The Stool and Oral Microbiome of Rheumatoid Arthritis Patients Taking Disease-Modifying Antirheumatic Drugs
Abstract
Objective: Rheumatoid arthritis (RA) is a common musculoskeletal condition with an estimated prevalence of 0.27% in Australia and New Zealand. Recently, interest in the role of the human microbiome regarding RA pathogenesis has increased. This study is aimed at determining the composition of the stool and oral microbiome of RA patients taking common disease-modifying antirheumatic drugs (DMARDs).
Methods: For this cross-sectional study, RA patients (n = 28) with established disease (> 12 months since diagnosis) and healthy individuals (n = 10) were recruited by the Australian Arthritis and Autoimmune Biobank Collaborative (A3BC). Stool samples and oral swabs were collected, processed, and sequenced to obtain 16s rRNA amplicon sequencing data. Data was processed using a QIIME2/DADA2 pipeline. Diversity and taxonomic analyses were performed using QIIME2 core metrics functions and RStudio packages.
Results: Diversity analyses identified differences in the RA stool microbiome, in comparison to healthy controls and between RA patients taking different DMARDs. This includes differences in microbial richness, diversity, and structure. For example, the microbiome of patients taking JAK inhibitors (JAKis) significantly differed to the microbiome of patients on combination therapy (TNF inhibitors [TNFi] +/− methotrexate [MTX]) or taking MTX only. Furthermore, patients taking MTX had a more similar microbiome to healthy individuals. In contrast, limited differences in the oral microbiome were observed for all diversity metrics, although a number of taxa previously linked to oral disease were identified as differentially abundant to healthy individuals.
Conclusion: Differences in the diversity and taxa of the stool and oral microbiome of patients with established RA were identified. Overall, there was some concordance with previous research. This work highlights the importance of further investigation into the impact of JAKi, TNFi, and MTX on the RA microbiome.
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that mainly affects the joints. RA is characterized by symmetric polyarticular arthritis, synovial inflammation, and pannus invasion, which is driven by a dysregulated immune response [1]. Recently, the prevalence of RA was reported as 0.27% in Australia and New Zealand and 0.46% globally [2]. Patient outcomes can include chronic pain and irreversible destruction of joints, leading to life-long disability and decreased quality of life [1]. In addition, chronic inflammation is linked to an increased comorbidity risk (e.g., cardiovascular disease, cancer, and gastrointestinal disorders) [3, 4]. The etiology of RA is currently unresolved. Genetic predisposition has been shown to increase the likelihood of developing RA by 40% [5]. Additionally, environmental triggers, such as infections, smoking, and host factors, such as gender, have also been linked to RA pathogenesis.
Recently, interest in the gastrointestinal microbiome in an RA context has increased. Involved in microbial–host cell interactions, production of molecules, and drug metabolization, the human microbiome is a cornerstone of immune system development and function [6–8]. Considering this complex interplay, dysbiosis of the microbiome may contribute either directly or indirectly to RA pathogenesis. This has been broadly highlighted previously, with overgrowth of particular bacterial groups (e.g., small intestinal bacteria overgrowth [9]) or species associated with other diseases (e.g., Clostridium difficile [10]). However, in an RA context, current evidence is varied and not always substantiated. In some cases, specific bacterial species have been identified as overabundant, including Prevotella copri [11], Lactobacillus salivarius, and Haemophilus species [12]. In some studies, upon treatment of patients receiving methotrexate (MTX) treatment, microbial profiles became more similar to those of healthy individuals [12]. Along with the heterogeneity of RA [13], heterogeneity of the human microbiome, experimental methodology, and the broad range of environmental factors patients are exposed to, varied conclusions are not surprising [14]. By furthering our understanding of host–microbe dynamics at various stages of RA disease progression, microbiome-focused interventions in clinical settings may be identified.
A range of drugs may be taken for RA disease management including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and various classes of disease-modifying antirheumatic drugs (DMARDs). MTX, a conventional synthetic DMARD (csDMARD), is the current first-line treatment with approximately a 40%–50% rate for RA remission or reduced activity [15]. RA microbiome research is often focused on the gut microbiota of DMARD-naive RA patients receiving MTX, which is suggested to be metabolized by gut microbiota [16]. Less is known regarding the impact of other commonly prescribed csDMARDs on the microbiome. If disease activity is not reduced with first-line treatment, additional DMARDs may be prescribed. This includes, but is not limited to, biological DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) which modulate immune pathways. Some of the most effective bDMARD therapies target TNF-α receptors, including adalimumab [17], etanercept [18], golimumab [19], and certolizumab [20], although each drug differs slightly in mechanism of action. More recently, one type of small-molecule Janus kinase (JAK) inhibitors (tsDMARDs) has been developed which inhibit JAK1/JAK3 (tofacitinib) and JAK1/JAK2 (baricitinib).
To date, few studies have examined the stool microbiome of RA patients prescribed various b/tsDMARDs. Furthermore, the investigation of the oral microbiome has not previously been integrated into such studies. Considering the current heterogeneity of RA microbiome data, it is worthwhile to investigate microbial stool and oral composition in these varied conditions. This study investigates the composition of the stool and oral microbiome of patients with established RA and explores the potential role common DMARDs play in shaping the RA microbiome.
2. Materials and Methods
2.1. Ethics
Human research ethics approval for this project was obtained from the Northern Sydney Local Health District HREC (2019/ETH10386, Australian Arthritis and Autoimmune Biobank Collaborative [A3BC]; 2019/ETH00270 [Project ResID3-PID1]).
2.2. Patient Recruitment
A convenient sample of RA patients (n = 28) attending the rheumatology outpatient clinic at Royal North Shore Hospital (RNSH) (Sydney, Australia) was recruited to the A3BC between 2018 and 2022. The A3BC is a national best practice biobank-registry network for multicenter prospective longitudinal collection, processing, and storage of broad patient-reported and clinical/health data and high-quality biospecimens from participants with inflammatory arthritis and autoimmune diseases. All patients were previously diagnosed with RA based on 2010 ACR/EULAR RA classification criteria, with > 12 months since diagnosis. Exclusion criteria included patients who were pregnant or who received antibiotics within the previous 3 months. RA patients’ current DMARDs at the time of microbiome sampling are shown in Table 1, along with additional demographic data. A convenient sample of unmatched healthy individuals (n = 10) with no reported autoimmune disease was recruited from within the RNSH campus precinct within the same time period. The study design was cross-sectional.
| Diagnosis (n) | Healthy (HC) | Rheumatoid arthritis (RA) |
|---|---|---|
| 10 | 28 | |
| Subject characteristics ∗ | ||
| Age (years, median) | 57 (53, 59) | 64 (60, 72) |
| Sex | F = 8, M = 2 | F = 26, M = 2 |
| Medications (n%) | ||
| b/tsDMARD | ||
| None | 10 (100%) | 9 (32%) |
| JAK inhibitor | 0 (0%) | 8 (29%) [baricitinib = 3 (11%); tofacitinib = 5 (18%)] |
| TNFα inhibitor | 0 (0%) | 9 (32%) [adalimumab = 3 (11%); certolizumab = 1 (3.6%); etanercept = 2 (7.1%); golimumab = 3 (11%)] |
| T cell costimulation modulator | 0 (0%) | 2 (7.1%) [abatacept = 2 (7.1%)] |
| csDMARD | ||
| Methotrexate | 0 (0%) | Yes = 16 (57%) |
| No = 12 (43%) | ||
| Hydroxychloroquine | 0 (0%) | Yes = 8 (29%) |
| No = 20 (71%) | ||
| Sulfasalazine | 0 (0%) | Yes = 5 (18%) |
| No = 23 (82%) | ||
| Leflunomide | 0 (0%) | Yes = 5 (18%) |
| No = 23 (82%) | ||
| GI disease reported | 0 (0%) | Not reported = 26 (68%); yes = 2 (5.3%) |
- ∗No significant difference in age or sex between RA and HC was detected when using the Student t-test.
2.3. Stool and Oral Sample Collection
During recruitment, participants were provided with a microbiome self-collection kit developed by the Microbiome Research Centre (MRC) (St George Hospital, UNSW, Sydney). The collection process and a brochure containing collection instructions were provided to patients during their appointment. In brief, participants collected one scoop of stool sample and homogenized the sample by shaking the sample in a collection tube containing Stool DNA Stabilizer (#1038111300, Stratec Molecular GmbH, Germany). Oral samples were collected by swabbing the inside of the mouth and tongue with a FLOQSwab followed by suspension of the swab in an eNAT tube containing 2 mL of guanidine–thiocyanate-based medium (COPAN diagnostics, California, United States). Stool and oral samples were returned to RNSH within 1–3 weeks of collection. Current medications, history of bowel disease, and dietary preference were self-reported by patients using a brief questionnaire.
2.4. DNA Extraction, Quality Control, and Sequencing
Stool and oral samples were aliquoted and stored at −80°C prior to batch DNA extraction. DNA extraction was performed using the PSP Spin Stool DNA Kit (#1038120300, Stratec Molecular GmbH, Germany) and the QIAamp DNA Mini Kit (#51306, Qiagen, United States) with BeadBug prefilled tubes, 2.0 mL capacity with 0.1 mm zirconium beads (#032-D1032-01 Pathtech, Australia), respectively, and according to the manufacturer’s instructions. Positive and negative controls were included for every batch of extractions. The positive controls included the ZymoBIOMICS microbiome community prep (#D6300; Zymo Research, United States) and previously aliquoted stool samples collected from one person. Multiple negative controls, containing either ultrapure water or the preservation buffer used to stabilize the stool or oral samples, were also included. An additional negative control was included during oral DNA extractions, where unused stabilization buffer exposed to an unused swab was extracted. All extracted controls were sequenced and analyzed alongside samples.
Prior to sequencing, DNA concentration was determined using a Qubit Fluorometer (Invitrogen) using a high-sensitivity dsDNA assay kit and appropriate assay tubes (#Q32854 and # Q32856, Thermo Fisher, United States). PCR amplification of the V3–V4 region of the 16S rRNA gene was performed using a DNA Engine Thermal Cycler (Bio-Rad) using conditions outlined in the 16s rRNA Illumina 16S Metagenomic Sequencing Library Preparation protocol. Amplicons were run on a 1.5% agarose gel and visualized using a UV transilluminator to confirm successful amplification. DNA was sent for sequencing in a sealed 96-well plate, on dry-ice to the Australian Genome Research Facility (AGRF) for microbial profiling using the 341F-806R primer target (5 ′ CCTAYGGGRBGCASCAG 3 ′ and 5 ′ GGACTACNNGGGTATCTAAT 3 ′, respectively). DNA was sequenced in batches, resulting in a total of 3 stools and 2 oral sequencing runs. An additional ZymoBIOMICS DNA microbiome community sequencing control was included per plate (#D6305, Zymo Research, United States).
2.5. Preprocessing of Data
Data was generated with the Illumina bcl2fastq pipeline Version 2.20.0.422. Paired-end sequences were returned as fastq.gz files. Data was uploaded to the University of Sydney Artemis High-Performance Computer and converted to a QIIME2 artifact. QIIME2 plugins were employed to remove primers, followed by denoising, dereplicating, and chimera filtered using Divisive Amplicon Denoising Algorithm 2 (DADA2) pipeline [21, 22]. Generated amplicon sequence variants (ASVs) and tables were used for downstream analyses. Taxonomy assignments were via the SILVA database [23]. QIIME2 Version 2020.2 was used during analysis [21].
2.6. Diversity and Statistical Analyses
Analyses were performed using validated QIIME2 plugins or with several RStudio packages. Alpha-diversity metrics were performed using the “microbiome” and “phyloseq” R (v 4.2.1) packages with visualizations constructed using “ggplot2” [24–26]. Statistical significance was determined with the Kruskal–Wallis nonparametric test with the p value adjusted by the “holm” method. Beta-diversity metrics (Jaccard, Bray–Curtis, and unweighted and weighted UniFrac results) were calculated using QIIME2 beta-diversity plugins and imported into RStudio for visualization. Permutational multivariate analysis of variance (PERMANOVA) was performed to determine p and q values using pairwise testing, with 999 permutations performed. Core microbiome tests were performed using the “microbiome” package. Differential abundance (DA) tests were performed using “Maaslin2,” “DESeq2,” “corncob,” “limma,” and “ANCOMBC” in R [27–31], following published scripts [32]. A Student t-test was performed to determine significant differences between group covariates including sex and age. For all analyses, p adjusted values of < 0.05 were considered significant.
3. Results
Following processing and quality control measures, a final total frequency of 1,640,431 and 3,234,472 sequences were generated via 16s rRNA amplicon sequencing of the V3–V4 region of stool and oral microbiome samples, respectively. Of these sequences, 2972 and 2599 unique ASVs were identified. For diversity metrics, sequences were rarefied to a depth of 20,000 sequences. Positive controls were used to identify uneven extraction of DNA from samples, batch effects, and, to a degree, accuracy of the trained SILVA classifier during taxonomic assignment. Negative controls from all batches produced a low number of sequences; however, the corresponding ASVs were either (1) not identified within the patient samples or (2) identified at a reduced number in comparison to real samples. This indicated that amplified DNA already existed in the negative controls and that some cross-contamination occurred during extraction and sequencing. Following exploration, both positive and negative controls were removed from the main dataset prior to analysis.
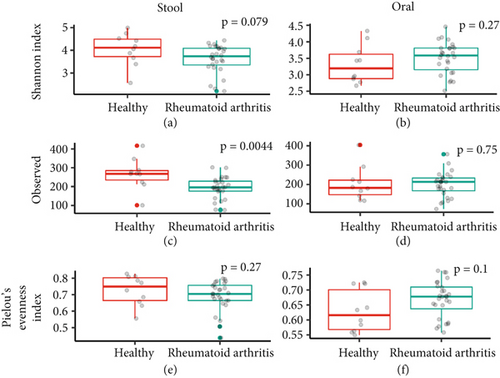
First, the stool and oral microbiome of RA patients and HC was analyzed for diversity, richness, and evenness (Figure 1). Comparison of the Shannon index (a measure of diversity factoring in both richness and evenness) of stool samples between groups identified a nonsignificant (p = 0.079) decrease in RA stool diversity (Figure 1a). Further investigation identified a significant decrease (p = 0.004) in observed ASVs (a measure of microbiome richness) in the RA microbiome (Figure 1c). Microbiome evenness, measured via Pielou’s evenness index, was similar for the RA and HC microbial community (Figure 1e). Oral samples which were collected within the same timeframe as stool samples (< 1 h) revealed no significant differences between groups in diversity, richness, or evenness (Figures 1b, 1d, and 1f). As such, the stool, but not oral, microbiome richness was decreased in the RA patients.
Afterward, participant samples were divided based on current medications including csDMARDs, bDMARDs, and tsDMARDs (Table 1). The same analyses (Shannon index, observed ASVs, and Pielou’s evenness index) were performed to understand microbiome diversity related to common RA treatment regimes.
The influence of MTX on alpha-diversity was explored first, with samples sorted into three groups (“MTX,” “none,” and “healthy,” denotated “M,” “X,” and “H”, respectively, in Figure 2). Of the 16 RA patients (57%) taking MTX (Group “M”), 10 of these patients were also prescribed a bDMARD. Among the patients who were not taking MTX (Group “X”), 9 were prescribed only a b/tsDMARD, and 3 were prescribed nothing.

The stool microbiome of the MTX group (M) had an overall higher microbial diversity compared to the “none” group (X) with no difference compared to the HC (Figure 2a: MTX vs. none p = 0.02; HC vs. MTX p = 0.42), although this similarity disappeared when taking into account observed ASVs (Figure 2e). Furthermore, both microbial diversity and richness of patients in the “none” group (N) was significantly decreased (Figure 2a: p = 0.014; Figure 2e: p = 0.0015) in comparison to the HC group, while microbial evenness was decreased in comparison to MTX patients (Figure 2i; p = 0.029). Again, no difference was observed for the oral microbiome of patients based on MTX prescription (Figures 2b, 2f, and 2j).
To further inquire the differences in RA treatment, additional grouping was performed based on the type of b/tsDMARD patients were taking (Figures 2c, 2g, and 2k). In this case, samples were grouped as HC (n = 10; denoted “H” group), RA patients taking JAKi (n = 8; denoted “J” group), patients taking TNFi (including patients also taking MTX [n = 8] or no csDMARD [n = 1; denoted “T” group] and herein referred to as TNFi [+/− MTX]) and RA patients not taking any b/tsDMARD (patients taking only MTX [n = 6]; denoted “X” group). Patients taking T cell inhibitors (n = 2) or no drugs (n = 3) were excluded from these analyses due to the low sample size. Regarding the stool microbiome, patients taking JAKi (J) revealed a decrease in diversity and richness in comparison to HC (p = 0.016 and p = 0.0062, respectively) with a trending decrease in diversity (p = 0.059) and evenness (p = 0.046) in comparison to patients taking TNFi (+/− MTX). Furthermore, no difference was observed between patients taking TNFi (+/− MTX) or no b/tsDMARDs, in comparison to HC richness and evenness; however, observed ASVs were reduced. In general, patients taking JAKi monotherapy presented overall lower alpha diversity metrics, in contrast to other groups.
Although the oral microbiome analyses previously revealed no differences between RA and HC, a trend toward an increased diversity and evenness in patients taking TNFi (+/− MTX) was observed, in contrast to all other groups. In regard to JAKi, although evenness was similar between the groups, richness was significantly higher in the TNFi (+/− MTX) group (Figure 2d, Shannon index [p = 0.027]). In contrast to stool microbiome analyses, no difference was observed in the oral microbiome between RA patients on JAKi and HC.
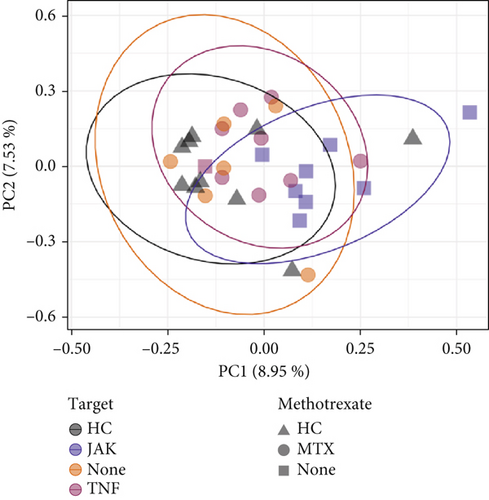
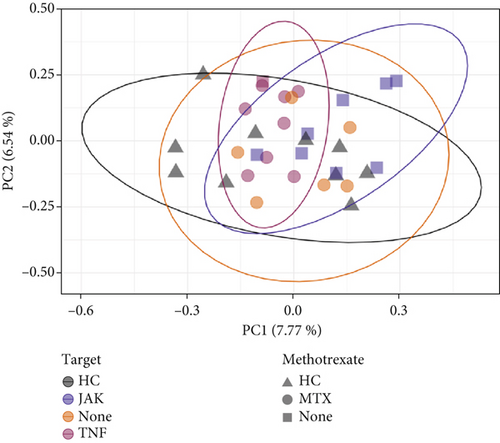
Beta-diversity metrics were applied to explore microbial community structure. Dissimilarity was observed in the RA stool microbiome when abundance was not taken into account, in contrast to the oral microbiome, whereby no difference was observed. When further grouping patients by JAKi versus TNFi (+/− MTX) status, microbial community structure based on both presence/absence and abundance data (Figure 3a and Supporting Information 1: Table S1) indicated significant dissimilarity (Bray–Curtis distance, p = 0.012). Furthermore, upon splitting groups based on MTX status, only JAKi revealed a different microbiota profile (p = 0.015), whereas patients only taking MTX versus HC or TNFi (+/− MTX) showed no difference in structure. Finally, the microbiome structure of patients taking JAK versus TNFi (+/− MTX) revealed significant dissimilarity upon analysis with the Jaccard metric (Figure 3b and Supporting Information 1: Table S2).
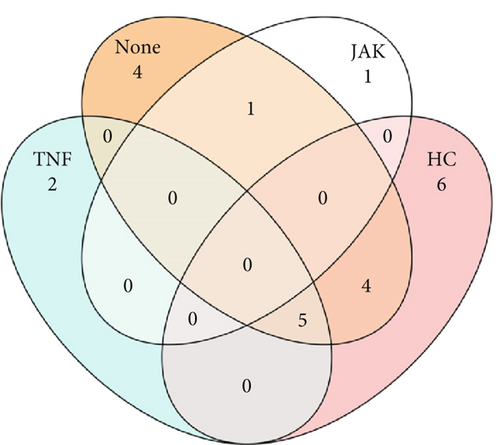
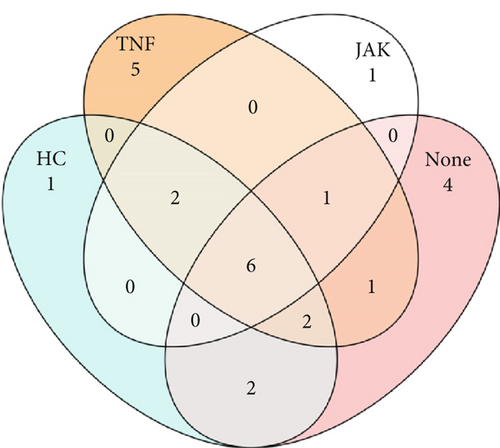
Similarities between groups based on predicted members of the core microbiome were determined. ASVs shared between samples were identified with a set prevalence of 70%. For the stool microbiome (Figure 4a), no ASVs in patients taking JAKi were found to be shared with HC; however, 4 and 5 ASVs were identified as shared with HC (“TNF” and “none” [i.e., MTX only] group, respectively). For the oral microbiome (Figure 4b), a higher number of ASVs were shared irrespective of group, which highlights the previously identified similarity of the oral microbiome. Taxonomic assignment of ASVs is presented in Supporting Information 2: Table S3.
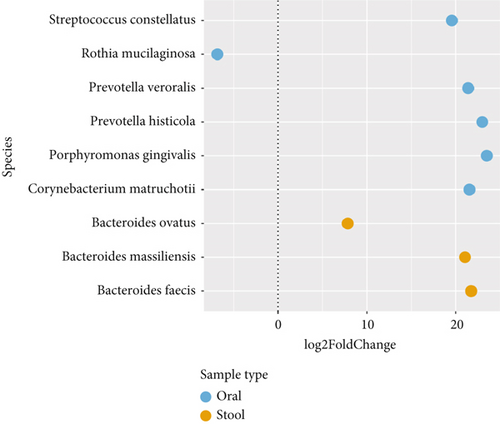
Considering the differences identified in the microbial community, DA testing was applied to determine differential ASVs between RA and HC patients. Due to lack of consensus on a “gold standard” DA statistical test, multiple methods were performed including ANCOM, ANCOM-BC, Maaslin2, Limma Voom, DeSeq2, and Corncob [32]. To improve certainty, only ASVs detected as DA by more than two methods are presented. ASVs identified as DA were deemed significant with an adjusted p value of < 0.05. Data was filtered to remove ASVs not present in less than 15% of samples and to remove sparse features. In this case, ANCOM-BC and Deseq2 identified 3 and 6 ASVs, in the stool and oral microbiome, respectively. All ASVs identified via ANCOM-BC were structural zeros. The log2fold produced by DESeq2 is presented with each ASV closest taxonomic assignment presented (Figure 5). Analyses of the stool microbiome identified three ASVs (genus Bacteroides: B. ovatus, B. massiliensis, and B. faecis) with an increased abundance in the RA stool microbiome, while five ASVs (Streptococcus constellatus, Prevotella veroralis, Prevotella histicola, Porphyromonas gingivalis, and Corynebacterium matruchotii) were increased in the RA oral microbiome. Only one ASV, Rothia mucilaginosa, was reduced in the oral microbiome.
4. Discussion
In this paper, we present our findings on the composition and structure of the stool and oral microbiome of patients with established RA. Sequencing data was generated via 16s rRNA amplicon sequencing of the V3–V4 region with analyses identifying compositional differences and differentially abundant taxa based on both diagnosis and medication groups. This research adds to cumulative knowledge on the RA microbiome and the potential impact of medication in shaping the gut and oral microbiome.
4.1. The Stool Microbiome of RA Patients
First, alpha- and beta-diversity analyses were performed to identify differences in the stool microbiome between RA patients and HC. Microbial richness and diversity (although not significantly) in the RA stool microbiome were decreased, while evenness remained similar between groups (Figures 1a, 1c, and 1e). Community structure was also significantly dissimilar based on the presence/absence of taxa identified in the samples (Figure 3a). These findings are not uncommon, with reduced microbial stool diversity in RA patients often reported, irrespective of the diversity metric employed [33, 34]. However, many studies focus on untreated, new, or early-RA patients, where differences in comparison to a healthy microbiome can be more distinct [11, 35]. Considering the majority of patients in our study had established RA with long-term management strategies, our result is not surprising. Indeed, in one prospective study, the stool microbiome of RA patients was more similar in composition to a healthy cohort following treatment [12]. However, it must be noted that the small sample size limits generalizability and statistical power. While these findings may provide a general understanding, increased sample size will be required for robust findings. Additionally, inquiry into longitudinal microbiome shifts in the microbiome would be of interest.
To obtain greater insights into the data, diversity analyses were repeated with patients grouped by medication. RA patients taking MTX (alone or as a combined therapy) revealed no difference in microbial diversity and evenness in comparison to the HC group, although a significant difference in observed ASVs was identified (Figures 2a, 2e, and 2i). In many cases, patients not taking MTX resulted in reduced diversity (i.e., richness, diversity, and evenness). Similar results were reported, where stool diversity was increased in patients taking MTX and hydroxychloroquine [36]. In addition, RA patients and healthy individuals taking a number of DMARDs (including MTX and bDMARDs) revealed no difference in Chao1 and Shannon index [37]. Together, with the data presented in this paper, there is evidence that MTX may contribute to a restored microbial diversity in RA patients.
Further exploration identified Bacteroides uniformis and Bacteroides thetaiotaomicron as core microbiome members for RA patients taking MTX. B. thetaiotaomicron is sensitive to MTX with gene expression markedly changed in the presence of MTX [38]. This includes protein synthesis pathways targeted by MTX [39]. It has also been suggested that MTX can be metabolized by specific stool microbes and that differential depletion of MTX may be used to predict clinical response [16]. Furthermore, a number of high and low abundance bacteria have been linked to MTX responders; in particular, multiple operational taxonomic units (OTUs) from the Bacteroides and Prevotella genera were increased in MTX responders [16]. Finally, one study has indicated that it is not the number but the composition of genes which can be linked to MTX responders/nonresponders [6]. Further investigation is warranted to determine potential mechanisms of action, bacterial strains of interest, and genes that may be involved. However, it appears to be agreed upon that specific taxa identified in samples may be related to MTX response and could potentially be used as “indicators” of drug efficiency.
To further delineate data, patients were divided by b/tsDMARD target where patients taking TNFi (+/− MTX) were compared to patients taking only MTX, JAKi, or no medication. Patients prescribed JAKi had a significantly reduced stool microbiome diversity in comparison to the HC group (Figure 2c,g). Furthermore, microbial community structure was significantly dissimilar in comparison to all other groups (Supporting Information 1: Table S1). Indeed, only two taxa, Clostridium leptum and an uncultured Oscillibacter, were identified as members of the core microbiome of the “JAKi” group, with only one of these taxa shared with another group (Figure 4a). C. leptum, in particular, is a known butyrate producer with a role in maintaining mucosal homeostasis. This could indicate that its presence in patients on a JAKi is in a protective capacity, although studies have noted that overproduction can result in destabilization of gut barrier permeability [40]. To the best of the authors’ knowledge, no studies have investigated the RA patient microbiome in response to JAKi; however, some clinical trials are currently underway [41]. However, in a spontaneous arthritis mouse model study, a reduction in disease activity and pathogenic bacteria (Proteobacteria and Chlamydiae) and alterations to mucosal immunity were identified after tofacitinib treatment [42]. Considering the reduction in stool microbiome diversity, structural dissimilarity, and overall heterogeneity observed, additional investigation into a relationship between the microbiome, JAKi, and mucosal immunity is warranted. In comparison to JAKi, a number of studies have investigated the microbiome of RA patients taking TNFi. In one study, the stool microbiome of psoriatic arthritis and spondylarthritis patients was analyzed pre- and post-TNFi treatment with no significant alterations in alpha-diversity identified [43]. Another study explored microbiome composition of patients taking either a TNFi (etanercept), MTX, or combined therapy, in comparison to treatment-naïve patients [44]. While no difference in alpha-diversity was identified between groups, differences in taxa present were identified. Overall, commonly used DMARDs appear to affect the stool microbiome in different ways; however, the downstream clinical implications of these findings are unknown and require further studies to investigate.
Due to low group numbers and the potential for false positives, DA tests were not performed based on individual medication. However, a number of DA taxa were identified between HC and RA groups in both the stool and oral microbiome. For the stool microbiome, only three Bacteroides were identified as DA in RA patients: B. ovatus, B. massiliensis, and B. faecis. However, these specific species are largely unreported in the RA literature, with Bacteroides predominantly noted as mutualistic with the ability to cause infection, especially B. fragilis species [45]. More generally, the Bacteroides–Prevotella ratio is well explored in RA patients, with one notable study identifying a decrease in Bacteroides abundance in early-RA patients, in contrast to an increase in Prevotella species [11, 35]. Considering the data is from patients treated for established RA, the DA Bacteroides identified is unsurprising.
4.2. The Oral Microbiome of RA Patients
In contrast to the RA stool microbiome, very few differences were identified in the oral microbiome of RA patients. Indeed, no notable differences were observed in alpha diversity (Figures 1b, 1d, and 1f). Research investigating the RA oral microbiome is more limited, but the majority of findings do not identify differences in alpha diversity [46], with only some minor exceptions [47]. Unlike the stool microbiome, no reduction in diversity was present between MTX groups. However, when further exploring the data, patients taking TNFi (+/− MTX) had an increased richness, diversity, and evenness in comparison to one or more groups tested (Figure 2) with structural dissimilarity identified between the TNFi (+/− MTX) and JAKi groups. The reason for the increased diversity in the TNFi (+/− MTX) group is unclear. The use of TNFi for cotreatment of periodontitis and RA has been investigated, with infliximab found to not aggravate downstream gingival inflammation and adalimumab leading to improved oral health [48, 49]. However, as disease activity was not linked to patient data, it is difficult to determine if increased diversity is a positive outcome.
Five taxa were identified as unique to the core microbiome of the TNFi (+/− MTX) group: Streptococcus sanguinis, Streptococcus gordonii, Prevotella histicola, an uncultured Bergeyella, and Porphyromonas spp. (Figure 4). Oral S. sanguinis is suggested to be involved in Behçet’s disease, an idiopathic systemic vasculitis with characteristics of autoimmune disorders and altered NF-κB pathway activation [50, 51]. In some cases, it may present as seronegative RA [50]. Considering the presence of S. sanguinis in patients receiving a TNFi, further investigation may be warranted. Also of interest is S. gordonii, with coadhesion between S. gordonii and P. gingivalis species leading to oral biofilm formation and chronic periodontitis [52]. S. gordonii has previously been found to be underrepresented in early-RA patients [53]. The almost ubiquitous presence of these potentially pathogenic bacteria may be of concern considering the increased risk of dental disease in RA patients and the elusive link between S. sanguinis and TNFα activation [54].
As previously mentioned, P. histicola was identified as a core microbe in patients taking TNFi (+/− MTX). Interestingly, P. histicola was also determined to be DA within the oral microbiome of RA patients, in comparison to HC (Figure 5). While commonly identified as part of the normal oral microflora, recent studies have reported its presence in the gut microbiome [55]. However, P. histicola was not present as an ASV within our stool microbiome dataset. Another taxon of interest is C. matruchotii which has previously been identified in patients with active RA [56]. Both S. constellatus and R. mucilaginosa have previously been identified in association with translocation or infection but are also abundant in the healthy microbiome [57].
Overall, the relative lack of oral microbiome dissimilarity may be related to the tumultuous nature of the oral environment, considering constant exposure to the external environment and frequent use of dental hygiene products. Additionally, due to the retrospective nature and scope of this work, an oral health check was unable to be completed to identify periodontitis in patients. Future work would benefit from this assessment to further understanding oral microbiome results. Furthermore, the sampling method employed (oral swab as opposed to subgingival sampling) or selected sequencing region (V3–V4) may also be related to the outcomes in this study. However, clearly there is an increase in notable oral taxa in RA patients, especially within patients taking TNFi (+/− MTX). Further investigation of DA and core microbiota based on medication status is a necessity and will complement current literature identifying drug responders.
4.3. Limitations of This Study
Several limitations exist in this study including the small sample population, grouping of medications with different mechanisms of action, and patient heterogeneity. In addition, limited conclusions are able to be reached when comparing this cross-sectional study to previously published prospective studies. As such, restraint when interpreting results and making comparisons to the literature is necessary. While some bias is expected in the dataset, to the best of ability of the authors, the dataset was cleaned and analyzed under strict conditions. Considering bias, future prospective research should utilize larger cohorts, analyzed by specific b/tsDMARD type. Additionally, potential confounding factors such as comorbidities, diet, and smoking data will be collected to improve analyses. Improvement of stool extraction methods may also lead to an increased frequency of stool ASVs. Despite these limitations, the authors consider this paper a foundation for future studies focused on JAKi in RA patients and other inflammatory diseases.
5. Summary
Our data suggests that JAKi have a significant influence on the gut microbiome, warranting further investigation. In contrast, patients prescribed TNFi (+/− MTX) appeared to have increased oral diversity, in comparison to different comparison groups (metric dependent). Furthermore, in some concordance with previously published work, MTX may lead to a more similar microbiome in healthy individuals. This highlights the importance of research on medications other than MTX, in a RA microbiome context. This research is important for understanding whether microbiome differences between cohorts persist despite ongoing treatment strategies and any potential downstream consequences. Further work investigating clinical relevancy and disease activity under longitudinal conditions is integral for realizing any potential for microbiome-centered interventions in the management of RA.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research is supported by the CLEARbridge Foundation, the National Health and Medical Research Council (10.13039/501100000925) (APP2006579), the Lincoln Centre, Janssen Australia, the Medical Research Future Fund (MRF2007502 and MRF2016105), and the NSW Ministry of Health.
Acknowledgments
This research (A3BC Access ID ResID3-PID1) has been made possible through use of samples and data from the Australian Arthritis and Autoimmune Biobank Collaborative (A3BC). The A3BC team is sincerely grateful for the generosity of the patient participants, carers, clinicians, and researchers in continuing to give their time and effort to allow research discovery such as that herein. The authors acknowledge the technical assistance provided by the Sydney Informatics Hub, a Core Research Facility of the University of Sydney, and provision of microbiome collection protocols provided by the Microbiome Research Centre, St George, UNSW.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The dataset used and analyzed in this study may be made available upon reasonable request to the A3BC steering committee (https://a3bc.org.au).




