PLGA-PEG-PLGA Polymer: From Synthesis to Advanced Pharmaceutical Applications
Abstract
This paper presents an in-depth analysis of the PLGA-PEG-PLGA polymer, focusing on its synthesis and applications in advanced drug delivery systems (DDSs). PLGA-PEG-PLGA, a triblock copolymer, gains attention due to its biodegradability, biocompatibility, and thermosensitive properties, making it suitable for encapsulating both hydrophilic and hydrophobic compounds. The polymer’s ability to undergo sol-to-gel at body temperature allows controlled and targeted drug release, significantly enhancing the solubility of poorly soluble drugs, such as paclitaxel and irinotecan. The paper discusses the polymer’s synthesis via ring-opening polymerization (ROP) and explores its optimization using various methods, including microwave-assisted techniques and supercritical CO2. Additionally, it examines the polymer’s cytotoxicity in in vitro and in vivo studies, emphasizing its low toxicity and ability to deliver chemotherapeutic agents more effectively. The study highlights the polymer’s potential in cancer therapy, biopharmaceutical delivery, and the development of dual-sensitive drug carriers.
1. Introduction
Biocompatible polymers began to be intensively studied in the mid-20th century. During this period, scientists focused on creating materials that could be used in medicine, such as surgical sutures, implants, and drug delivery systems (DDSs) [1, 2]. In the 1970s and 1980s, biodegradable polymers like polylactide (PLA) and polyglycolide (PGA) started to be used in medicine. They were particularly valued for their biodegradation in the body into nontoxic lactic and glycolic acid [2, 3]. The PLGA copolymer (PLA-co-glycolide) was developed as a combination of PLA and PGA, with PLA comprising lactic acid or lactide in the forms of L-, D-, or DL-isomers. This resulted in a material with varying biodegradation rates depending on molecular weight and the proportion of PLA and PGA. PLGA quickly became popular in medicine, especially in smart DDSs [2]. The addition of polyethylene glycol (PEG) to the PLGA copolymer increased the hydrophilicity and biocompatibility of PLGA by reducing protein and cell adsorption on the polymer’s surface [1]. The triblock copolymer PLGA-PEG-PLGA (poly (lactide-co-glycolide)-poly (ethyleneglycol)-poly (lactide-co-glycolide)) was developed as a modification of PLGA and PEG polymers in the 1990s. PLGA-PEG-PLGA is a thermosensitive hydrogel that can undergo sol-to-gel transition with an increase in temperature. This polymer was used to develop a DDS for the localized delivery of paclitaxel directly to tumors [4]. Previous reviews have addressed various aspects of PLGA-PEG-PLGA, focusing on biomedical applications [5]. In contrast, this review offers a new perspective by focusing on the classification of synthesis methods and the evaluation of cytotoxic areas that have not been fully explored in previous works. Additionally, this review provides an updated overview of the biomedical applications of PLGA-PEG-PLGA, reflecting the most recent advancements in the field. By focusing on these aspects, our paper offers a more comprehensive understanding of the polymer’s synthesis, safety profiles, and applications, aiming to provide fresh insights into its potential in biomedical fields.
2. Properties of the PLGA-PEG-PLGA Triblock Copolymer
PLGA-PEG-PLGA has an amphiphilic structure, consisting of hydrophobic PLGA chains and a hydrophilic PEG chain, which influences its solubility in water and organic solvents depending on the PLGA–PEG ratio. In aqueous solutions, it forms micelles, with a critical micelle concentration (CMC) typically ranging from 0.1 to 0.3 mg/mL [6, 7]. PLGA-PEG-PLGA is biodegradable, biocompatible, and thermosensitive [8]. In an aqueous solution at room temperature, it forms a sol, and as the temperature of the polymer solution increases, the interchain hydrogen bonding interactions between PEG weaken, while hydrophobic interactions between PLGA chains strengthen, leading to the formation of a three-dimensional hydrogel [9]. This polymer structure allows the encapsulation of low molecular weight hydrophilic and hydrophobic compounds, as well as larger structures, such as peptides and proteins [4, 8]. Thus, PLGA-PEG-PLGA is widely used as an intelligent DDS for low molecular weight compounds like curcumin, irinotecan, dexamethasone, and larger molecules like growth hormone, insulin, or exenatide (EXT) [10–14].
From the perspective of a DDS, it is essential that the obtained polymer formulation is sufficiently water-soluble, and the gelation temperature should be close to human body temperature. Another critical DDS parameter is the release kinetics of the substance encapsulated in the polymer structure. Improper formulation can lead to uncontrolled release (burst release), where a rapid and sudden release of the encapsulated substance is observed initially. For drugs with a narrow therapeutic index, this is an undesirable effect that can lead to increased toxicity [15]. The release rate of the substance and the gelation temperature of the polymer can be tailored to the application by adjusting the molar ratios of LA and GA, molecular weight of PEG, or the PLGA–PEG ratio [9, 16, 17]. Another method involves functionalizing the terminal hydroxyl groups of the polymer using chlorides or acid anhydrides and adding excipient substances, such as poloxamer, glycerin, zinc acetate, or PEG [6, 7, 9, 14, 18].
Over the past decades, many new chemotherapeutic agents have been discovered, but their poor water solubility posed a significant obstacle to further development. The issue of poor solubility prevents the intravenous (IV) administration of chemotherapeutic agents, and the use of high concentrations of solubility-enhancing surfactants often leads to undesirable side effects [19]. A commonly used method to enhance solubility is the application of polymer carriers. The use of the PLGA-PEG-PLGA carrier increased the solubility of paclitaxel, cyclosporine A, simvastatin, and indomethacin by 40–4000 times [4, 10, 20–22]. Another advantage of this carrier is its ability to influence the chemical form of the administered drug. Irinotecan, a camptothecin derivative and topoisomerase I inhibitor, is primarily used as an anticancer drug. Irinotecan exists in two forms: the active (lactone) and inactive (carboxylate) forms, with the latter dominating at physiological pH [23]. A DDS consisting of PLGA-PEG-PLGA polymer and irinotecan was developed, and its effectiveness was tested in vivo through subcutaneous administration to mice with SW620 colon tumors. The use of the polymer favored the active form of irinotecan in the thermogel, which amounted to approximately 50%, and the increased active lactone fraction led to higher drug efficacy and reduced side effects [11].
- •
A biodegradable and biocompatible carrier for low and high-molecular-weight compounds;
- •
An intelligent temperature-responsive polymer (thermosensitive);
- •
An amphiphilic polymer with a low CMC, making it soluble in water and organic solvents;
- •
Capable of increasing the solubility of poorly soluble compounds;
- •
Able to encapsulate multiple compounds, making it suitable for combination therapies;
- •
Its properties can be modified by altering substrate ratios, functionalizing terminal groups, or adding excipients.
3. Polymer Synthesis
There is a significant amount of research on the synthesis of PLGA-PEG-PLGA in the global literature. Table S1 lists the methods and techniques used by various researchers for the synthesis of PLGA-PEG-PLGA copolymers. The original synthesis of the triblock copolymer PLGA-PEG-PLGA is based on chain growth following the ring-opening polymerization (ROP) mechanism of the cyclic lactide derivative of lactic acid and the cyclic glycolide derivative of glycolic acid. The reaction also involves PEG and Sn (Oct)2. Two theories have been proposed regarding the mechanism of Sn (Oct)2 action. The first suggests that Sn (Oct)2 coordinates with the alcohol and cyclic ester monomer, followed by ROP. In this mechanism, Sn (Oct)2 acts as a catalyst, while the alcohol serves as the initiator. The second theory suggests that the true initiators of the reaction are OctSn-OR and Sn (OR)2 compounds, formed in situ through a reaction between Sn (Oct)2 and ROH [24, 25].
The synthesis of PLGA-PEG-PLGA polymer is carried out in the melt state under an inert gas atmosphere at a high vacuum, typically between 130 and 400 Pa [26]. Glycolide reactivity in the presence of Sn (Oct)2 is higher than that of lactide, so the ring opening of glycolide, which first attaches to both ends of PEG, is favored [7, 27]. During polymerization, the process of transesterification can lead to changes in the arrangement of monomers within the polymer chains, significantly impacting the polymer’s properties, such as gelation temperature [7, 25]. The transesterification process, illustrated in Figure 1, occurs over time and is strongly influenced by the reaction temperature. Depending on these conditions, the monomer sequence can shift from an ordered to a more randomized arrangement [28]. This highlights the importance of precisely controlling both the polymerization time and temperature to ensure a reproducible polymer structure with the desired properties.
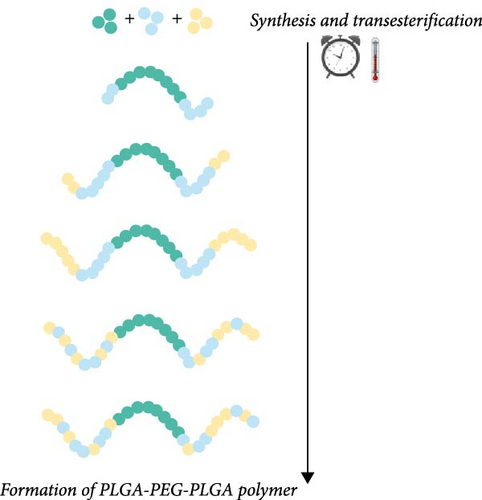
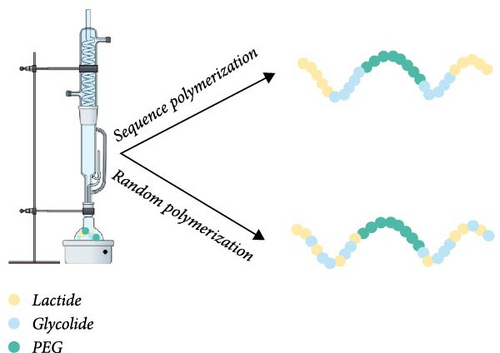
In addition to random copolymers, it is possible to obtain sequenced polymers. The triblock copolymers produced in this manner significantly differ from PLGA-PEG-PLGA synthesized by the random attachment of glycolic and lactic acid units. The ability to produce PLGA-PEG-PLGA with a specific (programed) sequence of monomers is particularly important because the sequence of LA and GA in the polymer influences the swelling and erosion of the polymer, which are among the primary mechanisms of drug release from polymer carriers [15, 29]. Sequenced PLGA microparticles degraded at a slower and more consistent rate compared to random copolymers with identical lactic acid to glycolic acid ratios. In vitro studies showed that sequenced copolymers released rhodamine-B gradually, in contrast to the more abrupt release observed with random sequence copolymers. Therefore, polymer sequencing can be a tool to control the release kinetics of the active substance and reduce the burst release effect [30].
The synthesis method commonly employed in the literature involves using an oil bath with Sn (Oct)2 as a catalyst. The use of an oil bath ensures precise temperature control within the reaction vessel, which can improve the control over polymerization degree, reduce the amount of unreacted monomers, and enhance the efficiency and reproducibility of the process [31–33].
Microwave-assisted ROP has been utilized as a novel technique, with the synthesis results compared to those obtained using conventional polymerization methods. Microwave-assisted synthesis achieved higher efficiency, reduced the synthesis time to 5 min, and resulted in polymers with dispersity <1.5, lower gelation temperatures, and a broader gelation window compared to the conventional method [34].
Another innovation is the method involving supercritical CO2. The optimization of pressure, temperature, and synthesis time was performed, and the results were compared with the previously developed microwave-assisted method [35]. It was observed that the polymer’s molecular weight increased with extended synthesis time (from 0.59 to 17.41 h) and within a pressure range of 266–367 bar. Within a temperature range of 116°C–144°C, the molecular weight gradually increased, reaching a maximum value of 7100 g/mol. However, further temperature increases to 184°C led to a slight decrease in molecular weight, likely due to monomer degradation. The LA/GA molar ratio in the polymerization process using supercritical CO2 precisely matched the theoretical value. In the microwave-assisted method, it was lower than expected, but the synthesis of triblock copolymers using microwave irradiation was significantly faster than with supercritical CO2 [35].
Besides the ROP method, PLGA-PEG-PLGA polymer can be obtained through the activation of PLGA and subsequent reaction with PEG-bis-amine (NH2-PEG-NH2). The synthesis of PLGA-PEG-PLGA involves activating PLGA using N, N′-dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (NHS) in dichloromethane (DCM), enabling the formation of reactive end groups. The activated PLGA then reacts with PEG-bis-amine in DCM, forming the triblock copolymer PLGA-PEG-PLGA. DCC acts as a condensing agent, and NHS enhances reaction efficiency, leading to a stable final product [20, 36].
PLGA-PEG-PLGA was also obtained via polycondensation of lactic acid, glycolic acid, and PEG in an inert gas atmosphere under vacuum for 10 h at 150°C, using H2SO4 as a catalyst. The resulting polymer was mixed with maghemite (Fe2O3) and co-trimoxazole in the molten state, producing a nanocomposite capable of drug release through the application of a magnetic field [37]. Polycondensation also allows for the production of linear-dendritic (barbell-like) PLGA-PEG-PLGA polymers. The first step involved obtaining tetra- and octa-hydroxy PEG, followed by polycondensation at 165°C–180°C under 1300 Pa between PEG and lactic and glycolic acid in the presence of SnCl2 as a catalyst [38]. Another polycondensation synthesis involved obtaining PLGA through the condensation of lactic and glycolic acid in the presence of Sn (Oct)2 at 150°C for 7 h, followed by reaction with PEG 6000 g/mol at 170°C for 10 h under an inert gas atmosphere using various PLGA weight ratios [39]. The high temperature and long synthesis time led to depolymerization, resulting in PLGA-PEG-PLGA polymers with a molecular weight (Mw) of approximately 2500.
A crucial step after the synthesis of PLGA-PEG-PLGA is its purification, which aims to remove unreacted low molecular weight compounds, including the catalyst, which is often highly toxic. This process can be conducted in two ways. The first involves dissolving the polymer in water and then precipitating it at elevated temperatures [4, 34, 40]. An alternative purification method is solvent precipitation, which involves dissolving the polymer in an organic solvent such as DCM, chloroform, methylene chloride, or tetrahydrofuran, followed by precipitation through the addition of a second solvent [26, 38, 41, 42]. The second method is particularly useful and effective in removing the tin catalyst (Sn (Oct)2), which is insoluble in water. The effect of the precipitating solvent on the polymer’s thermogelling properties was examined. The polymer was first dissolved in DCM, and then one of the selected precipitating solvents was used: diethyl ether, hexane, or methanol [43]. Precipitation with hexane and diethyl ether resulted in polymers with similar molecular weight (Mn) of 5500 and 5430, while methanol precipitation produced polymers with higher Mn of 6,590 and larger particle size. This difference can be attributed to the poor solubility of PLGA in methanol, combined with the excellent solubility of PEG, especially when the PEG blocks are short. Additionally, methanol precipitation led to polymers with lower dispersion, lower gelation temperature, and lower CMC.
- 1.
ROP in the molten state, polymerization in an inert gas atmosphere at atmospheric pressure.
- 2.
ROP in the molten state, polymerization under vacuum conditions.
- 3.
ROP in the molten state, polymerization in an inert gas atmosphere at atmospheric pressure in an oil bath with very precise temperature control.
- 4.
ROP in the molten state, synthesis assisted by microwave irradiation at atmospheric pressure.
- 5.
ROP in solvent—supercritical CO2 under high-pressure conditions.
- 6.
ROP in organic solvent at the boiling temperature of the solvent, e.g., toluene.
- 7.
Polycondensation reaction with variously activated PLGA and PEG.
The synthesis methods presented vary significantly in terms of their efficiency, polymer properties, and reaction conditions. ROP methods, including both traditional and microwave-assisted approaches, offer fast synthesis times, with microwave irradiation providing better control over polymer properties such as molecular weight and dispersity. Supercritical CO2-based polymerization provides the advantage of precise control over molecular weight but requires longer synthesis times and specialized equipment. Polycondensation methods, though slower and prone to lower molecular weights, allow for the synthesis of linear-dendritic copolymers and enable functionalization with nanomaterials. Purification methods are essential for removing toxic catalysts and unreacted compounds, with solvent precipitation techniques being more effective in removing metal catalysts. Each method has its advantages, and the choice of technique depends on the desired polymer characteristics, such as molecular weight, dispersity, gelation properties, and the speed of synthesis. However, when characterizing these polymers using NMR, the literature often lacks crucial information regarding the L-G-L sequence that governs transesterification, a key factor in the stabilization of the three-dimensional gel structure. This sequence plays a significant role in determining the gelation properties and the stability of the polymer network [16, 27, 44].
4. Cytotoxicity Assessment of the Polymer
- •
Direct method, where the tested material has direct contact with the cell culture;
- •
Diffusion method, where the tested material is in direct contact with agar and indirect contact with the cell culture;
- •
Extract method involves the use of an extract from the materials obtained through incubation.
The approach to testing polymer cytotoxicity should reflect its actual application as closely as possible, considering the potential site of use [45].
The biodegradability and biocompatibility of PLGA-PEG-PLGA polymer have been repeatedly demonstrated in many applications [4, 8, 10, 20, 46, 47]. However, interpreting in vitro cytotoxicity test results can be complicated due to the specific conditions of these tests. One factor complicating cytotoxicity assessment is the three-dimensional structure of the polymer. A high dose of hydrogel used during the viability test can lead to cell suffocation, which can result in false positive results. Significant cell death was observed when monolayer cells attached to the plate surface were exposed to direct contact with the polymer solution. This could be due to the dense and impermeable polymer structure, which prevented the diffusion of nutrients and oxygen from the medium, leading to cell death [48, 49]. Another factor disrupting in vitro polymer cytotoxicity measurement is degradation of the polymer. The degradation of PLGA-PEG-PLGA hydrogel generates glycolic and lactic acid, leading to a pH decrease that can introduce false-positive cytotoxicity results in vitro. [11]. Variations in buffer capacity, metabolism, and tissue distribution between in vitro and in vivo systems contribute to these discrepancies. Studies have shown that PLGA-PEG-PLGA hydrogels significantly reduce monocyte viability in vitro (<20% after 48 h, p < 0.001), with cytotoxic effects primarily attributed to acidic degradation products rather than inherent polymer toxicity. Buffering the medium with Tris has been reported to improve cell viability (p < 0.01), further supporting the pH-dependent nature of the observed effects [50]. In vivo, physiological buffering and metabolic clearance mitigate these changes, accounting for the polymer’s established biocompatibility. These findings underscore the need to refine in vitro cytotoxicity assays to more accurately reflect in vivo conditions.
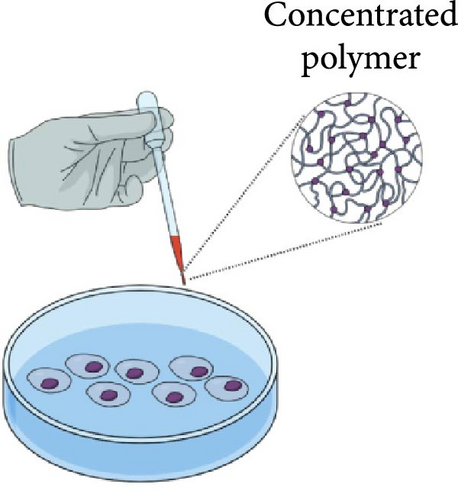
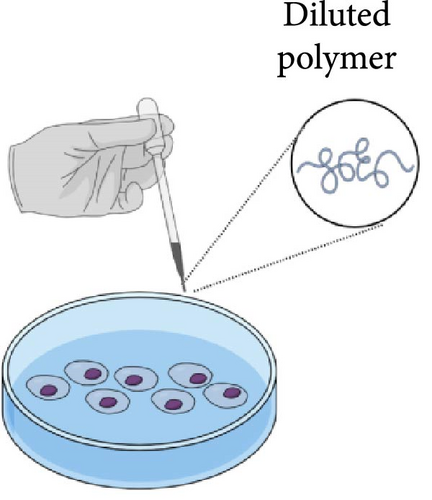
Three primary approaches have been employed in cytotoxicity studies of PLGA-PEG-PLGA polymer, as described in Figure 2 and Table 1.
| Cytotoxicity testing method | Polymer concentration [mg/mL] | Active substance | Type of test | Cell type | Reference |
|---|---|---|---|---|---|
| Gel state polymer testing | 150–250 | Dexamethasone, ketorolac, idebenone, and tocopherol | MTT | hTERT (RPE-1) | [12] |
| 200 | Metformin and levofloxacin | CCK8 | HCEC | [51] | |
| 200 | Curcumin | WST-1 | L929, N2a | [10] | |
| 200 | Doxorubicin and PLK1shRNA | MTT | Saos-2, MG-63 | [46] | |
| Diluted polymer testing | n/a | TGF-β1 | MTT | h-DPSC | [42] |
| 0.031–0.250 | Doxorubicin | CCK8 | L02 | [52] | |
| 1 | US597 | MTT | HepG-2, B16F10, SW620 | [20] | |
| 0.012 | Doxorubicin | MTT | MCF-7 | [33] | |
| 0.01–10 | n/a | CCK8 | MC3Tc | [11] | |
| 0.1–10 | Temozolomide | MTT | U87-MG/Luc | [48] | |
| 0.005–0.32 | Andrographolide | MTT | MAD-MB-231 | [36] | |
| 0.06–4 | n/a | MTT | U251 | [53] | |
| 0.0625–4 | Polyethyleneimine | MTT | Chondrocytes and Synoviocytes | [54] | |
| Polymer extract testing | 300 | n/a | MTT | BALB/3T3 | [13] |
| 500 | Risperidone | MTT | L929 | [55] | |
- Note: US597, 3β-acetoxy-urs-12-en-28-oic acid hexamethylenediamine.
- Abbreviations: n/a, not available; TGF-β1, transforming growth factor beta.
The first method involves testing the polymer at a sufficiently high concentration to enable gel formation at the site of application [46]. Cytotoxicity was evaluated for PLGA-PEG-PLGA polymers with GA:LA ratios of 10:1 and 15:1. The cytotoxicity was assessed using the hTERT (RPE-1) cell line, with polymer concentrations ranging from 15% to 25%. The cell viability for the tested polymers and concentrations exceeded 80%, with slightly higher viability observed for the polymer with the higher GA ratio. The MTT, CCK8, and WST-1 assays are all commonly used methods to assess cell viability, with MTT measuring mitochondrial activity, CCK8 evaluating cell proliferation, and WST-1 assessing metabolic activity. After selecting the optimal polymer concentration, formulations containing dexamethasone, ketorolac, idebenone, and tocopherol were prepared. Some cell viability results exceeded 100%, suggesting not only a lack of cytotoxicity but also a potential proliferative effect of certain formulations [12]. Low cytotoxicity was also noted for polymer formulations containing metformin (MET) and levofloxacin (LFH) on the HCEC cell line [51]. No significant difference in cell viability was observed for the polymer formulation containing curcumin on the L929 and N2a cell lines [10 ]. Testing the polymer in its gel state allows for the evaluation of the polymer’s properties in the actual form it will assume under application conditions, enabling the study of local interactions between the gel and cells, which is crucial for assessing biocompatibility. However, increasing the polymer concentration also increases its viscosity, potentially complicating the accurate sampling of both the polymer and the encapsulated substances, which may affect measurement accuracy.
The second method involves analyzing the polymer in a highly diluted state. Using a diluted polymer solution does not result in significant cytotoxicity, either compared to PEG or PLGA polymers [11, 52, 53]. An oral formulation of PLGA-PEG-PLGA triblock micelles was developed. The polymer showed no noticeable cytotoxicity on HepG-2, B16F10, and SW620 cell lines, and at low concentrations, the cytotoxicity of free US597 in HepG-2 cells was slightly higher than that of US597 micelles [20]. Empty copolymer micelles did not exhibit cytotoxicity against MCF-7 cells after 48 h of incubation. However, PLGA-PEG-PLGA micelles containing doxorubicin showed slightly lower cytotoxicity at all pH levels compared to the DOX solution [33]. The polymer did not exhibit cytotoxicity within the 0.1–10 mg/mL range on the U87-MG/Luc cell line, and the use of the carrier increased the IC50 to 2390 µg/mL compared to an IC50 of 1080 µg/mL for the group that received temozolomide alone [48]. This method is characterized by simplicity, easier standardization, and greater dosing reproducibility. The main drawback is the lack of representativeness associated with the form of the polymer in which it is tested. A lower concentration polymer does not accurately reflect the actual application conditions, which may lead to different degradation behavior, thereby altering the kinetics of active substance release and toxicity.
The third method involves analyzing the extract from the polymer in its gel state. The developed thermosensitive polymer was extracted with PBS after 10 days of incubation at 37°C or 70°C. The obtained extracts were neutralized to pH 7.4 and diluted with medium in ratios ranging from 1:1 to 1:16. Cytotoxicity was assessed using the MTT test on the BALB/3T3 cell line. No significant differences were observed in cell viability in media diluted with extracts compared to the control for all dilutions [13]. A similar experiment was conducted where the polymer containing risperidone was extracted with PBS at 37°C in a stirred bath for a period ranging from 2 h to 35 days. The cytotoxicity of the extracts was assessed using the MTT test on the L929 cell line. PLGA-PEG-PLGA polymer without risperidone exhibited minimal cytotoxicity throughout the study period, with cell viability slightly lower compared to the control group [55]. The extract testing method simulates physiologically degraded material, based on the fact that hydrolysis is one of the degradation mechanisms of the polymer, allowing for direct evaluation of the impact of polymer degradation products on cells while simulating conditions within the body. The drawback of this method is the time-consuming nature due to additional steps required to prepare extracts and the variability in the reproducibility of the obtained extract. In Table 1, the different cell lines are presented. The various cell types used in these assays, such as hTERT (RPE-1), HepG-2, B16F10, SW620, MCF-7, L929, N2a, and BALB/3T3, vary in terms of origin, tissue type, and response to the tested substances, which may explain differences in cytotoxicity results and methodologies.
5. In Vivo Applications of the Polymer
The PLGA-PEG-PLGA polymer, due to its biodegradable and biocompatible properties, finds wide application in biomedicine as a carrier for various active substances, becoming an intelligent DDS (Figure 3, Table S2).
The first group of in vivo applications for this polymer involves the development of biodegradable implants for controlled release. Delivering drugs to the eyes presents challenges due to the presence of selective biological barriers, such as the tight junctions of the corneal epithelium. One of the most commonly used forms of drug administration is eye drops, which are rapidly cleared by mechanisms like tears, leading to low bioavailability and necessitating higher and more frequent doses of the drug [56]. The use of PLGA-PEG-PLGA polymer as a carrier for dexamethasone acetate significantly improved the bioavailability of this active substance. A sevenfold increase in Cmax and an eightfold higher AUC was achieved compared to eye drops [57]. A controlled release system for treating posterior segment eye diseases, such as diabetic retinopathy, was also developed. The polymer formulation exhibited prolonged insulin release, and polymer degradation did not cause retinal damage after subconjunctival injection [58]. The effectiveness of a formulation containing MET and LFH in treating corneal neovascularization (CNV) was also studied. A single subconjunctival administration of the polymer with MET and LFH resulted in controlled release of both substances and demonstrated synergistic effects, significantly inhibiting CNV formation [51].
The polymer has also been applied as a controlled release system in addiction therapy. In vivo pharmacokinetic studies showed an extended half-life of naltrexone hydrochloride [59]. The prolonged half-life allows for reduced drug side effects and decreased dosing frequency, leading to better therapeutic efficacy. A similar effect was achieved with curcumin delivery. The use of the polymer carrier improved curcumin’s bioavailability after IV administration, increasing AUC(0–∞), t1/2α, t1/2β, and MRT by 1.3-, 2.5-, 4.5-, and 2.7-fold, respectively, compared to the curcumin solution. Moreover, in the group administered polymer micelles, reduced drug uptake by the liver and spleen, and increased distribution in the brain and lungs were observed [60]. Thanks to increased brain distribution, micelles can be effectively used in treating various neurological and oncological diseases. The PLGA-PEG-PLGA carrier enables more effective, safer, and precise therapies that were previously difficult to achieve due to limitations in delivering drugs to the central nervous system [10].
Biopharmaceuticals are gaining increasing popularity due to their high efficacy and precise action in treating various diseases. Their ability to target specific areas and reduce the risk of side effects makes them increasingly preferred by physicians. For these molecules, the polymer carrier can protect the encapsulated substance from proteolytic enzymes, the immune system, and adverse environmental conditions, such as pH, which accelerate drug metabolism. This can lead to the need for higher and more frequent doses of often expensive biopharmaceuticals [61, 62]. The amphiphilic structure of PLGA-PEG-PLGA enables the encapsulation of biopharmaceuticals via self-assembly in an aqueous solution, preserving the full activity of the encapsulated substance. A single subcutaneous injection of polymer-loaded pGH at a dose of 70 mg/mL resulted in weight gain equivalent to daily injections of 5 mg pGH over 14 consecutive days [4]. Compared to intravenously administered pGH, the overall bioavailability of pGH for the group using subcutaneous polymer was 38.20% to 86.03%. This was approximately 4.5–14.5 times more than subcutaneous pGH administration [13]. In a pharmacokinetic study conducted on animals, subcutaneous injection of bispecific T-cell engaging (BiTE) antibodies tripled the half-life compared to IV injection. This allowed BiTE levels to remain optimally low with smaller fluctuations in blood levels in live mice, resulting in almost zero cytokine release [63].
Reviewing the literature, it can be concluded that among all applications, the PLGA-PEG-PLGA polymer is most frequently used in cancer therapies (Table S2). To date, it has been used in treating cancers of the brain, lungs, colon, liver, bone, and breast [11, 36, 48, 64–68]. One reason limiting the effectiveness of cancer treatments is the low solubility of hydrophobic compounds in water. The use of the polymer carrier increases the solubility of highly hydrophobic compounds [11, 64]. Controlled and localized release of the active substance by the polymer enables increased bioavailability, maintaining active substance concentration between the minimum effective concentration (MEC) and the minimum toxic concentration (MTC), and reducing systemic toxicity [11, 20, 36, 39, 48]. Chemoresistance is the primary reason for the failure of conventional chemotherapy. Combination therapy, which uses a combination of drugs with different mechanisms of action, can overcome chemoresistance through synergistic effects, reduced toxicity, and prevent cancer cells from developing resistance to multiple drugs simultaneously [46, 68].
The development of intelligent drug carriers based on the PLGA-PEG-PLGA polymer goes beyond synthesis alone. Introducing modifications to these carriers aims to enhance their efficacy and safety in drug delivery. The most popular method of modifying the polymer’s properties is by altering substrates during synthesis. However, other methods include using hybrid carriers with liposomes, adding excipients, and various functionalization techniques. The addition of the polymer to liposomal systems reduced the initial drug burst, increased mean residence time, and demonstrated controlled release [66, 69, 70]. PEGylation of camptothecin increased its solubility from 1.3 µg/mL to over 150 mg/mL; however, it also increased polymer viscosity and lowered the sol–gel transition temperature [67]. The addition of glycerol to the polymer solution increased the release rate, while adding poloxamer decreased it. Adding both substances lowered the gelation temperature [71]. The addition of poloxamer or polysorbate increased BBB penetration, which could significantly improve the effectiveness of neurological disease treatments [72]. The use of thermoreversible hydrogel (PLGA-PEG-PLGA) slowed EXT release, but a significant drug burst effect was observed in the initial stage. Introducing zinc acetate slowed EXT release by forming insoluble Zn-EXT complexes, which led to too slow a release. The synergistic action of excipients (zinc acetate, PEG, and sucrose) overcame these issues, ensuring prolonged drug release for 1 week [14]. The polymer’s terminal functional groups can be functionalized to alter polymer properties, such as gelation temperature or release kinetics [73–75]. A synergistic therapy for hepatoblastoma was developed by combining an acid anhydride with DOX and subsequently conjugating it with the terminal hydroxyl groups of the PLGA-PEG-PLGA polymer, into which docetaxel was later introduced. In the resulting DDS, faster release was observed, supported by low pH, increased anticancer efficacy, and favorable in vivo safety [68]. A similar drug delivery system based on PLGA-PEG-PLGA was created, where the terminal groups were functionalized with L-histidine, which accelerated degradation in reduced pH conditions [6, 33]. Such a system could be targeted for drug delivery to many diseases, such as cancers, where lower pH is observed, leading to increased efficacy and reduced side effects. Additionally, other dual-sensitive systems based on PLGA-PEG-PLGA have been developed that, in addition to thermosensitivity, also respond to external stimuli such as UV radiation, magnetic fields, or electric fields [37, 76, 77]. Such advanced DDSs open new therapeutic possibilities and allow for more precise and effective treatment of various diseases.
6. Conclusions
One of the key stages in developing a polymer formulation is the synthesis of the PLGA-PEG-PLGA polymer. Due to the risk of transesterification and depolymerization, which can affect the polymer’s properties, the synthesis process must be conducted under strictly controlled conditions [7, 39, 78]. The hydrophilic-hydrophobic balance and the gelation temperature of the polymer can be modified by altering substrate proportions, PEG molecular weight, or through functionalization [9, 16]. This article presented and discussed the existing approaches to synthesizing PLGA-PEG-PLGA polymer.
The next stage involves encapsulating the active substance and testing the polymer’s cytotoxicity in in vitro studies. The cytotoxicity testing method should be accurate, reproducible, and appropriate for the intended polymer application. The cytotoxicity testing methods include gel state polymer testing, diluted polymer testing, and extract testing.
The PLGA-PEG-PLGA polymer has found wide application as a controlled DDS. The release time of the encapsulated substance can range from 1 day to as long as 60 days [58, 69, 79]. The use of PLGA-PEG-PLGA as a DDS offers benefits such as increased solubility of hydrophobic compounds, the ability to encapsulate labile compounds, reduction of initial burst release, increased bioavailability, and the possibility of combination therapy through encapsulation of multiple compounds [11, 12, 66, 69, 70]. It is also possible to develop dual-sensitive systems in which the active substance is released more rapidly in an environment specific to cancer cells, leading to better therapeutic effects and reduced side effects [76, 77]. The release kinetics should be tailored to the intended purpose and site of application, which can be achieved through the use of hybrid systems, varying polymer concentrations, or the addition of excipients.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The research for this project was funded by ASLAB Science.
Acknowledgments
The authors have nothing to report.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The source data are available upon reasonable request.




