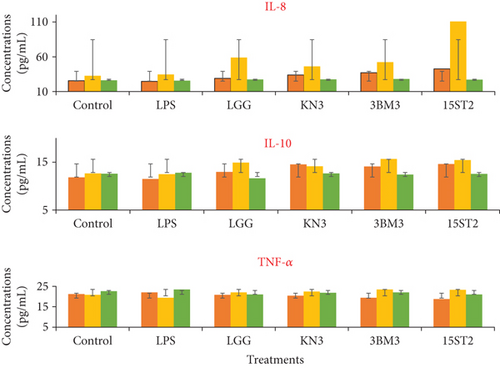
 , LPS-LAB coculture incubation;
, LPS-LAB coculture incubation;  , LPS prechallenge incubation;
, LPS prechallenge incubation;  , LPS postchallenge incubation. Results are presented as average of three different values with error bars from the standard deviations (mean ± SD).
, LPS postchallenge incubation. Results are presented as average of three different values with error bars from the standard deviations (mean ± SD).4. Conclusion
The present study showed that cell surface hydrophobicity and intestinal adhesion are strain-dependent properties and are not completely interlinked. The test strains showed significantly higher adhesion to the intestinal cells before the pathogen infection. Similar results were found in transcriptional and translation studies that, during pre- and postchallenge models, the expression as well as secretion of IL-8 proinflammatory marker was upregulated compared to the LPS alone model, while the coculture model showed significant upregulation in the expression of proinflammatory marker TNF-α and TLR2 by test strains in the coculture model. However, during prechallenge incubation, no significant modulation of IL-10 and TNF-α secretion was recorded under all the test conditions. Both TNF-α and IL-8 protein cytokines are known to play very important biological roles in the regulation of the inflammatory responses of macrophages. The lactobacilli strains analyzed in this study have immune-stimulatory effects and their administration during infection (coculture) and preinfection (preculture) may decrease the expression of TNF-α and IL-8, while at the same time enhancing the expression of anti-inflammatory cytokines. The results therefore suggested that test lactobacillus strains could regulate the immune response via mediating IL-8 expression and secretion through TLR pathway signaling.
Nomenclature
-
- GRAS
-
- generally recognized as safe
-
- IL
-
- interleukin
-
- LAB
-
- lactic acid bacteria
-
- LPS
-
- lipopolysaccharide
-
- MRS
-
- de Man Rogosa Sharpe
-
- PBS
-
- phosphate-buffered saline
-
- ssDNA
-
- single-stranded deoxyribonucleic acid
-
- TNF
-
- tumor necrosis factor
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Lewis I. Ezeogu: conceptualization, methodology, supervision, software, validation, review and editing. Harsh Panwar: conceptualization, methodology, resources, supervision, investigation, software, review and editing. Rachael T. Duche: performed experiments, investigation, preparation, visualization, data curation, funding acquisition, writing – original draft. Tochukwu N. T. Nwagu: supervision, writing, review and editing. Manvesh Kumar Sihag: resources, supervision, review and editing. Anamika Singh: performed experiments, investigation, writing. Jaspreet Kaur: writing, review and editing. Vikas Sangwan: supervision, review and editing.
Funding
The study is supported by the Tertiary Education Trust Fund (10.13039/501100008895), 2016/2017 (merged) AT&D intervention (UAM/DOL/ACA/01/VOL.1), Applied Microbiology International, United Kingdom, PhD Hardship Grant (2021 Award).
Acknowledgments
We remain grateful to the Department of Dairy Microbiology, College of Dairy Science and Technology, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana (Punjab), India, for providing laboratory facilities for this study.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




