Design and Fabrication of Bone Scaffolds With Regular and Irregular Voronoi Architectures: A Comparative Study
Abstract
Bone diseases and consequent defects present a significant challenge in the orthopedics. Synthetic scaffolds mimic bone porose structures and can be substituted in bone defects. In this study, we designed and evaluated four scaffold models with different architectures (regular Voronoi (Rv), irregular Voronoi (Iv), Star (S), and Vintiles (V) structures). Additionally, the scaffolds were designed with four different porosities (50%, 60%, 70%, and 80%), and 16 scaffold models were designed and manufactured using the three-dimensional (3D) printing (3DP) method. The models were fabricated using two photosensitive resins (50% PLA-Pro resin and 50% P-CROWN [zirconia and ceramic]). Thus, the models’ mechanical properties were tested using compression tests. The results showed porosity plays an essential role in scaffold mechanical behavior. Moreover, the architecture was effective in the mechanical performance of the models. The elastic modulus of the models was 4–30 MPa, which is close to trabecular bone mechanical properties. The S-50 model showed a maximum stress of 17.75 MPa, which was 20 times higher than the S-80 model. Similar results were visible in other groups of scaffolds. In all four groups, 50% and 80% porosity scaffolds showed the highest and lowest mechanical strength, respectively. The results of this study showed that the Voronoi structure mimics bone morphology with a stochastic porosity and demonstrated a mechanical property similar to the scaffold with regular structures, which confirms its compatibility with bone tissue engineering. The outcomes of this study shed more light on scaffold design and fabrication for bone defects.
1. Introduction
Bone defect treatment has become a major clinical operation as a result of trauma, tumor resections, degenerative diseases, infections, and accidents. Millions of people suffer from bone defects resulting from the diseases above, which have caused significant clinical challenges [1]. The tissue and organ defects are mainly treated using allografts, autografts, and xenografts. Sometimes, it is not possible to find a donor or arrange for growth after traumatic events for damaged organs or bones. Moreover, these sources for tissue replacement are always accompanied by problems [2] that prompt biomedical and tissue engineers to develop artificial synthetic tissues that facilitate the regeneration of tissues, such as bone [3]. Recently, bone replacement tissue engineering has presented some successful approaches. Design and manufacturing scaffolds for bone that mimic bone’s natural structure have attracted the attention of researchers in the field.
The mechanical and physical properties of a scaffold should satisfy host bone necessities. An ideal scaffold should be stiff enough against biomechanical loads and contain porosity to promote cell differentiation and proliferation. Therefore, most studies in recent years have focused on scaffold architecture and manufacturing that controls these requirements [4, 5]. The success of a scaffold directly depends on having an architecture similar to the host bone structure in the first place. Therefore, tissue engineers make great efforts to mimic bone architecture in these constructs as closely as possible because the scaffold architecture determines the final structure of the newly generated bone [6].
Furthermore, it has to possess interconnected porosity networks allowing vascularization, cell migration, and nutrient delivery [7, 8]. Lattice structures, with their intricate network of interlinked struts, have gained significant attention in various fields, particularly biomedical engineering. These structures offer a unique combination of properties, including a high strength-to-weight ratio, high porosity, and the ability to tailor their mechanical properties for specific applications. In addition to regular and classical scaffold architectures, scaffolds with random morphology have shown promising results in accelerating the healing process of bone defects.
The development of additive manufacturing technologies allows tissue engineers to design and manufacture scaffolds with precise size and control the effective parameters for scaffolds’ success. Voronoi architecture is one of the random morphologies widely used in scaffold design [9]. The unique structure of Voronoi scaffolds can help simulate the extracellular matrix found in natural tissues, encouraging cells to grow more naturally and functionally [2]. This is especially important in tissue engineering for developing implants that can integrate seamlessly into the body. The porous structure of Voronoi scaffolds facilitates the movement of fluids (e.g., blood or lymph) and nutrients throughout the scaffold, ensuring that the cells within the scaffold remain nourished and functional [4]. In some material applications, Voronoi scaffolds can be designed to respond to environmental changes, such as stress or strain. The structural pattern can enable self-healing or adaptive behavior, which helps develop dynamic materials that adjust to changing conditions [5]. The unique structure of Voronoi scaffolds can help simulate the extracellular matrix found in natural tissues, encouraging cells to grow more naturally and functionally [10, 11].
Despite all the advantages of Voronoi scaffolds, unresolved issues still deserve further research. For instance, difficulty in fabrication arising from their irregular structure is challenging for tissue engineers. Stress shielding and interconnectivity control of pores are also other issues that remain unsolved [12]. Where numerical solution and simulation for regular scaffolds are powerful in artificial tissue design, Voronoi scaffold modeling is not a straightforward task because of their complex morphology [13]. In conclusion, the main problems with Voronoi scaffolds revolve around their complexity in structure and manufacturing, the need to optimize material properties, and the challenge of ensuring proper connectivity and uniformity. However, these issues can often be mitigated through computational methods and advanced manufacturing techniques [9]. The Vinitles architecture showed irregular mechanical performance, likely due to nonuniform stress distribution arising from its asymmetric design and variable node-to-strut ratios. Unlike more symmetric TPMS-based structures, Vintiles (V) lacks consistent load pathways, which may lead to early localized failure or unpredictable deformation modes. Similar findings have been reported in lattice structure studies where irregular architectures exhibited inferior mechanical behavior due to geometric discontinuities and reduced redundancy in load-bearing pathways.
This study offers a number of contributions, some of which are as follows: We designed different types of scaffolds for bone, namely regular Voronoi (Rv), irregular Voronoi (Iv), star (S), and tin tiles, to test their mechanical properties. We manufactured these models at four porosities to examine the effect of porosity on their strength. The results of this study shed light on the selection and design of scaffolds for bone in tissue engineering.
2. Material and Methods
2.1. Design of the Scaffolds
The selected porosity levels (50%, 60%, 70%, and 80%) were chosen based on a balance between biological and mechanical requirements for bone tissue engineering. Literature indicates that porosities above 50% are essential to facilitate sufficient vascularization, nutrient transport, and bone ingrowth [14]. However, excessively high porosity can compromise mechanical stability. The range chosen aligns with previous studies that report optimal bone regeneration and mechanical integrity within this porosity window [15]. Including this rationale in the revised manuscript enhances the biological relevance of our scaffold design.
The Rhino 8 and Grasshopper were implemented to design three-dimensional (3D) scaffold models. The software parametric design tools enable us to measure the model’s porosity and specific surface area while also providing precise control and easy-to-use of the model’s structural features. Four designs of scaffolds were namely Rv, Iv, S, and tin tiles scaffolds [16–19]. The Voronoi–Tessellation scaffold design method, as shown in Figure 1 is a typical modeling method. In particular, to construct the basic architecture, the model geometric space box is determined, the density of seed points (250), and the seed points are randomly distributed within the design volume to fulfill a space partition in regions. The Voronoi scaffold structure is generated by extracting and constructing the Voronoi cells from the space created by the Voronoi 3D in Grasshopper. Figure 2 shows the groups of scaffolds 3D models.
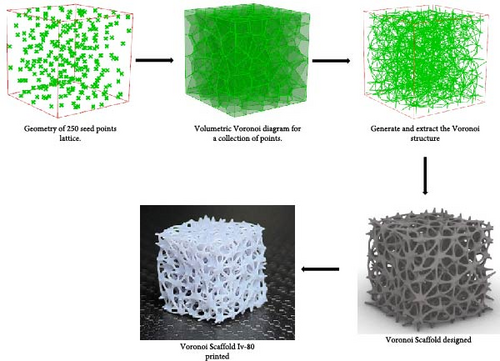
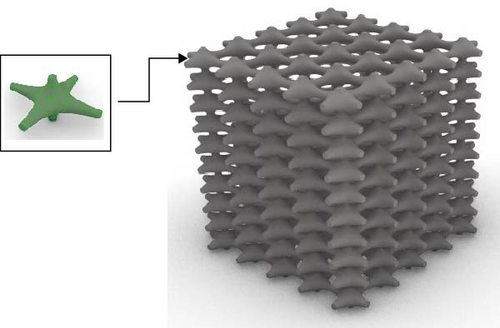
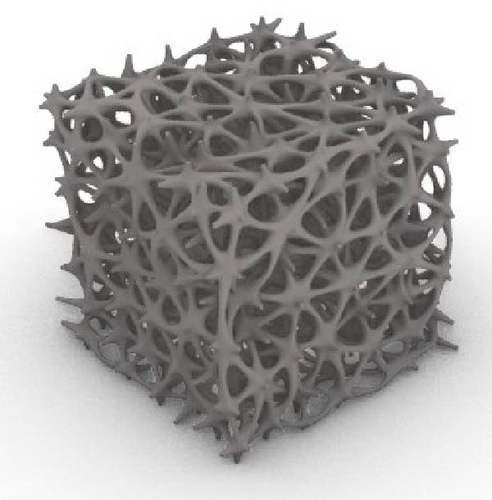
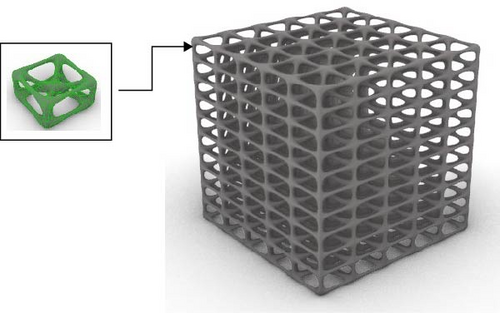
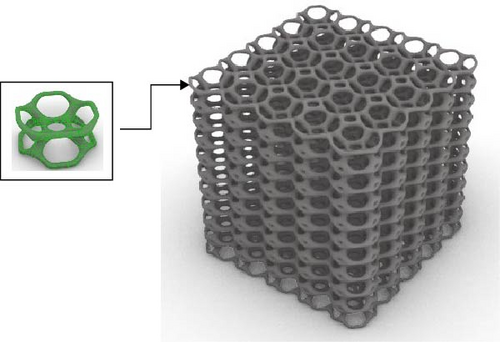
Models were built by repeating a distinguished unit cell in three axes except for scaffolds with Iv architecture and no repeating unit cell.
To understand the relation between porosity and node size, the node size of each model was presented in Table 1.
| Symbol | Porosity % (T−S/T) ×100 | Node size (mm) |
|---|---|---|
| Rv-50 | 50 | 0.51 |
| Rv-60 | 60 | 0.44 |
| Rv-70 | 70 | 0.37 |
| Rv-80 | 80 | 0.29 |
| Iv-50 | 50 | 0.28 |
| Iv-60 | 60 | 0.24 |
| Iv-70 | 70 | 0.2 |
| Iv-80 | 80 | 0.15 |
| S-50 | 50 | 0.188 |
| S-60 | 60 | 0.166 |
| S-70 | 70 | 0.14 |
| S-80 | 80 | 0.135 |
| V-50 | 50 | 0.23 |
| V-60 | 60 | 0.195 |
| V-70 | 70 | 0.162 |
| V-80 | 80 | 0.14 |
The 50:50 mixture of PLA-Pro and P-CROWN (zirconia and ceramic) was selected to leverage the complementary properties of both materials. PLA-Pro offers biodegradability and moderate mechanical strength, while P-CROWN contributes enhanced hardness, wear resistance, and bioinert ceramic stability. The combination is intended to optimize scaffold stiffness and support load-bearing applications while maintaining acceptable biocompatibility. Studies have shown that the inclusion of zirconia ceramics in polymer matrices can significantly improve osteoconductivity and cell adhesion [20]. This rationale has been added to the Materials and Methods section.
The scaffolds were designed at four different porosity 50%, 60%, 70%, and 80%. The generated scaffolds are shown in Figure 3. Table 1 also represents the node size and volume values for each model. The node size is essential in developing scaffolds with specific mechanical, biological, and structural properties. The node size is the diameter or physical dimensions of connected regions in a lattice structure, the scaffold’s overall stiffness and strength are determined by node size. Larger nodes improve mechanical stability but might reduce porosity, an essential parameter for better nutrient and oxygen diffusion [21]. The designed models were exported as Stereolithography (STL) files to manufacturing using the 3D printing (3DP) method. Based on scaffold architecture and porosity, the models were denoted as Rv-50, Rv-60, Rv-70, and Rv-80; Iv-50, Iv-60, Iv-70, and Iv-80; S-50, S-60, S-70, and S-80; and V-50, V-60, V-70, and V-80. For example, the Rv-70 model represents a scaffold with a Rv architecture and a porosity of 70% (Figure 3).
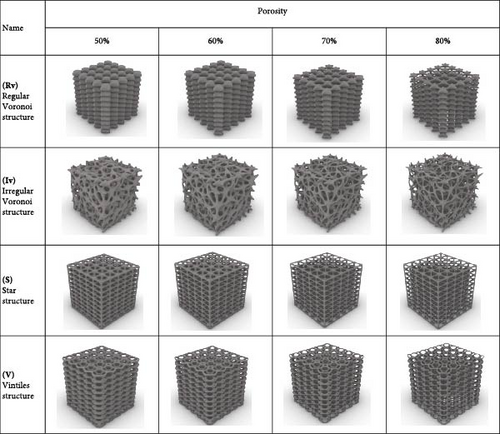
2.2. Fabrication of the Scaffolds
Postcuring was performed using a UV chamber (Anycubic Wash and Cure 2.0) at a wavelength of 405 nm and intensity of 60 mW/cm2 for 30 min. The postcuring temperature was maintained at 40°C to optimize polymer crosslinking without thermal deformation. These parameters were selected based on the manufacturer’s guidelines for PLA-Pro and the known photo-curing behavior of ceramic-containing resins. Post-curing ensures improved mechanical integrity and dimensional stability of the printed scaffolds.
The STL files were imported into Chitubox 3D slicing software, where they were processed to generate the necessary support structures and slicing parameters for 3DP. The Phrozen Sonic Mini 8K S Resin 3D Printer was used for scaffold fabrication. A blend of two photosensitive resins were used: 50% PLA-Pro resin and 50% P-CROWN (zirconia and ceramic) resin [22], and setting the various parameters, such as layer height: 0.050 mm, exposure time: 3.500 s, transition type: linear, bottom lift distance: 3.000 mm, bottom lift speed: 40.000 mm/min, and lifting speed: 40.000 mm/min. The PLA-Pro resin, sourced from Shenzhen Esun Industrial Co., Ltd., offers high precision, toughness, strength, and a smooth printing surface, with the following specifications: gray color, viscosity of 200–300 mPas, density of 1.09–1.10 g/cm3, tensile strength of 37–48 MPa, elongation at break of 25%–28%, flexural strength of 36–49 MPa, impact strength of 32–36 J/m, and tear strength as referenced [23]. P-CROWN resin is a zirconia and ceramic hybrid radiopaque material, CE-certified for 3DP of permanent single crowns, inlays, onlays, and veneers. It is a biocompatible medical class IIA resin with high strength, surface hardness, and low shrinkage. Its properties include a flexural strength of 440.00 MPa, flexural modulus more fabulous than 9500 MPa, water sorption less than 1.2 µg/mm, water solubility less than 0.09 µg/mm, and surface hardness of 95. It is noncytotoxic, nonirritant, nonsensitive, and nongenotoxic [24].
After printing, the scaffolds were subjected to a series of treatments to optimize their properties. Initially, the support structures were removed to reveal the samples in their final form. The samples were then immersed in a 99% pure ethanol solution and subjected to ultrasonic cleaning using a DK Sonic Digital Ultrasonic Cleaner. This process effectively removed any residual impurities and ensured the surfaces were clean. The scaffolds underwent postcuring treatment following ultrasonic cleaning to enhance their mechanical properties. The form cure device was employed, which precisely combined heat and UV light (405 nm) for approximately 10 min to consistently postcure the prints [25]. This treatment optimized the material properties of the STL prints, resulting in enhanced part strength and overall performance. The printed samples with symbols are shown in Figure 4.
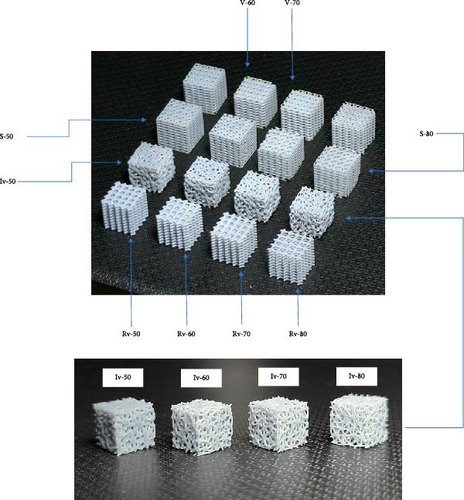
2.3. Quasi-Static Compression Test
Compression tests were indeed performed to evaluate the mechanical strength of the scaffolds. The tests were conducted using a universal testing machine (e.g., Instron 5967) under a crosshead speed of 1 mm/min until failure. The cylindrical scaffold specimens (10 mm diameter × 15 mm height) were used, and the compressive strength and modulus were recorded. These details have now been included in the Methods section for clarity and reproducibility. A compressive strength test was performed on scaffolds using an electromechanical compression machine (GESTER Universal Tensile Strength Tester model GT-C02-1 A). An increment compressive force was applied to the scaffold, allowing for the determination of the maximum load it can withstand before failure (Figure 5). All experiments adhered to the same environmental conditions as humidity and temperature.

The scaffolds were subjected to a progressive compressive load increase, while monitoring the resulting deformation. The resulting stress–strain curve provides valuable insights into the material and structural properties of the scaffolds. This curve reveals the relationship between the applied stress and the corresponding deformation, ultimately characterizing the compressive strength and stiffness of the scaffolds.
3. Results and Discussion
3.1. Accuracy of 3DP
Where complicated methods like micro-CT visualization are expensive techniques to evaluate the 3DP quality of scaffolds [26], mass measurement is a simple approach to understanding the accuracy of 3D printed samples [27]. This study measured the masses of 3D models and printed samples with a resolution of 0.01 g to check the printing accuracy. The result is shown in Table 2. Three samples of each model were printed, and their average mass was calculated. The mass of the CAD models was calculated by multiplying their volumes seen in the Rhinoceros software by the density of the selected materials (the density of a cubic sample with the size of 10 mm3 was determined as 1.17 g/mm3).
| Sample | CAD model mass (g) | Printed model mass (g) | Error (%) |
|---|---|---|---|
| Bulk model | — | 1.17 | — |
| Rv-50 | 0.58 | 0.59 | 1.72 |
| Rv-60 | 0.47 | 0.48 | 2.12 |
| Rv-70 | 0.35 | 0.37 | 5.71 |
| Rv-80 | 0.24 | 0.25 | 4.16 |
| Iv-50 | 0.58 | 0.62 | 6.89 |
| Iv-60 | 0.47 | 0.49 | 6.38 |
| Iv-70 | 0.35 | 0.37 | 5.71 |
| Iv-80 | 0.24 | 0.25 | 4.16 |
| S-50 | 0.58 | 0.61 | 5.17 |
| S-60 | 0.47 | 0.50 | 6.38 |
| S-70 | 0.35 | 0.37 | 5.71 |
| S-80 | 0.24 | 0.25 | 4.16 |
| V-50 | 0.58 | 0.60 | 3.44 |
| V-60 | 0.47 | 0.49 | 4.25 |
| V-70 | 0.35 | 0.36 | 2.85 |
| V-80 | 0.24 | 0.25 | 4.16 |
Table 2 data shows the printed samples are in good agreement with their original CAD models only with a maximum error of 6.9%. Ensuring the print quality of the samples enabled us to perform mechanical testing on them.
3.2. Mechanical Properties
The compression test results are crucial for determining the scaffold’s suitability for supporting bone regeneration and its capability to endure biomechanical loads in vivo. (Figure 6).
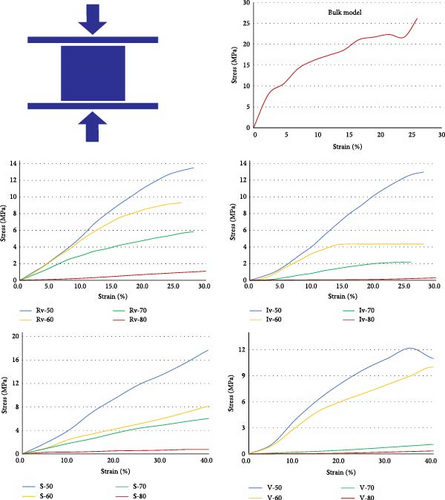
Regardless of the scaffold architecture, as the porosity increased, the compression strength decreased in all the designs. For example, in samples with S architecture, the S-50 model showed a maximum stress of 17.75 MPa, which was 20 times higher than the S-80 model. Similar results were visible in other groups of scaffolds. In all four groups, 50% and 80% porosity scaffolds showed the highest and lowest mechanical strength, respectively. Porosity has a primary role in governing scaffolds’ mechanical performance. Different trends were observed regarding the sensitivity of mechanical behavior to porosity in the scaffold groups. For example, mechanical resistance decreased in a regular pattern in the Rv, Iv, and S groups of scaffolds as porosity increased, but this is not observable in the V group models. Moreover, the scaffolds with Voronoi architecture were broken completely at the strain rate of 30%, and the other two groups resisted 40% of the strain. The architecture was also significant in the scaffold’s compression strength. The models with 60%, 70%, and 80% porosity Rv and S scaffolds showed relatively close mechanical performance. For instance, the S-50 models performed at a higher strength than their counter parts in other groups of scaffolds. Additionally, V-70 and V-80 showed lower strength than their counterparts in other scaffold groups.
Using Hooke’s law (σ = Eε), the elastic modulus of the scaffolds was determinate. The results are shown in Figure 7.
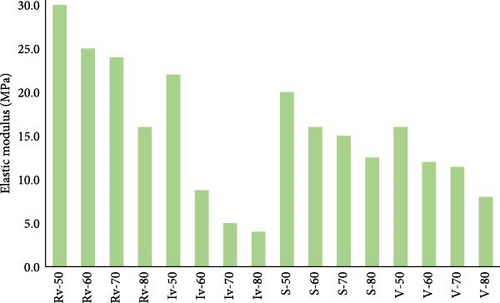
As seen in Figure 7, similar to compression strength, the porosity governed the models’ stiffens, which is more evident in the Iv group of scaffolds. Moreover, architecture was also a primary factor in the model. For example, the Rv group of scaffolds showed higher elastic modulus than others.
Although in vitro or in vivo biological tests were not performed in this study, we have expanded the discussion to include relevant literature supporting the biocompatibility and osteoconductive potential of PLA and zirconia-based composites. PLA is widely used in biomedical applications due to its biodegradability and minimal inflammatory response [20], while zirconia and ceramic reinforcements are known to promote cell attachment, proliferation, and osseointegration. These references are now cited in the discussion to support our material choices and the intended bone tissue applications.
The scaffold architectures were compared based on mechanical properties, porosity distribution, and manufacturability. Scaffold A showed the highest compressive strength and stiffness, suitable for load-bearing use. Scaffold B offered greater flexibility, while Scaffold C provided a balanced performance. Porosity in Scaffold A was the most uniform, aiding nutrient flow and cell migration. Scaffold B had variable porosity, and Scaffold C maintained consistent but slightly lower porosity. Scaffold A printed with high accuracy and minimal defects. Scaffold B required extra supports, while Scaffold C was faster to produce but needed more postprocessing. These results highlight design trade-offs relevant to biomedical applications.
We used 3DP technology to obtain composite scaffolds with different architectures and porosities. Then, the mechanical properties of scaffolds with regular and Voronoi architectures were investigated. The 3DP results showed an acceptable similarity between CAD models and fabricated scaffolds. The discrepancy between CAD and manufactured models is avoidable because of scaffold shrinkage after printing and postprocessing, like ultrasonic cleaning [28, 29]. The mechanical test results showed porosity was pivotal in scaffolds’ mechanical performance. However, in some models, the architecture was also effective in their mechanical behavior. Porosity causes voids in the scaffold structure and weakens the material resistance [30]. The study results agree with similar works in the literature [31, 32]. The literature reported a Young’s modulus of 5–50 MPa for the trabecular bone [33]. The elastic modulus of the models in this study showed an elastic modulus of 4–30 MPa, which is suitable for bone defect replacement. Regarding new and biomimetic structures, Voronoi models have already demonstrated good biocompatibility performance [9]. This study showed that the mechanical properties of the Voronoi structure were also similar to regular scaffolds, making it an outstanding model for bone replacements [34, 35].
4. Conclusion
- –
PLA-Pro resin and P-CROWN zirconia composition are suitable for precise 3DP.
- –
Voronoi structures with stochastic architecture show close mechanical properties to scaffolds with regular structures and cancellous bone, which makes it a potential bone-mimetic structure.
- –
Models with S and V architectures showed similar stiffness, which arises from their regular architectures.
- –
Although, the results of this study shed more light on scaffold design for bone tissue replacement, there is a need for more theoretical and experimental studies to obtain models with more biocompatibility.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




